Fruit and Vegetable Consumption and Sarcopenia among Older Adults in Low- and Middle-Income Countries
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Survey
2.2. Sarcopenia
2.3. Fruit and Vegetable Consumption
2.4. Covariates
2.5. Statistical Analysis
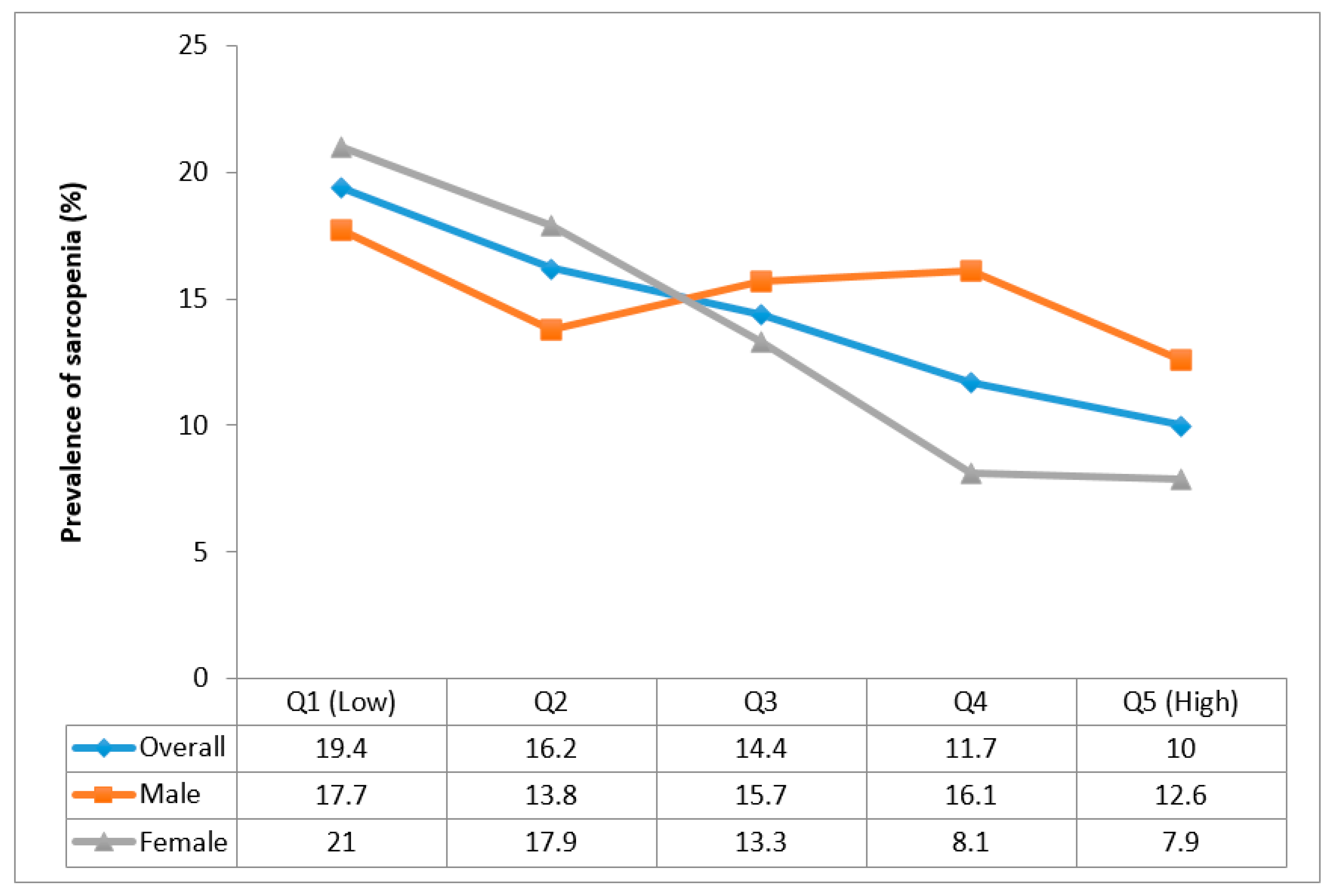
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127 (Suppl. 5), 990s–991s. [Google Scholar] [CrossRef] [Green Version]
- Patel, H.P.; Syddall, H.E.; Jameson, K.; Robinson, S.; Denison, H.; Roberts, H.C.; Edwards, M.; Dennison, E.; Cooper, C.; Aihie Sayer, A. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: Findings from the Hertfordshire Cohort Study (HCS). Age Ageing 2013, 42, 378–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.J.; Liu, S.L.; Lei, S.F.; Papasian, C.J.; Deng, H.W. Molecular genetic studies of gene identification for sarcopenia. Hum. Genet. 2012, 131, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary Patterns, Skeletal Muscle Health, and Sarcopenia in Older Adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United Nations. World Population Ageing 2019; United Nations: San Francisco, CA, USA, 2019. [Google Scholar]
- Woo, J. Sarcopenia. Clin. Geriatr. Med. 2017, 33, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Hao, Q.; Hai, S.; Wang, H.; Cao, L.; Dong, B. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: A systematic review and meta-analysis. Maturitas 2017, 103, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Bonjour, J.P.; Boonen, S.; Burckhardt, P.; Degens, H.; El Hajj Fuleihan, G.; Josse, R.; Lips, P.; Morales Torres, J.; Rizzoli, R.; et al. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteroporosis Int. 2013, 24, 1555–1566. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef]
- Abiri, B.; Vafa, M. Nutrition and sarcopenia: A review of the evidence of nutritional influences. Crit. Rev. Food Sci. Nutr. 2019, 59, 1456–1466. [Google Scholar] [CrossRef]
- Bloom, I.; Shand, C.; Cooper, C.; Robinson, S.; Baird, J. Diet Quality and Sarcopenia in Older Adults: A Systematic Review. Nutrients 2018, 10, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, R.; Leung, J.; Woo, J. Association Between Estimated Net Endogenous Acid Production and Subsequent Decline in Muscle Mass Over Four Years in Ambulatory Older Chinese People in Hong Kong: A Prospective Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 905–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.S.; Kim, K.M. Association of vegetables and fruits consumption with sarcopenia in older adults: The Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2015, 44, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, R.; Leung, J.; Woo, J. A Prospective Cohort Study to Examine the Association Between Dietary Patterns and Sarcopenia in Chinese Community-Dwelling Older People in Hong Kong. J. Am. Med. Dir. Assoc. 2016, 17, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Neville, C.E.; Young, I.S.; Gilchrist, S.E.; McKinley, M.C.; Gibson, A.; Edgar, J.D.; Woodside, J.V. Effect of increased fruit and vegetable consumption on physical function and muscle strength in older adults. Age 2013, 35, 2409–2422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.N.; Moore, S.; Harper, S.B.; Lynch, J.W. Global variability in fruit and vegetable consumption. Am. J. Prev. Med. 2009, 36, 402–409.e405. [Google Scholar] [CrossRef]
- Frank, S.M.; Webster, J.; McKenzie, B.; Geldsetzer, P.; Manne-Goehler, J.; Andall-Brereton, G.; Houehanou, C.; Houinato, D.; Gurung, M.S.; Bicaba, B.W.; et al. Consumption of Fruits and Vegetables Among Individuals 15 Years and Older in 28 Low- and Middle-Income Countries. J. Nutr. 2019, 149, 1252–1259. [Google Scholar] [CrossRef]
- Kowal, P.; Chatterji, S.; Naidoo, N.; Biritwum, R.; Fan, W.; Lopez Ridaura, R.; Maximova, T.; Arokiasamy, P.; Phaswana-Mafuya, N.; Williams, S.; et al. Data resource profile: The World Health Organization Study on global AGEing and adult health (SAGE). Int. J. Epidemiol. 2012, 41, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Tyrovolas, S.; Koyanagi, A.; Olaya, B.; Ayuso-Mateos, J.L.; Miret, M.; Chatterji, S.; Tobiasz-Adamczyk, B.; Koskinen, S.; Leonardi, M.; Haro, J.M. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: A multi-continent study. J. Cachexia Sarcopenia Muscle 2016, 7, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.T.; Peters, K.W.; Fragala, M.; Cawthon, P.M.; Harris, T.B.; McLean, R.; Shardell, M.; Alley, D.E.; Kenny, A.; Ferrucci, L.; et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Wang, Z.; Heo, M.; Ross, R.; Janssen, I.; Heymsfield, S.B. Total-body skeletal muscle mass: Development and cross-validation of anthropometric prediction models. Am. J. Clin. Nutr. 2000, 72, 796–803. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Tyrovolas, S.; Koyanagi, A.; Olaya, B.; Ayuso-Mateos, J.L.; Miret, M.; Chatterji, S.; Tobiasz-Adamczyk, B.; Koskinen, S.; Leonardi, M.; Haro, J.M. The role of muscle mass and body fat on disability among older adults: A cross-national analysis. Exp. Gerontol. 2015, 69, 27–35. [Google Scholar] [CrossRef]
- Capistrant, B.D.; Glymour, M.M.; Berkman, L.F. Assessing mobility difficulties for cross-national comparisons: Results from the world health organization study on global ageing and adult health. J. Am. Geriatr. Soc. 2014, 62, 329–335. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Bull, F.C.; Maslin, T.S.; Armstrong, T. Global physical activity questionnaire (GPAQ): Nine country reliability and validity study. J. Phys. Health 2009, 6, 790–804. [Google Scholar] [CrossRef] [Green Version]
- Rippe, J.M.; Angelopoulos, T.J. Sucrose, high-fructose corn syrup, and fructose, their metabolism and potential health effects: What do we really know? Adv. Nutr. 2013, 4, 236–245. [Google Scholar] [CrossRef]
- Le, K.A.; Faeh, D.; Stettler, R.; Debard, C.; Loizon, E.; Vidal, H.; Boesch, C.; Ravussin, E.; Tappy, L. Effects of four-week high-fructose diet on gene expression in skeletal muscle of healthy men. Diabetes Metab. 2008, 34, 82–85. [Google Scholar] [CrossRef]
- Koyanagi, A.; Lara, E.; Stubbs, B.; Carvalho, A.F.; Oh, H.; Stickley, A.; Veronese, N.; Vancampfort, D. Chronic Physical Conditions, Multimorbidity, and Mild Cognitive Impairment in Low- and Middle-Income Countries. J. Am. Geriatr. Soc. 2018, 66, 721–727. [Google Scholar] [CrossRef]
- Koyanagi, A.; Garin, N.; Olaya, B.; Ayuso-Mateos, J.L.; Chatterji, S.; Leonardi, M.; Koskinen, S.; Tobiasz-Adamczyk, B.; Haro, J.M. Chronic conditions and sleep problems among adults aged 50 years or over in nine countries: A multi-country study. PLoS ONE 2014, 9, e114742. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr. 1995, 62 (Suppl. 6), 1315s–1321s. [Google Scholar] [CrossRef]
- Wang, J.; Leung, K.S.; Chow, S.K.; Cheung, W.H. Inflammation and age-associated skeletal muscle deterioration (sarcopaenia). J. Orthop. Transl. 2017, 10, 94–101. [Google Scholar] [CrossRef]
- Meng, S.J.; Yu, L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef] [Green Version]
- Conti, V.; Izzo, V.; Corbi, G.; Russomanno, G.; Manzo, V.; De Lise, F.; Di Donato, A.; Filippelli, A. Antioxidant Supplementation in the Treatment of Aging-Associated Diseases. Front. Pharmacol. 2016, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Saito, K.; Yokoyama, T.; Yoshida, H.; Kim, H.; Shimada, H.; Yoshida, Y.; Iwasa, H.; Shimizu, Y.; Kondo, Y.; Handa, S.; et al. A Significant Relationship between Plasma Vitamin C Concentration and Physical Performance among Japanese Elderly Women. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 67, 295–301. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Bartali, B.; Cherubini, A.; Penninx, B.W.; Williams, G.R.; Atkinson, H.; Martin, A.; Guralnik, J.M.; Ferrucci, L. Antioxidants and physical performance in elderly persons: The Invecchiare in Chianti (InCHIANTI) study. Am. J. Clin. Nutr. 2004, 79, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Welch, A.A.; MacGregor, A.J.; Skinner, J.; Spector, T.D.; Moayyeri, A.; Cassidy, A. A higher alkaline dietary load is associated with greater indexes of skeletal muscle mass in women. Osteoporos. Int. 2013, 24, 1899–1908. [Google Scholar] [CrossRef]
- Baker, A.H.; Wardle, J. Sex differences in fruit and vegetable intake in older adults. Appetite 2003, 40, 269–275. [Google Scholar] [CrossRef]
- Anderson, L.J.; Liu, H.; Garcia, J.M. Sex Differences in Muscle Wasting. Adv. Exp. Med. Biol. 2017, 1043, 153–197. [Google Scholar]
- Gheller, B.J.; Riddle, E.S.; Lem, M.R.; Thalacker-Mercer, A.E. Understanding Age-Related Changes in Skeletal Muscle Metabolism: Differences Between Females and Males. Annu. Rev. Nutr. 2016, 36, 129–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Smith, L.; Hamer, M. Gender-specific risk factors for incident sarcopenia: 8-year follow-up of the English longitudinal study of ageing. J. Epidemiol. Community Health 2019, 73, 86–88. [Google Scholar] [CrossRef]
- Roberts, B.M.; Lavin, K.M.; Many, G.M.; Thalacker-Mercer, A.; Merritt, E.K.; Bickel, C.S.; Mayhew, D.L.; Tuggle, S.C.; Cross, J.M.; Kosek, D.J.; et al. Human neuromuscular aging: Sex differences revealed at the myocellular level. Exp. gerontol. 2018, 106, 116–124. [Google Scholar] [CrossRef]
- Rivera, J.d.J.; Fonseca-Sanchez, M.A.; Rodriguez, P.; Garcia, J.M.; Palma, I.; Aristi, G.; Flores-Luce, A.; Garcia, L.; Trujillo, Y.; Queipo, G. Physical Activity Protects Men but Not Women for Sarcopenia Development. J. Gerontol. Geriatr. Med. 2016, 2, 233372141666787. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Rech, C.R.; Dellagrana, R.A.; Marucci, M.D.F.N.; Petroski, E.L. Validity of anthropometric equations for th eestimation of muscle mass in elderly. Rev. Bras. Cineantropom. Desempenho Hum. 2012, 14, 23–31. [Google Scholar]
- Naseeb, M.A.; Volpe, S.L. Protein and exercise in the prevention of sarcopenia and aging. Nutr. Res. 2017, 40, 1–20. [Google Scholar] [CrossRef]
- Fulton, S.L.; McKinley, M.C.; Young, I.S.; Cardwell, C.R.; Woodside, J.V. The Effect of Increasing Fruit and Vegetable Consumption on Overall Diet: A Systematic Review and Meta-analysis. Crit. Rev. Food Sci. Nutr. 2016, 56, 802–816. [Google Scholar] [CrossRef] [Green Version]

| Sarcopenia | |||||
|---|---|---|---|---|---|
| Overall | No | Yes | p-Value a | ||
| Sex | Female | 55.0 | 54.5 | 55.1 | <0.769 |
| Male | 45.0 | 45.5 | 44.9 | ||
| Age | Mean (SD) | 72.6 (11.5) | 71.4 (10.1) | 76.1 (12.4) | <0.001 |
| Education | Primary | 63.7 | 64.8 | 77.5 | <0.001 |
| Secondary | 29.9 | 28.9 | 19.2 | ||
| Tertiary | 6.4 | 6.2 | 3.4 | ||
| Wealth | Poorest | 21.7 | 19.8 | 32.1 | <0.001 |
| Poorer | 21.0 | 20.1 | 22.5 | ||
| Middle | 20.4 | 20.4 | 17.9 | ||
| Richer | 17.5 | 18.9 | 15.0 | ||
| Richest | 19.4 | 20.9 | 12.6 | ||
| Physical activity | High | 35.2 | 38.6 | 29.0 | <0.001 |
| Moderate | 25.2 | 26.1 | 25.6 | ||
| Low | 39.6 | 35.3 | 45.4 | ||
| Smoking | No | 70.7 | 68.9 | 69.9 | 0.705 |
| Yes | 29.3 | 31.1 | 30.1 | ||
| Alcohol consumption | No | 86.1 | 85.2 | 89.9 | 0.001 |
| Yes | 13.9 | 14.8 | 10.1 | ||
| BMI (kg/m2) | <18.5 | 19.3 | 20.1 | 20.2 | 0.061 |
| 18.5–24.9 | 46.4 | 47.3 | 45.1 | ||
| 25.0–29.9 | 23.9 | 23.7 | 21.8 | ||
| ≥30 | 10.4 | 9.0 | 12.9 | ||
| No. of chronic conditions | Mean (SD) | 2.4 (2.9) | 2.3 (2.9) | 2.6 (2.9) | <0.001 |
| Overall | Male | Female | |||||
|---|---|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | OR | 95%CI | ||
| Fruit consumption a | Q1 (Low) | 1.00 | 1.00 | 1.00 | |||
| Q2 | 0.85 | (0.62, 1.16) | 0.74 | (0.50, 1.10) | 0.87 | (0.55, 1.38) | |
| Q3 | 0.74 * | (0.55, 1.00) | 0.86 | (0.58, 1.28) | 0.56 ** | (0.37, 0.83) | |
| Q4 | 0.64 * | (0.43, 0.95) | 1.12 | (0.71, 1.77) | 0.38 ** | (0.21, 0.69) | |
| Q5 (High) | 0.60 ** | (0.42, 0.84) | 0.81 | (0.53, 1.25) | 0.42 ** | (0.24, 0.73) | |
| Vegetable consumption b | Q1 (Low) | 1.00 | 1.00 | 1.00 | |||
| Q2 | 0.85 | (0.63, 1.15) | 0.96 | (0.57, 1.63) | 0.86 | (0.53, 1.38) | |
| Q3 | 0.91 | (0.60, 1.38) | 0.95 | (0.52, 1.74) | 0.97 | (0.51, 1.85) | |
| Q4 | 0.89 | (0.59, 1.33) | 0.81 | (0.41, 1.56) | 1.09 | (0.61, 1.95) | |
| Q5 (High) | 0.99 | (0.62, 1.58) | 1.00 | (0.49, 2.03) | 1.07 | (0.55, 2.09) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koyanagi, A.; Veronese, N.; Solmi, M.; Oh, H.; Shin, J.I.; Jacob, L.; Yang, L.; Haro, J.M.; Smith, L. Fruit and Vegetable Consumption and Sarcopenia among Older Adults in Low- and Middle-Income Countries. Nutrients 2020, 12, 706. https://doi.org/10.3390/nu12030706
Koyanagi A, Veronese N, Solmi M, Oh H, Shin JI, Jacob L, Yang L, Haro JM, Smith L. Fruit and Vegetable Consumption and Sarcopenia among Older Adults in Low- and Middle-Income Countries. Nutrients. 2020; 12(3):706. https://doi.org/10.3390/nu12030706
Chicago/Turabian StyleKoyanagi, Ai, Nicola Veronese, Marco Solmi, Hans Oh, Jae Il Shin, Louis Jacob, Lin Yang, Josep Maria Haro, and Lee Smith. 2020. "Fruit and Vegetable Consumption and Sarcopenia among Older Adults in Low- and Middle-Income Countries" Nutrients 12, no. 3: 706. https://doi.org/10.3390/nu12030706








