Effects of an Alkalizing or Acidizing Diet on High-Intensity Exercise Performance under Normoxic and Hypoxic Conditions in Physically Active Adults: A Randomized, Crossover Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
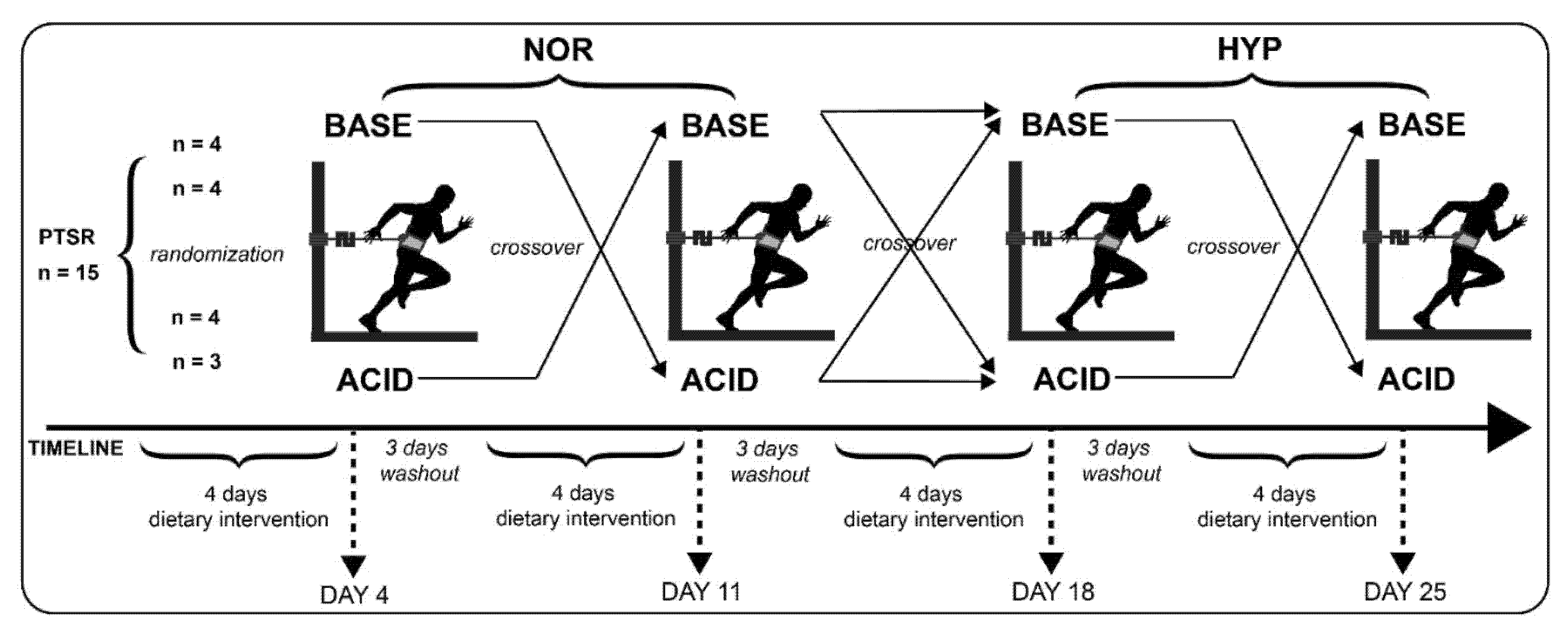
2.2. Experimental Design
2.3. Dietary Interventions
2.4. Urinary pH
2.5. High-Intensity Anaerobic Performance Test
2.6. Blood Gas Analysis
2.7. Anthropometric Characteristics
2.8. Hypoxic Conditions
2.9. Statistical Analysis
3. Results
3.1. Dietary Intervention
3.2. Urinary pH
3.3. High-Intensity Anaerobic Performance Test
3.4. Blood Gas Analysis
3.5. Regression Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Dataset
Conflicts of Interest
References
- Losnegard, T.; Myklebust, H.; Hallen, J. Anaerobic capacity as a determinant of performance in sprint skiing. Med. Sci. Sports Exerc. 2012, 44, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Hydren, J.R.; Kraemer, W.J.; Volek, J.S.; Dunn-Lewis, C.; Comstock, B.A.; Szivak, T.K.; Hooper, D.R.; Denegar, C.R.; Maresh, C.M. Performance changes during a weeklong high-altitude alpine ski-racing training camp in lowlander young athletes. J. Strength Cond. Res. 2013, 27, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Wirnitzer, K.C.; Kornexl, E. Exercise intensity during an 8-day mountain bike marathon race. Eur. J. Appl. Physiol. 2008, 104, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- McSharry, P.E. Effect of altitude on physiological performance: A statistical analysis using results of international football games. BMJ 2007, 335, 1278–1281. [Google Scholar] [CrossRef] [Green Version]
- Hamlin, M.J.; Hopkins, W.G.; Hollings, S.C. Effects of altitude on performance of elite track-and-field athletes. Int. J. Sports Physiol. Perform. 2015, 10, 881–887. [Google Scholar] [CrossRef]
- Watts, P.B. Physiology of difficult rock climbing. Eur. J. Appl. Physiol. 2004, 91, 361–372. [Google Scholar] [CrossRef]
- Bertuzzi, R.C.; Franchini, E.; Kokubun, E.; Kiss, M.A. Energy system contributions in indoor rock climbing. Eur. J. Appl. Physiol. 2007, 101, 293–300. [Google Scholar] [CrossRef]
- Baertsch, P. Innere Medizin: Hoehenanpassung. Dtsch. Z. Sportmed. 2000, 51, 139–140. [Google Scholar]
- Mazzeo, R.S. Physiological responses to exercise at altitude: An update. Sports Med. 2008, 38, 1–8. [Google Scholar] [CrossRef]
- West, J. High Altitude Medicine and Physiology, 5th ed.; CRC Press: Hoboken, NJ, USA, 2012; ISBN 9781444154320. [Google Scholar]
- Swenson, E.R. Hypoxia and its acid-base consequences: From mountains to malignancy. Adv. Exp. Med. Biol. 2016, 903, 301–323. [Google Scholar] [CrossRef]
- Zubieta-Calleja, G.; Zubieta-Castillo, G.; Zubieta-Calleja, L.; Ardaya-Zubieta, G.; Paulev, P.-E. Do over 200 million healthy altitude residents really suffer from chronic acid-base disorders? Indian J. Clin. Biochem. 2011, 26, 62–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez-Sandoval, J.C.; Castilla-Peon, M.F.; Gotes-Palazuelos, J.; Vazquez-Garcia, J.C.; Wagner, M.P.; Merelo-Arias, C.A.; Vega-Vega, O.; Rincon-Pedrero, R.; Correa-Rotter, R. Bicarbonate values for healthy residents living in cities above 1500 meters of altitude: A theoretical model and systematic review. High Alt. Med. Biol. 2016, 17, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Duffin, J. Role of acid-base balance in the chemoreflex control of breathing. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 2005, 99, 2255–2265. [Google Scholar] [CrossRef] [PubMed]
- Osnes, J.B.; Hermansen, L. Acid-base balance after maximal exercise of short duration. J. Appl. Physiol. 1972, 32, 59–63. [Google Scholar] [CrossRef]
- Cerretelli, P.; Samaja, M. Acid–base balance at exercise in normoxia and in chronic hypoxia. Revisiting the “lactate paradox”. Eur. J. Appl. Physiol. 2003, 90, 431–448. [Google Scholar] [CrossRef]
- Coudert, J. Anaerobic performance at altitude. Int. J. Sports Med. 1992, 13 (Suppl. 1), 5. [Google Scholar] [CrossRef]
- Yoshida, T.; Udo, M.; Chida, M.; Makiguchi, K.; Ichioka, M.; Muraoka, I. Arterial blood gases, acid-base balance, and lactate and gas exchange variables during hypoxic exercise. Int. J. Sports Med. 1989, 10, 279–285. [Google Scholar] [CrossRef]
- Rusko, H.K.; Tikkanen, H.O.; Peltonen, J.E. Altitude and endurance training. J. Sports Sci. 2004, 22, 928. [Google Scholar] [CrossRef]
- Sweeting, A.J.; Billaut, F.; Varley, M.C.; Rodriguez, R.F.; Hopkins, W.G.; Aughey, R.J. Variations in hypoxia impairs muscle oxygenation and performance during simulated team-sport running. Front. Physiol. 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatterer, H.; Menz, V.; Untersteiner, C.; Klarod, K.; Burtscher, M. Physiological factors associated with declining repeated sprint performance in hypoxia. J. Strength Cond. Res. 2017, 33, 211–216. [Google Scholar] [CrossRef]
- Dekerle, J.; Mucci, P.; Carter, H. Influence of moderate hypoxia on tolerance to high-intensity exercise. Eur. J. Appl. Physiol. 2012, 112, 327–335. [Google Scholar] [CrossRef] [PubMed]
- McLellan, T.M.; Cheung, S.S.; Meunier, M.R. The effect of normocapnic hypoxia and the duration of exposure to hypoxia on supramaximal exercise performance. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 66, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.P.; Jones, A.M.; Skiba, P.F.; Vanhatalo, A.; Wilkerson, D. Influence of hypoxia on the power-duration relationship during high-intensity exercise. Int. J. Sports Med. 2015, 36, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Morales-Alamo, D.; Ponce-Gonzalez, J.G.; Guadalupe-Grau, A.; Rodriguez-Garcia, L.; Santana, A.; Cusso, M.R.; Guerrero, M.; Guerra, B.; Dorado, C.; Calbet, J.A.L. Increased oxidative stress and anaerobic energy release, but blunted Thr172-AMPKalpha phosphorylation, in response to sprint exercise in severe acute hypoxia in humans. J. Appl. Physiol. (1985) 2012, 113, 917–928. [Google Scholar] [CrossRef] [Green Version]
- Burtscher, M.; Faulhaber, M.; Flatz, M.; Likar, R.; Nachbauer, W. Effects of short-term acclimatization to altitude (3200 m) on aerobic and anaerobic exercise performance. Int. J. Sports Med. 2006, 27, 629–635. [Google Scholar] [CrossRef]
- McLellan, T.M.; Kavanagh, M.F.; Jacobs, I. The effect of hypoxia on performance during 30 s or 45 s of supramaximal exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 60, 155–161. [Google Scholar] [CrossRef]
- Ogura, Y.; Katamoto, S.; Uchimaru, J.; Takahashi, K.; Naito, H. Effects of low and high levels of moderate hypoxia on anaerobic energy release during supramaximal cycle exercise. Eur. J. Appl. Physiol. 2006, 98, 41–47. [Google Scholar] [CrossRef]
- Oguri, K.; Fujimoto, H.; Sugimori, H.; Miyamoto, K.; Tachi, T.; Nagasaki, S.; Kato, Y.; Matsuoka, T. Pronounced muscle deoxygenation during supramaximal exercise under simulated hypoxia in sprint athletes. J. Sports Sci. Med. 2008, 7, 512–519. [Google Scholar]
- Ge, R.-L.; Babb, T.G.; Sivieri, M.; Resaland, G.K.; Karlsen, T.; Stray-Gundersen, J.; Levine, B.D. Urine acid-base compensation at simulated moderate altitude. High Alt. Med. Biol. 2006, 7, 64–71. [Google Scholar] [CrossRef]
- Luks, A.M. Physiology in Medicine: A physiologic approach to prevention and treatment of acute high-altitude illnesses. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 2015, 118, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Deb, S.K.; Gough, L.A.; Sparks, S.A.; McNaughton, L.R. Determinants of curvature constant (W’) of the power duration relationship under normoxia and hypoxia: The effect of pre-exercise alkalosis. Eur. J. Appl. Physiol. 2017, 117, 901–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyand, P.G.; Lee, C.S.; Martinez-Ruiz, R.; Bundle, M.W.; Bellizzi, M.J.; Wright, S. High-speed running performance is largely unaffected by hypoxic reductions in aerobic power. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1999, 86, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Goods, P.S.R.; Slattery, K.M. High-intensity exercise in hypoxia: Is increased reliance on anaerobic metabolism important? Front. Physiol. 2016, 7, 637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deb, S.K.; Gough, L.A.; Sparks, S.A.; McNaughton, L.R. Sodium bicarbonate supplementation improves severe-intensity intermittent exercise under moderate acute hypoxic conditions. Eur. J. Appl. Physiol. 2018, 118, 607–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gough, L.A.; Deb, S.K.; Brown, D.; Sparks, S.A.; McNaughton, L.R. The effects of sodium bicarbonate ingestion on cycling performance and acid base balance recovery in acute normobaric hypoxia. J. Sports Sci. 2019, 37, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Gough, L.A.; Brown, D.; Deb, S.K.; Sparks, S.A.; McNaughton, L.R. The influence of alkalosis on repeated high-intensity exercise performance and acid-base balance recovery in acute moderate hypoxic conditions. Eur. J. Appl. Physiol. 2018, 118, 2489–2498. [Google Scholar] [CrossRef] [Green Version]
- Shannon, O.M.; Duckworth, L.; Barlow, M.J.; Deighton, K.; Matu, J.; Williams, E.L.; Woods, D.; Xie, L.; Stephan, B.C.M.; Siervo, M.; et al. Effects of dietary nitrate supplementation on physiological responses, cognitive function, and exercise performance at moderate and very-high simulated altitude. Front. Physiol. 2017, 8, 401. [Google Scholar] [CrossRef]
- Shannon, O.M.; McGawley, K.; Nybäck, L.; Duckworth, L.; Barlow, M.J.; Woods, D.; Siervo, M.; O’Hara, J.P. “Beet-ing” the mountain: A review of the physiological and performance effects of dietary nitrate supplementation at simulated and terrestrial altitude. Sports Med. 2017, 47, 2155–2169. [Google Scholar] [CrossRef]
- McNaughton, L.R.; Gough, L.; Deb, S.; Bentley, D.; Sparks, S.A. Recent developments in the use of sodium bicarbonate as an ergogenic aid. Curr. Sports Med. Rep. 2016, 15, 233–244. [Google Scholar] [CrossRef]
- Siegler, J.C.; Marshall, P.W.M.; Bishop, D.; Shaw, G.; Green, S. Mechanistic insights into the efficacy of sodium bicarbonate supplementation to improve athletic performance. Sports Med. Open 2016, 2, 41. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.J.; Slater, G.J.; Gore, C.J.; Dawson, B.; Burke, L.M. Effect of sodium bicarbonate on HCO3-, pH, and gastrointestinal symptoms. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Feriche Fernandez-Castanys, B.; Delgado-Fernandez, M.; Alvarez Garcia, J. The effect of sodium citrate intake on anaerobic performance in normoxia and after sudden ascent to a moderate altitude. J. Sports Med. Phys. Fit. 2002, 42, 179–185. [Google Scholar]
- Hausswirth, C.; Bigard, A.X.; Lepers, R.; Berthelot, M.; Guezennec, C.Y. Sodium citrate ingestion and muscle performance in acute hypobaric hypoxia. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 71, 362–368. [Google Scholar] [CrossRef] [PubMed]
- McLellan, T.; Jacobs, I.; Lewis, W. Acute altitude exposure and altered acid-base states. II. Effects on exercise performance and muscle and blood lactate. Eur. J. Appl. Physiol. Occup. Physiol. 1988, 57, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Flinn, S.; Herbert, K.; Graham, K.; Siegler, J.C. Differential effect of metabolic alkalosis and hypoxia on high-intensity cycling performance. J. Strength Cond. Res. 2014, 28, 2852–2858. [Google Scholar] [CrossRef] [PubMed]
- Saunders, B.; Sale, C.; Harris, R.C.; Sunderland, C. Sodium bicarbonate and high-intensity-cycling capacity: Variability in responses. Int. J. Sports Physiol. Perform. 2014, 9, 627–632. [Google Scholar] [CrossRef]
- Caciano, S.L.; Inman, C.L.; Gockel-Blessing, E.E.; Weiss, E.P. Effects of dietary acid load on exercise metabolism and anaerobic exercise performance. J. Sports Sci. Med. 2015, 14, 364–371. [Google Scholar]
- Remer, T. Influence of nutrition on acid-base balance—Metabolic aspects. Eur. J. Nutr. 2001, 40, 214–220. [Google Scholar] [CrossRef]
- Poupin, N.; Calvez, J.; Lassale, C.; Chesneau, C.; Tome, D. Impact of the diet on net endogenous acid production and acid-base balance. Clin. Nutr. 2012, 31, 313–321. [Google Scholar] [CrossRef]
- Niekamp, K.; Zavorsky, G.S.; Fontana, L.; McDaniel, J.L.; Villareal, D.T.; Weiss, E.P. Systemic acid load from the diet affects maximal-exercise RER. Med. Sci. Sports Exerc. 2012, 44, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Remer, T.; Manz, F. Potential renal acid load of foods and its influence on urine pH. J. Am. Diet. Assoc. 1995, 95, 791–797. [Google Scholar] [CrossRef]
- Deriemaeker, P.; Aerenhouts, D.; Hebbelinck, M.; Clarys, P. Nutrient based estimation of acid-base balance in vegetarians and non-vegetarians. Plant Foods Hum. Nutr. 2010, 65, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Aerenhouts, D.; Deriemaeker, P.; Hebbelinck, M.; Clarys, P. Dietary acid-base balance in adolescent sprint athletes: A follow-up study. Nutrients 2011, 3, 200–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arciero, P.J.; Miller, V.J.; Ward, E. Performance enhancing diets and the PRISE Protocol to optimize athletic performance. J. Nutr. Metab. 2015, 2015, 715859. [Google Scholar] [CrossRef] [Green Version]
- Hietavala, E.-M.; Puurtinen, R.; Kainulainen, H.; Mero, A.A. Low-protein vegetarian diet does not have a short-term effect on blood acid-base status but raises oxygen consumption during submaximal cycling. J. Int. Soc. Sports Nutr. 2012, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Ball, D.; Greenhaff, P.L.; Maughan, R.J. The acute reversal of a diet-induced metabolic acidosis does not restore endurance capacity during high-intensity exercise in man. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 105–112. [Google Scholar] [CrossRef]
- Ball, D.; Maughan, R.J. The effect of sodium citrate ingestion on the metabolic response to intense exercise following diet manipulation in man. Exp. Physiol. 1997, 82, 1041–1056. [Google Scholar] [CrossRef]
- Rios Enriquez, O.; Guerra-Hernandez, E.; Feriche Fernandez-Castanys, B. Efectos de la alcalosis metabolica inducida por la dieta en el rendimiento anaerobico de alta intensidad. Nutr. Hosp. 2010, 25, 768–773. [Google Scholar]
- Hietavala, E.-M.; Stout, J.R.; Hulmi, J.J.; Suominen, H.; Pitkanen, H.; Puurtinen, R.; Selanne, H.; Kainulainen, H.; Mero, A.A. Effect of diet composition on acid-base balance in adolescents, young adults and elderly at rest and during exercise. Eur. J. Clin. Nutr. 2015, 69, 399–404. [Google Scholar] [CrossRef]
- Welch, A.A.; Mulligan, A.; Bingham, S.A.; Khaw, K.-T. Urine pH is an indicator of dietary acid-base load, fruit and vegetables and meat intakes: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk population study. Br. J. Nutr. 2008, 99, 1335–1343. [Google Scholar] [CrossRef] [Green Version]
- Limmer, M.; Berkholz, A.; de Marées, M.; Platen, P. Reliability and validity of a new portable tethered sprint running test as a measure of maximal anaerobic performance. J. Strength Cond. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, D.R.; Meksawan, K.; Limprasertkul, A.; Fisher, N.M. Influence of exercise on nutritional requirements. Eur. J. Appl. Physiol. 2011, 111, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Applegate, C.; Mueller, M.; Zuniga, K.E. Influence of dietary acid load on exercise performance. Int. J. Sport Nutr. Exerc. Metab. 2017, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H. The role of acidosis in fatigue: Pro perspective. Med. Sci. Sports Exerc. 2016, 48, 2335–2338. [Google Scholar] [CrossRef]
- Limmer, M.; Eibl, A.D.; Platen, P. Enhanced 400-m sprint performance in moderately trained participants by a 4-day alkalizing diet: A counterbalanced, randomized controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 25. [Google Scholar] [CrossRef] [Green Version]
- Couto, P.G.; Bertuzzi, R.; de Souza, C.C.; Lima, H.M.; Kiss, M.A.; de-Oliveira, F.R.; Lima-Silva, A.E. High carbohydrate diet induces faster final sprint and overall 10,000-m times of young runners. Pediatr. Exerc. Sci. 2015, 27, 355–363. [Google Scholar] [CrossRef]
- Skein, M.; Duffield, R.; Kelly, B.T.; Marino, F.E. The effects of carbohydrate intake and muscle glycogen content on self-paced intermittent-sprint exercise despite no knowledge of carbohydrate manipulation. Eur. J. Appl. Physiol. 2012, 112, 2859–2870. [Google Scholar] [CrossRef]
- Heil, D.P. Acid-base balance and hydration status following consumption of mineral-based alkaline bottled water. J. Int. Soc. Sports Nutr. 2010, 7, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wynn, E.; Krieg, M.-A.; Aeschlimann, J.-M.; Burckhardt, P. Alkaline mineral water lowers bone resorption even in calcium sufficiency: Alkaline mineral water and bone metabolism. Bone 2009, 44, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Inbar, O.; Amon, E. A new field-test for the assessment of the human anaerobic and repeated sprints capability–reliability and validity. Harefuah 2016, 155, 335–339, 388. [Google Scholar] [PubMed]
- Semenick, D. Anaerobic testing: Practical applications. Strength Cond. J. 1984, 6, 45. [Google Scholar]
- Zagatto, A.M.; Beck, W.R.; Gobatto, C.A. Validity of the running anaerobic sprint test for assessing anaerobic power and predicting short-distance performances. J. Strength Cond. Res. 2009, 23, 1820–1827. [Google Scholar] [CrossRef] [Green Version]
- Girard, O.; Brocherie, F.; Millet, G.P. Effects of altitude/hypoxia on single- and multiple-sprint performance: A comprehensive review. Sports Med. 2017, 47, 1931–1949. [Google Scholar] [CrossRef]
- Gastin, P.B. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001, 31, 725–741. [Google Scholar] [CrossRef]
- Calbet, J.A.L.; de Paz, J.A.; Garatachea, N.; Cabeza de Vaca, S.; Chavarren, J. Anaerobic energy provision does not limit Wingate exercise performance in endurance-trained cyclists. J. Appl. Physiol. 2003, 94, 668–676. [Google Scholar] [CrossRef]
- Ogawa, T.; Ohba, K.; Nabekura, Y.; Nagai, J.; Hayashi, K.; Wada, H.; Nishiyasu, T. Intermittent short-term graded running performance in middle-distance runners in hypobaric hypoxia. Eur. J. Appl. Physiol. 2005, 94, 254–261. [Google Scholar] [CrossRef]
- Duffield, R.; Dawson, B.; Goodman, C. Energy system contribution to 400-metre and 800-metre track running. J. Sports Sci. 2005, 23, 299–307. [Google Scholar] [CrossRef]
- Stewart, P.A. Independent and dependent variables of acid-base control. Respir. Physiol. 1978, 33, 9–26. [Google Scholar] [CrossRef]



| PO2 [mmHg] | PCO2 [mmHg] | SaO2 [%] | pHb | [HCO3−] [mmol/L] | BE [mmol/L] | |||
|---|---|---|---|---|---|---|---|---|
| N O R | ACID | PRE PTSR | 85.7 ± 7.6 # | 37.6 ± 2.2 | 98.4 ± 1.1 # | 7.40 ± 0.02 | 22.9 ± 1.1 | −0.7 ± 1.3 |
| POST PTSR | 91.5 ± 9.7 # | 42.2 ± 5.0 | 96.9 ± 1.4 # | 7.20 ± 0.05 | 15.7 ± 2.0 | −11.4 ± 2.6 | ||
| BASE | PRE PTSR | 85.6 ± 4.0 # | 38.9 ± 3.5 | 98.3 ± 0.9 # | 7.41 ± 0.02 | 24.3 ± 1.7 * | 0.1 ± 1.3 | |
| POST PTSR | 89.9 ± 7.4 # | 43.1 ± 5.5 | 97.0 ± 13 # | 7.23 ± 0.04 | 17.2 ± 2.1 * | −10.8 ± 2.8 | ||
| H Y P | ACID | PRE HYP | 90.1 ± 9.0 # | 39.8 ± 3.5 # | 98.6 ± 0.9 # | 7.39 ± 0.02 | 23.7 ± 1.5 # | −0.6 ± 1.2 |
| PRE PTSR | 67.8 ± 4.3 | 36.2 ± 3.8 | 94.4 ± 1.3 | 7.41 ± 0.02 | 22.5 ± 2.0 | −1.2 ± 1.5 | ||
| POST PTSR | 72.8 ± 5.9 | 38.1 ± 5.7 | 91.5 ± 2.9 | 7.22 ± 0.06 | 15.1 ± 1.8 | −12.0 ± 2.4 | ||
| BASE | PRE HYP | 90.3 ± 7.9 # | 41.1 ± 2.9 # | 98.5 ± 0.6 # | 7.41 ± 0.01 | 25.5 ± 1.6 #* | 1.2 ± 1.3 * | |
| PRE PTSR | 66.3 ± 4.6 | 37.1 ± 3.3 | 93.3 ± 1.4 | 7.43 ± 0.01 * | 24.0 ± 1.8 | 0.4 ± 1.4 | ||
| POST PTSR | 70.3 ± 5.6 | 40.1 ± 6.4 | 90.8 ± 2.1 | 7.24 ± 0.06 | 16.5 ± 2.0 | −10.3 ± 2.5 | ||
| Predictor Variable | R2 | Corrected R2 | F | p | Standardized β | T | p | |
|---|---|---|---|---|---|---|---|---|
| Lamax | Model | 0.200 | 0.184 | 12.746 | 0.001 * | |||
| ∑ PRAL | 0.073 | 0.539 | 0.592 | |||||
| ∑ Fluid | 0.030 | 0.241 | 0.811 | |||||
| ∑ CAL | 0.145 | 1.163 | 0.250 | |||||
| pHu | −0.212 | −1.689 | 0.097 | |||||
| pHb PRE PTSR | −0.224 | −1.823 | 0.074 | |||||
| [HCO3-] PRE PTSR | 0.447 | 3.570 | 0.001 * | |||||
| BE PRE PTSR | −0.304 | −1.021 | 0.312 | |||||
| HR | Model | 0.091 | 0.073 | 5.084 | 0.028 * | |||
| ∑ PRAL | −0.176 | −1.255 | 0.215 | |||||
| ∑ fluid | 0.065 | 0.480 | 0.633 | |||||
| ∑ CAL | 0.168 | 1.265 | 0.212 | |||||
| pHu | 0.089 | 0.603 | 0.550 | |||||
| pHb PRE PTSR | −0.301 | −2.255 | 0.028 * | |||||
| [HCO3−] PRE PTSR | 0.172 | 1.294 | 0.202 | |||||
| BE PRE PTSR | 0.183 | 1.367 | 0.178 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limmer, M.; Sonntag, J.; de Marées, M.; Platen, P. Effects of an Alkalizing or Acidizing Diet on High-Intensity Exercise Performance under Normoxic and Hypoxic Conditions in Physically Active Adults: A Randomized, Crossover Trial. Nutrients 2020, 12, 688. https://doi.org/10.3390/nu12030688
Limmer M, Sonntag J, de Marées M, Platen P. Effects of an Alkalizing or Acidizing Diet on High-Intensity Exercise Performance under Normoxic and Hypoxic Conditions in Physically Active Adults: A Randomized, Crossover Trial. Nutrients. 2020; 12(3):688. https://doi.org/10.3390/nu12030688
Chicago/Turabian StyleLimmer, Mirjam, Juliane Sonntag, Markus de Marées, and Petra Platen. 2020. "Effects of an Alkalizing or Acidizing Diet on High-Intensity Exercise Performance under Normoxic and Hypoxic Conditions in Physically Active Adults: A Randomized, Crossover Trial" Nutrients 12, no. 3: 688. https://doi.org/10.3390/nu12030688





