Exploring the Association between Vascular Dysfunction and Skeletal Muscle Mass, Strength and Function in Healthy Adults: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
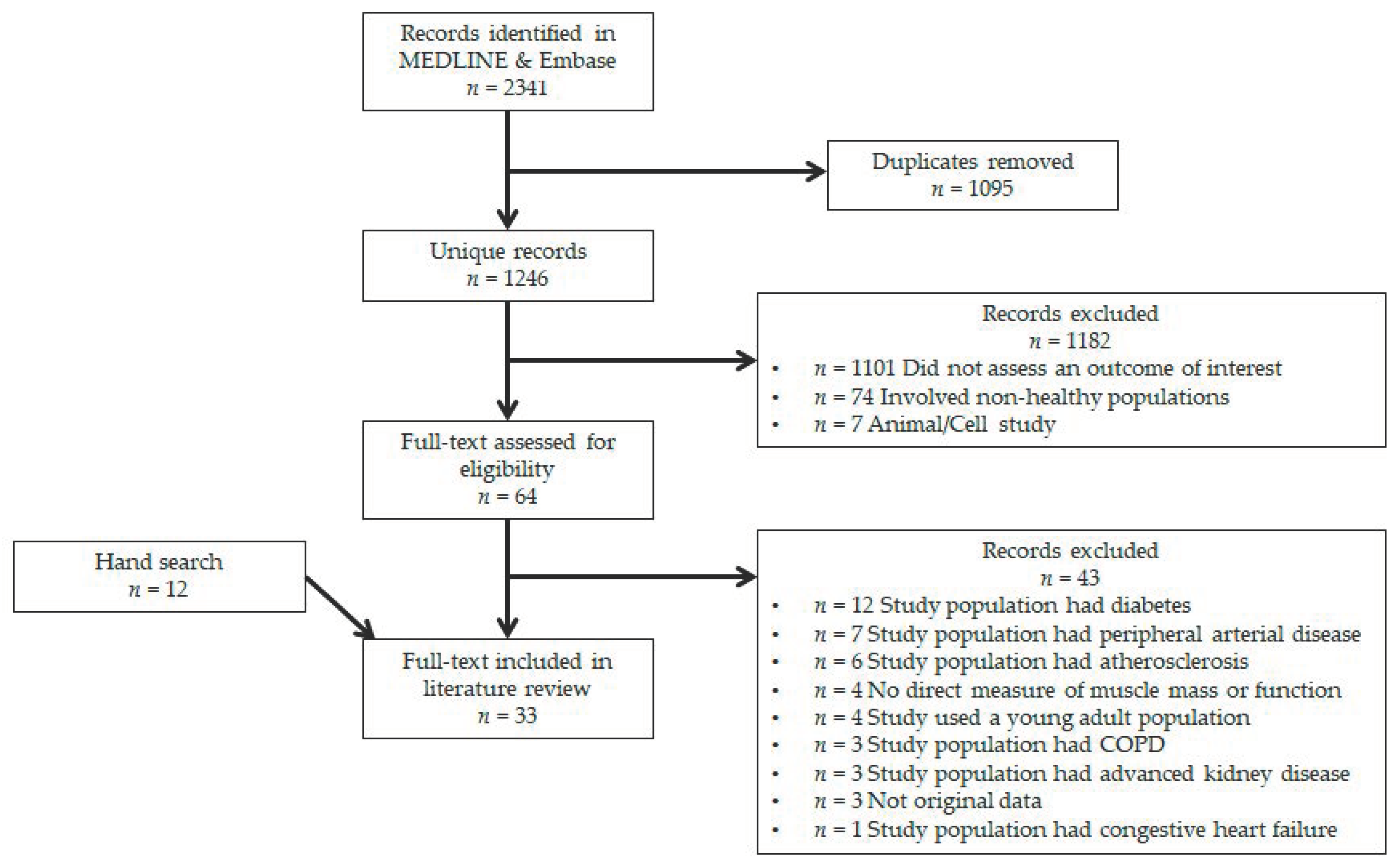
2.1. Literature Search
2.2. Inclusion Criteria
2.3. Data Extraction
2.4. Study Details and Sample Demographics
2.5. Assessment of Skeletal Muscle Health
2.6. Assessment of Vascular Health
3. Results
3.1. Macrovascular Studies
3.2. Microvascular Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, M.R. Effects of aging on muscle fibre type and size. Sports Med. 2004, 34, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Rizzoli, R.; Bruyere, O.; Reginster, J.Y.; Biver, E. Sarcopenia: Burden and challenges for public health. Arch. Public Health 2014, 72, 45. [Google Scholar] [CrossRef]
- Scott, D.; Daly, R.M.; Sanders, K.M.; Ebeling, P.R. Fall and Fracture Risk in Sarcopenia and Dynapenia With and Without Obesity: The Role of Lifestyle Interventions. Curr. Osteoporos. Rep. 2015, 13, 235–244. [Google Scholar] [CrossRef]
- Goates, S.; Du, K.; Arensberg, M.B.; Gaillard, T.; Guralnik, J.; Pereira, S.L. Economic Impact of Hospitalizations in US Adults with Sarcopenia. J. Frailty Aging 2019, 8, 93–99. [Google Scholar] [CrossRef]
- Locquet, M.; Beaudart, C.; Petermans, J.; Reginster, J.Y.; Bruyere, O. EWGSOP2 Versus EWGSOP1: Impact on the Prevalence of Sarcopenia and Its Major Health Consequences. J. Am. Med. Dir. Assoc. 2019, 20, 384–385. [Google Scholar] [CrossRef]
- Rodriguez-Rejon, A.I.; Ruiz-Lopez, M.D.; Wanden-Berghe, C.; Artacho, R. Prevalence and Diagnosis of Sarcopenia in Residential Facilities: A Systematic Review. Adv. Nutr. 2019, 10, 51–58. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Laslett, L.J.; Alagona, P., Jr.; Clark, B.A., III; Drozda, J.P., Jr.; Saldivar, F.; Wilson, S.R.; Poe, C.; Hart, M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012, 60, S1–S49. [Google Scholar] [CrossRef]
- Smith, S.C., Jr.; Collins, A.; Ferrari, R.; Holmes, D.R., Jr.; Logstrup, S.; McGhie, D.V.; Ralston, J.; Sacco, R.L.; Stam, H.; Taubert, K.; et al. Our time: A call to save preventable death from cardiovascular disease (heart disease and stroke). J. Am. Coll. Cardiol. 2012, 60, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.L.; Whincup, P.H.; Morris, R.W.; Lennon, L.T.; Papacosta, O.; Wannamethee, S.G. Sarcopenic obesity and risk of cardiovascular disease and mortality: A population-based cohort study of older men. J. Am. Geriatr. Soc. 2014, 62, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.Y.; Chang, H.Y.; Lee, M.S.; Chia-Yu Chen, R.; Pan, W.H. Skeletal muscle mass and risk of death in an elderly population. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Spahillari, A.; Mukamal, K.J.; DeFilippi, C.; Kizer, J.R.; Gottdiener, J.S.; Djousse, L.; Lyles, M.F.; Bartz, T.M.; Murthy, V.L.; Shah, R.V. The association of lean and fat mass with all-cause mortality in older adults: The Cardiovascular Health Study. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Uberoi, A.; Wray, D.W.; Nishiyama, S.; Lawrenson, L.; Richardson, R.S. Differential effects of aging on limb blood flow in humans. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H272–H278. [Google Scholar] [CrossRef]
- Skilton, M.R.; Lai, N.T.; Griffiths, K.A.; Molyneaux, L.M.; Yue, D.K.; Sullivan, D.R.; Celermajer, D.S. Meal-related increases in vascular reactivity are impaired in older and diabetic adults: Insights into roles of aging and insulin in vascular flow. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1404–H1410. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Sorensen, K.E.; Spiegelhalter, D.J.; Georgakopoulos, D.; Robinson, J.; Deanfield, J.E. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J. Am. Coll. Cardiol. 1994, 24, 471–476. [Google Scholar] [CrossRef]
- Seals, D.R.; Moreau, K.L.; Gates, P.E.; Eskurza, I. Modulatory influences on ageing of the vasculature in healthy humans. Exp. Gerontol. 2006, 41, 501–507. [Google Scholar] [CrossRef]
- Clark, M.G.; Wallis, M.G.; Barrett, E.J.; Vincent, M.A.; Richards, S.M.; Clerk, L.H.; Rattigan, S. Blood flow and muscle metabolism: A focus on insulin action. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E241–E258. [Google Scholar] [CrossRef]
- Timmerman, K.L.; Dhanani, S.; Glynn, E.L.; Fry, C.S.; Drummond, M.J.; Jennings, K.; Rasmussen, B.B.; Volpi, E. A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am. J. Clin. Nutr. 2012, 95, 1403–1412. [Google Scholar] [CrossRef]
- Rodriguez, A.J.; Karim, M.N.; Srikanth, V.; Ebeling, P.R.; Scott, D. Lower muscle tissue is associated with higher pulse wave velocity: A systematic review and meta-analysis of observational study data. Clin. Exp. Pharmacol. Physiol. 2017, 44, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.G. Impaired microvascular perfusion: A consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E732–E750. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.G.; Rattigan, S.; Barrett, E.J. Nutritive blood flow as an essential element supporting muscle anabolism. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.J.; Casperson, S.L.; Dillon, E.L.; Keske, M.A.; Paddon-Jones, D.; Sanford, A.P.; Hickner, R.C.; Grady, J.J.; Sheffield-Moore, M. Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J. 2010, 24, 4117–4127. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.K.; Phillips, B.E.; Williams, J.P.; Rankin, D.; Smith, K.; Lund, J.N.; Atherton, P.J. Development of a new Sonovue contrast-enhanced ultrasound approach reveals temporal and age-related features of muscle microvascular responses to feeding. Physiol. Rep. 2013, 1, e00119. [Google Scholar] [CrossRef] [PubMed]
- Brightwell, C.R.; Markofski, M.M.; Moro, T.; Fry, C.S.; Porter, C.; Volpi, E.; Rasmussen, B.B. Moderate-intensity aerobic exercise improves skeletal muscle quality in older adults. Transl. Sports Med. 2019, 2, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Snijders, T.; Holloway, T.M.; Van Kranenburg, J.; Van Loon, L.J. Resistance Training Increases Skeletal Muscle Capillarization in Healthy Older Men. Med. Sci. Sports Exerc. 2016, 48, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.J. Anabolic resistance: The effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl. Physiol. Nutr. Metab. 2009, 34, 377–381. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef]
- Wilkes, E.A.; Selby, A.L.; Atherton, P.J.; Patel, R.; Rankin, D.; Smith, K.; Rennie, M.J. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am. J. Clin. Nutr. 2009, 90, 1343–1350. [Google Scholar] [CrossRef]
- Phillips, B.E.; Atherton, P.J.; Varadhan, K.; Limb, M.C.; Williams, J.P.; Smith, K. Acute cocoa flavanol supplementation improves muscle macro- and microvascular but not anabolic responses to amino acids in older men. Appl. Physiol. Nutr. Metab. 2016, 41, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Fryar, C.D.; Ostchega, Y.; Hales, C.M.; Zhang, G.; Kruszon-Moran, D. Hypertension Prevalence and Control among Adults: United States, 2015–2016. In NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2017; pp. 1–8. [Google Scholar]
- Barnouin, Y.; McPhee, J.S.; Butler-Browne, G.; Bosutti, A.; De Vito, G.; Jones, D.A.; Narici, M.; Behin, A.; Hogrel, J.Y.; Degens, H. Coupling between skeletal muscle fiber size and capillarization is maintained during healthy aging. J. Cachexia Sarcopenia Muscle 2017, 8, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.; Williams, J.; Atherton, P.; Smith, K.; Hildebrandt, W.; Rankin, D.; Greenhaff, P.; Macdonald, I.; Rennie, M.J. Resistance exercise training improves age-related declines in leg vascular conductance and rejuvenates acute leg blood flow responses to feeding and exercise. J. Appl. Physiol. 2012, 112, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Bunout, D.; de la Maza, M.P.; Leiva, L.; Hirsch, S. Carotid ultrasound examination as an aging and disability marker. Geriatr. Gerontol. Int. 2014, 14, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Kim, M.; Jin, Y.; Kim, Y.; Hong, J. Fitness as a determinant of arterial stiffness in healthy adult men: A cross-sectional study. J. Sports Med. Phys. Fit. 2018, 58, 150–156. [Google Scholar] [CrossRef]
- den Ouden, M.E.; Schuurmans, M.J.; Arts, I.E.; Grobbee, D.E.; Bots, M.L.; van den Beld, A.W.; Lamberts, S.W.; van der Schouw, Y.T. Atherosclerosis and physical functioning in older men, a longitudinal study. J. Nutr. Health Aging 2013, 17, 97–104. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Chen, H.Y.; Barinas-Mitchell, E.; McClure, C.; Selzer, F.; Karvonen-Gutierrez, C.; Jackson, E.A.; Ylitalo, K.R.; Sternfeld, B. Simple physical performance measures and vascular health in late midlife women: The Study of Women’s Health across the nation. Int. J. Cardiol. 2015, 182, 115–120. [Google Scholar] [CrossRef]
- Fahs, C.A.; Thiebaud, R.S.; Rossow, L.M.; Loenneke, J.P.; Bemben, D.A.; Bemben, M.G. Relationships between central arterial stiffness, lean body mass, and absolute and relative strength in young and older men and women. Clin. Physiol. Funct. Imaging 2018, 38, 676–680. [Google Scholar] [CrossRef]
- Gonzales, J.U.; Wiberg, M.; Defferari, E.; Proctor, D.N. Arterial stiffness is higher in older adults with increased perceived fatigue and fatigability during walking. Exp. Gerontol. 2015, 61, 92–97. [Google Scholar] [CrossRef]
- Heffernan, K.S.; Chale, A.; Hau, C.; Cloutier, G.J.; Phillips, E.M.; Warner, P.; Nickerson, H.; Reid, K.F.; Kuvin, J.T.; Fielding, R.A. Systemic vascular function is associated with muscular power in older adults. J. Aging Res. 2012, 2012, 386387. [Google Scholar] [CrossRef]
- Im, I.J.; Choi, H.J.; Jeong, S.M.; Kim, H.J.; Son, J.S.; Oh, H.J. The association between muscle mass deficits and arterial stiffness in middle-aged men. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Kohara, K.; Okada, Y.; Ochi, M.; Ohara, M.; Nagai, T.; Tabara, Y.; Igase, M. Muscle mass decline, arterial stiffness, white matter hyperintensity, and cognitive impairment: Japan Shimanami Health Promoting Program study. J. Cachexia Sarcopenia Muscle 2017, 8, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Youm, Y.; Kim, C.O.; Lee, W.J.; Choi, W.; Chu, S.H.; Park, Y.R.; Kim, H.C. Association between skeletal muscle mass and radial augmentation index in an elderly Korean population. Arch. Gerontol. Geriatr. 2014, 59, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef]
- Ochi, M.; Kohara, K.; Tabara, Y.; Kido, T.; Uetani, E.; Ochi, N.; Igase, M.; Miki, T. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis 2010, 212, 327–332. [Google Scholar] [CrossRef]
- Prior, S.J.; Ryan, A.S.; Blumenthal, J.B.; Watson, J.M.; Katzel, L.I.; Goldberg, A.P. Sarcopenia Is Associated With Lower Skeletal Muscle Capillarization and Exercise Capacity in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2016, 71, 1096–1101. [Google Scholar] [CrossRef]
- Sanada, K.; Miyachi, M.; Tanimoto, M.; Yamamoto, K.; Murakami, H.; Okumura, S.; Gando, Y.; Suzuki, K.; Tabata, I.; Higuchi, M. A cross-sectional study of sarcopenia in Japanese men and women: Reference values and association with cardiovascular risk factors. Eur. J. Appl. Physiol. 2010, 110, 57–65. [Google Scholar] [CrossRef]
- Shiotsu, Y.; Watanabe, Y.; Tujii, S.; Yanagita, M. Effect of exercise order of combined aerobic and resistance training on arterial stiffness in older men. Exp. Gerontol. 2018, 111, 27–34. [Google Scholar] [CrossRef]
- Suwa, M.; Imoto, T.; Kida, A.; Yokochi, T.; Iwase, M.; Kozawa, K. Association of body flexibility and carotid atherosclerosis in Japanese middle-aged men: A cross-sectional study. BMJ Open 2018, 8, e019370. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kawano, H.; Gando, Y.; Iemitsu, M.; Murakami, H.; Sanada, K.; Tanimoto, M.; Ohmori, Y.; Higuchi, M.; Tabata, I.; et al. Poor trunk flexibility is associated with arterial stiffening. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1314–H1318. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Maeda, S.; Miyaki, A.; Misono, M.; Saito, Y.; Tanabe, K.; Kuno, S.; Ajisaka, R. Effect of 12 weeks of moderate-intensity resistance training on arterial stiffness: A randomised controlled trial in women aged 32–59 years. Br. J. Sports Med. 2009, 43, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.I.; Kim, M.J.; Na, J.B.; Chun, Y.H.; Park, Y.J.; Park, Y.; Hah, Y.S.; Ha, Y.C.; Park, K.S. Relationship between endothelial function and skeletal muscle strength in community dwelling elderly women. J. Cachexia Sarcopenia Muscle 2018, 9, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, Q.; Feng, B.L.; Wang, C.Y.; Han, P.P.; Hu, J.; Sun, X.D.; Zeng, W.F.; Zheng, Z.X.; Li, H.S.; et al. A Cross-Sectional Study of the Association between Arterial Stiffness and Sarcopenia in Chinese Community-Dwelling Elderly Using the Asian Working Group for Sarcopenia Criteria. J. Nutr. Health Aging 2019, 23, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Dipla, K.; Triantafyllou, A.; Koletsos, N.; Papadopoulos, S.; Sachpekidis, V.; Vrabas, I.S.; Gkaliagkousi, E.; Zafeiridis, A.; Douma, S. Impaired Muscle Oxygenation and Elevated Exercise Blood Pressure in Hypertensive Patients: Links With Vascular Stiffness. Hypertension 2017, 70, 444–451. [Google Scholar] [CrossRef]
- Gueugneau, M.; Coudy-Gandilhon, C.; Meunier, B.; Combaret, L.; Taillandier, D.; Polge, C.; Attaix, D.; Roche, F.; Feasson, L.; Barthelemy, J.C.; et al. Lower skeletal muscle capillarization in hypertensive elderly men. Exp. Gerontol. 2016, 76, 80–88. [Google Scholar] [CrossRef]
- Lima-Junior, D.; Farah, B.Q.; Germano-Soares, A.H.; Andrade-Lima, A.; Silva, G.O.; Rodrigues, S.L.C.; Ritti-Dias, R. Association between handgrip strength and vascular function in patients with hypertension. Clin. Exp. Hypertens. 2018, 41, 692–695. [Google Scholar] [CrossRef]
- Sampaio, R.A.; Sewo Sampaio, P.Y.; Yamada, M.; Yukutake, T.; Uchida, M.C.; Tsuboyama, T.; Arai, H. Arterial stiffness is associated with low skeletal muscle mass in Japanese community-dwelling older adults. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 109–114. [Google Scholar] [CrossRef]
- Shimizu, Y.; Sato, S.; Koyamatsu, J.; Yamanashi, H.; Nagayoshi, M.; Kadota, K.; Kawashiri, S.Y.; Inoue, K.; Nagata, Y.; Maeda, T. Handgrip strength and subclinical carotid atherosclerosis in relation to platelet levels among hypertensive elderly Japanese. Oncotarget 2017, 8, 69362–69369. [Google Scholar] [CrossRef]
- Wong, A.; Figueroa, A.; Son, W.M.; Chernykh, O.; Park, S.Y. The effects of stair climbing on arterial stiffness, blood pressure, and leg strength in postmenopausal women with stage 2 hypertension. Menopause 2018, 25, 731–737. [Google Scholar] [CrossRef]
- Groen, B.B.; Hamer, H.M.; Snijders, T.; van Kranenburg, J.; Frijns, D.; Vink, H.; van Loon, L.J. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J. Appl. Physiol. 2014, 116, 998–1005. [Google Scholar] [CrossRef]
- Wust, R.C.; Gibbings, S.L.; Degens, H. Fiber capillary supply related to fiber size and oxidative capacity in human and rat skeletal muscle. Adv. Exp. Med. Biol. 2009, 645, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Bodur, H.; Yilmaz, O.; Keskin, D. Hand disability and related variables in patients with rheumatoid arthritis. Rheumatol. Int. 2006, 26, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Escalante, A.; Haas, R.W.; del Rincon, I. Measurement of global functional performance in patients with rheumatoid arthritis using rheumatology function tests. Arthritis Res. Ther. 2004, 6, R315–R325. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Abellan van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef]
- Afilalo, J.; Eisenberg, M.J.; Morin, J.F.; Bergman, H.; Monette, J.; Noiseux, N.; Perrault, L.P.; Alexander, K.P.; Langlois, Y.; Dendukuri, N.; et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J. Am. Coll. Cardiol. 2010, 56, 1668–1676. [Google Scholar] [CrossRef]
- Blain, H.; Carriere, I.; Sourial, N.; Berard, C.; Favier, F.; Colvez, A.; Bergman, H. Balance and walking speed predict subsequent 8-year mortality independently of current and intermediate events in well-functioning women aged 75 years and older. J. Nutr. Health Aging 2010, 14, 595–600. [Google Scholar] [CrossRef]
- Ries, J.D.; Echternach, J.L.; Nof, L.; Gagnon Blodgett, M. Test-retest reliability and minimal detectable change scores for the timed “up & go” test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys. Ther. 2009, 89, 569–579. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- Rodriguez, A.J.; Scott, D.; Ebeling, P.R. Exploring the Links Between Common Diseases of Ageing—Osteoporosis, Sarcopenia and Vascular Calcification. Clin. Rev. Bone Miner. Metab. 2019, 17, 23. [Google Scholar] [CrossRef]
- Andrade, J.; Er, L.; Ignaszewski, A.; Levin, A. Exploration of association of 1,25-OH2D3 with augmentation index, a composite measure of arterial stiffness. Clin. J. Am. Soc. Nephrol. 2008, 3, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Nowson, C.A.; Daly, R.M.; English, D.R.; Hodge, A.M.; Giles, G.G.; Ebeling, P.R. Higher Dietary Calcium Intakes Are Associated With Reduced Risks of Fractures, Cardiovascular Events, and Mortality: A Prospective Cohort Study of Older Men and Women. J. Bone Miner. Res. 2015, 30, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.J.; Scott, D.; Srikanth, V.; Ebeling, P. Effect of vitamin D supplementation on measures of arterial stiffness: A systematic review and meta-analysis of randomized controlled trials. Clin. Endocrinol. 2016, 84, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.J.; Kruszka, B.; Delaney, J.A.; He, K.; Burke, G.L.; Alonso, A.; Bild, D.E.; Budoff, M.; Michos, E.D. Calcium Intake From Diet and Supplements and the Risk of Coronary Artery Calcification and its Progression Among Older Adults: 10-Year Follow-up of the Multi-Ethnic Study of Atherosclerosis (MESA). J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoon, J.W.; Kim, K.W.; Lee, E.J.; Lee, W.; Cho, S.H.; Shin, C.S. Increased dietary calcium intake is not associated with coronary artery calcification. Int. J. Cardiol. 2012, 157, 429–431. [Google Scholar] [CrossRef]
- Nocon, M.; Hiemann, T.; Muller-Riemenschneider, F.; Thalau, F.; Roll, S.; Willich, S.N. Association of physical activity with all-cause and cardiovascular mortality: A systematic review and meta-analysis. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 239–246. [Google Scholar] [CrossRef]
- Sofi, F.; Capalbo, A.; Cesari, F.; Abbate, R.; Gensini, G.F. Physical activity during leisure time and primary prevention of coronary heart disease: An updated meta-analysis of cohort studies. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 247–257. [Google Scholar] [CrossRef]
- Melo, X.; Santa-Clara, H.; Santos, D.A.; Pimenta, N.M.; Minderico, C.S.; Fernhall, B.; Sardinha, L.B. Independent Association of Muscular Strength and Carotid Intima-Media Thickness in Children. Int. J. Sports Med. 2015, 36, 624–630. [Google Scholar] [CrossRef]
- Badrov, M.B.; Freeman, S.R.; Zokvic, M.A.; Millar, P.J.; McGowan, C.L. Isometric exercise training lowers resting blood pressure and improves local brachial artery flow-mediated dilation equally in men and women. Eur. J. Appl. Physiol. 2016, 116, 1289–1296. [Google Scholar] [CrossRef]
| Study & Year | Design | Sample | Country | n | Male (%) | Age | Exclusion |
|---|---|---|---|---|---|---|---|
| Barnouin 2017 [33] | Cross-sectional | Healthy adults | UK | 47 | 77 | 22–74 | Cardiovascular, neuromuscular, or respiratory diseases |
| Barrera 2014 [35] | Cross-sectional | Healthy adults | Chile | 259 | 49 | 29–88 | Undernutrition BMI < 18, cancer, autoimmune disease, kidney, liver or cardiac failure, diabetes, cognitive impairment, steroids or HRT |
| Brightwell 2018 [26] | RCT | Healthy adults | USA | 23 | 30 | 65–82 | Diabetes, cancer, smoking, CVD, kidney disease, uncontrolled high blood pressure, low daily protein intake |
| Chung 2018 [36] | Cross-sectional | Healthy adults | Korea | 1590 | 78 | 40–79 | Metabolic syndrome, HRT, any medication |
| den Ouden 2013 [37] | Cross-sectional | Healthy adults | The Netherlands | 403 | 100 | 73–91 | Inability to visit the study center independently |
| Dipla 2017 [55] | Cross-sectional | Healthy and hypertensive adults | Greece | 91 | 60 | 31–55 | CVD, diabetes |
| Fahs 2017 [39] | Cross-sectional | Healthy adults | USA | 71 | 51 | 18–75 | Hypertension, participation in regular exercise, HRT |
| Gonzales 2015 [40] | Cross-sectional | Healthy adults | USA | 45 | 44 | 60–78 | CVD, diabetes, pulmonary disease, HRT, obesity, medication for blood pressure or cholesterol |
| Groen 2014 [61] | Cross-sectional | Healthy and T2DM adults | The Netherlands | 45 | 100 | 23–71 | Impaired renal or liver function, obesity, CVD, hypertension, advanced diabetes, insulin therapy |
| Gueugneau 2016 [56] | Cross-sectional | Healthy and hypertensive adults | France | 37 | 100 | 21–74 | Prior myocardial infarction or stroke, heart failure, atrial fibrillation, diabetes, morbid obesity, Parkinson’s disease |
| Heffernan 2012 [41] | Cross-sectional | Healthy adults | USA | 24 | 46 | 70–85 | Acute/terminal illness, coronary heart disease, myocardial infarction, hypertension, neuromuscular disease, HRT, diabetes, renal disease, BMI > 32.5 |
| Im 2017 [42] | Cross-sectional | Healthy adults | Korea | 3356 | 100 | 40–64 | ABI < 0.9, high WBC count, cancer |
| Khoudary 2015 [38] | Cross-sectional | Healthy adults | USA | 1103 | 0 | 56–62 | Stroke, angina or myocardial infarction, hysterectomy or bilateral oophorectomy, pregnancy, HRT |
| Kohara 2017 [43] | Cross-sectional | Healthy adults | Japan | 1518 | 40 | 60–74 | CVD, PAD, stroke, coronary heart disease, and congestive heart failure |
| Lee 2014 [44] | Cross-sectional | Healthy adults | Korea | 427 | 42 | 52–95 | n/r or unclear |
| Lima-Junior 2018 [57] | Cross-sectional | Hypertensive adults | Brazil | 72 | 28 | 48–68 | <18 years, smoking, diabetics, CVD, inability to perform isometric handgrip, enrolled in physical activity program |
| Mitchell 2013 [25] | RCT | Healthy adults | UK | 36 | 100 | 18–75 | Diabetes, CVD, BMI < 18 or >28 |
| Ochi 2010 [46] | Cross-sectional | Healthy adults | Japan | 496 | 36 | n/r | Stroke, TIA, coronary heart disease and congestive heart failure |
| Phillips 2012 [34] | RCT | Healthy adults | UK | 51 | 57 | 21–72 | Muscle wasting, metabolic or respiratory diseases, CVD, chronic diseases |
| Prior 2016 [47] | Cross-sectional | Healthy adults | USA | 76 | 42 | 45–80 | Coronary artery disease, heart failure, PAD, stroke, liver, kidney or lung disease, smoking |
| Sampaio 2014 [58] | Cross-sectional | Healthy and hypertensive adults | Japan | 175 | 48 | 70–77 | Moderate cognitive impairment, uncontrolled cardiovascular, pulmonary or metabolic diseases, stroke, Parkinson’s disease, PAD, orthopedic disease |
| Sanada 2010 [48] | Cross-sectional | Healthy adults | Japan | 1488 | 29 | 18–85 | CVD, beta-blockers, HRT, athletes |
| Shimizu 2017 [59] | Cross-sectional | Hypertensive adults | Japan | 795 | 57 | 60–89 | Participants without hypertension, BMI < 18.5, high BP, history of stroke |
| Shiotsu 2018 [49] | RCT | Healthy adults | Japan | 45 | 100 | 63–85 | Current participation in structured exercise program, CVD, musculoskeletal disease, diabetes |
| Suwa 2018 [50] | Cross-sectional | Healthy adults | Japan | 1354 | 100 | 35–59 | CVD, history of stroke |
| Timmerman 2012 [20] | RCT | Healthy older adults | USA | 6 | 50 | 67–73 | Obesity, chronic diseases |
| Verdijk 2016 [27] | RCT | Healthy adults | The Netherlands | 30 | 100 | 19–83 | CVD, PAD, diabetes, inability to participate in an exercise program |
| Wong 2018 [60] | RCT | Hypertensive adults | Korea | 41 | 0 | 49–67 | Pre-menopause, CVD, diabetes, HRT, smoking, exercise, endocrine disorders, psychiatric disorders |
| Wüst 2009 [62] | Cross-sectional | Adults | UK | 11 | 45 | n/r | n/r or unclear |
| Yamamoto 2009 [51] | Cross-sectional | Healthy adults | Japan | 526 | 34 | 20–83 | Obesity, chronic diseases, smoking, ABI < 0.9, any medication |
| Yoo 2018 [53] | Cross-sectional | Community-dwelling older adults | Korea | 236 | 0 | 67–79 | CVD, cognitive disorder, malignancy |
| Yoshizawa 2009 [52] | RCT | Healthy adults | Japan | 35 | 0 | 32–59 | Chronic diseases, smoking, any medication |
| Zhang 2019 [54] | Cross-sectional | Community-dwelling older adults | China | 1002 | 42 | 65–81 | n/r or unclear |
| Study & Year | Parameter | Region | Modality | Device |
|---|---|---|---|---|
| Brightwell 2018 * [26] | Muscular strength | Appendicular | Peak leg torque | Biodex isokinetic dynamometer |
| Chung 2018 [36] | Muscular strength | Appendicular | Hand-grip strength | Hand-grip dynamometer |
| den Ouden 2013 [37] | Muscular strength | Appendicular | Hand-grip strength | JAMAR hand-grip dynamometer |
| Dipla 2017 [55] | Muscular strength | Appendicular | Hand-grip strength | Biopac hand-grip dynamometer |
| Lima-Junior 2018 [57] | Muscular strength | Appendicular | Hand-grip strength | Hand-grip dynamometer |
| Shimizu 2017 [59] | Muscular strength | Appendicular | Hand-grip strength | Smedley hand-grip dynamometer |
| Wong 2018 [60] | Muscular Strength | Appendicular | 8-RM | Cybex dynamometer |
| Yoo 2018 [53] | Muscular strength | Appendicular | Hand-grip strength | T.K.K hand-grip dynamometer |
| Im 2017 [42] | Muscle mass | Whole body | BIA | Inbody |
| Kohara 2017 [43] | Muscle mass | Whole body | BIA, CT | Omron, GE |
| Lee 2014 [44] | Muscle mass | Whole body | BIA | InBody |
| Mitchell 2013 * [25] | Muscle mass | Appendicular | DXA | Lunar |
| Sampaio 2014 [58] | Muscle mass | Whole body | BIA | n/r |
| Sanada 2010 [48] | Muscle mass | Whole body | DXA | Hologic |
| Timmerman 2012 * [20] | Muscle anabolic response | Appendicular | Muscle biopsy | Bergström needle |
| Zhang 2019 [54] | Muscle mass | Whole body | BIA | InBody |
| Barnouin 2017 * [33] | Muscle CSA | Appendicular | Muscle biopsy | Bergström needle |
| Gueugneau 2016 * [56] | Muscle CSA | Appendicular | Muscle biopsy | Bergström needle |
| Ochi 2010 [46] | Muscle CSA | Appendicular | CT | GE |
| Wüst 2009 * [62] | Muscle CSA | Appendicular | Muscle biopsy | Percutaneous needle |
| Gonzales 2015 [40] | Muscular function | Whole body | 400 m walk | Polar monitor (to track HR during walk) |
| Khoudary 2015 [38] | Muscular function | Whole body | 40-foot walking speed, sit-to-stand test | n/r |
| Suwa 2018 [50] | Muscular function | Appendicular | Arm extensibility test | n/r |
| Yamamoto 2009 [51] | Muscular function | Whole body | Sit and reach test | Takei Scientific digital flexibility testing device |
| Yoshizawa 2009 [52] | Muscular function | Whole body | 1-RM, aerobic capacity | Selectorized weight machines, cycle ergometer |
| Barrera 2014 [35] | Muscular strength and function | Whole body | 12-min walk, TUG, hand-grip strength | Digital force transducer and hand-grip dynamometer |
| Fahs 2017 [39] | Muscular strength and muscle mass | Whole body | 1-RM, DXA | Selectorized weight machines, Hologic |
| Groen 2014 * [61] | Muscle mass and CSA | Appendicular | CT, muscle biopsy | n/r, percutaneous needle |
| Heffernan 2012 [41] | Muscle strength and power | Appendicular | 1-RM | Keiser Sports |
| Phillips 2012 [34] | Muscle mass and strength | Whole body | DXA, leg extension 75% 1-RM | Lunar, Leisure Lines |
| Prior 2016 * [47] | Muscle mass and CSA | Whole body | DXA, CT | Lunar, Siemens |
| Shiotsu 2018 [49] | Muscular strength and function | Whole body | 1-RM, hand-grip strength, 10-m walk, sit and reach test | Leg press/curl, chest/shoulder press, seated row, hand-grip dynamometer |
| Verdijk 2016 * [27] | Muscular strength and muscle mass | Whole body | 1-RM, CT, DXA, muscle biopsy | Technogym, Phillips Medical, GE, percutaneous needle |
| Study & Year | Parameter | Vascular Site | Method | Device |
|---|---|---|---|---|
| Chung 2018 [36] | PWV | Brachial-ankle | Volume-plethysmographic apparatus | Colin Medical |
| Fahs 2017 [39] | PWV | Carotid-femoral | Applanation tonometry | SphygmoCor |
| Gonzales 2015 [40] | PWV | Carotid-femoral | Applanation tonometry | SphygmoCor |
| Im 2017 [42] | PWV | Carotid-ankle | Oscillometric | Fukuda Denshi |
| Kohara 2017 [43] | PWV | Brachial-ankle | Volume-plethysmographic apparatus | Omron |
| Ochi 2010 [46] | PWV | Brachial-ankle | Volume-plethysmographic apparatus | Omron |
| Sampaio 2014 [58] | PWV | Carotid-ankle | Oscillometric | Fukuda Denshi |
| Sanada 2010 [48] | PWV | Brachial-ankle | Volume-plethysmographic apparatus | Colin Medical |
| Shiotsu 2018 [49] | PWV | Carotid-femoral | Oscillometric | Fukuda Denshi |
| Wong 2018 [60] | PWV | Brachial-ankle | Volume-plethysmographic apparatus | Colin Medical |
| Yamamoto 2009 [51] | PWV | Brachial-ankle | Volume-plethysmographic apparatus | Omron |
| Yoshizawa 2009 [52] | PWV | Carotid-femoral | Volume-plethysmographic apparatus | Colin Medical |
| Zhang 2019 [54] | PWV | Brachial-ankle | Volume-plethysmographic apparatus | Omron |
| Barrera 2014 [35] | CIMT | Carotid | Ultrasound | GE |
| den Ouden 2013 [37] | CIMT | Carotid | Ultrasound | ATL Ultramark IV |
| Khoudary 2015 [38] | CIMT | Carotid | Ultrasound | Teratech Corp |
| Shimizu 2017 [59] | CIMT | Carotid | Ultrasound | GE |
| Suwa 2018 [50] | CIMT | Carotid | Ultrasound | Aplio |
| Heffernan 2012 [41] | Aix | Radial | Applanation tonometry | Omron |
| Lee 2014 [44] | Aix | Radial | Applanation tonometry | SphygmoCor |
| Lima-Junior 2018 [57] | Aix | Radial | Applanation tonometry | EndoPAT |
| Dipla 2017 [55] | FMD | Brachial | Muscle oxygenation apparatus | NIRS Artinis |
| Yoo 2018 [53] | FMD | Brachial | Applanation tonometry | EndoPAT |
| Phillips 2012 [34] | LBF | Femoral | Doppler ultrasound | Toshiba |
| Barnouin 2017 * [33] | C: F Ratio | Femoral | Immunohistochemistry | Bergström needle |
| Brightwell 2018 * [26] | C: F Ratio | Femoral | Immunohistochemistry | Bergström needle |
| Groen 2014 * [61] | C: F Ratio | Femoral | Immunohistochemistry | Percutaneous needle |
| Gueugneau 2016 * [56] | C: F Ratio | Femoral | Immunohistochemistry | Bergström needle |
| Prior 2016 * [47] | C: F Ratio | Femoral | Immunohistochemistry | Percutaneous needle |
| Verdijk 2016 * [27] | C: F Ratio | Femoral | Immunohistochemistry | Percutaneous needle |
| Wüst 2009 * [62] | C: F Ratio | Femoral | Immunohistochemistry | Percutaneous needle |
| Mitchell 2013 * [25] | MBF | Femoral | Contrast-enhanced ultrasound | Sonovue |
| Timmerman 2012 * [20] | MBF | Femoral | Doppler ultrasound | Philips ATL |
| Study & Year | Sample | Age | Muscle and Vascular Association | Type of Association | Finding |
|---|---|---|---|---|---|
| Barrera 2014 [35] | Healthy adults | 29–88 | Muscular strength/function and CIMT | Difference between groups (p < 0.05) † | In older adults, CIMT is negatively associated with muscular strength and function |
| Chung 2018 [36] | Healthy adult men | 40–79 | Muscular strength and PWV | Difference between groups (p < 0.05) † | In middle-aged and older adults, arterial stiffness is negatively associated with muscular strength and function |
| den Ouden 2013 [37] | Healthy older men | 73–91 | Muscular strength and CIMT | Correlation (r = −0.17; p < 0.05) | In older men, CIMT is negatively associated with muscular strength |
| Dipla 2017 # [55] | Healthy and hypertensive adults | 31–55 | Muscular strength and muscle perfusion | Difference between groups (p < 0.05) † | Hypertensive adults have reduced tissue oxygen saturation compared to healthy controls; to produce same amount of torque compared to healthy controls requires a two-fold increase in BP |
| Fahs 2017 [39] | Healthy adults | 18–75 | Muscular strength and PWV | Correlation (r = −0.230/−0.484; p < 0.05) | In adults, arterial stiffness is negatively correlated with absolute and relative muscular strength |
| Gonzales 2015 [40] | Healthy older adults | 60–78 | Muscular function and PWV | Beta coefficient (p < 0.05) | In older adults, arterial stiffness is positively correlated with muscle fatigue |
| Heffernan 2012 [41] | Healthy older adults | 70–85 | Muscular power and augmentation index | Correlation (r = -0.54; p < 0.05) | In older adults, arterial stiffness is negatively associated with muscular power |
| Im 2017 [42] | Healthy adult men | 40–64 | Muscle mass and PWV | Correlation (p < 0.05) | In middle-aged men, arterial stiffness is negatively correlated with muscle mass |
| Khoudary 2015 [38] | Healthy older women | 56–62 | Muscle function and CIMT | Beta coefficient (0.028; p < 0.05) | In older women, CIMT is negatively associated with muscle function |
| Kohara 2017 [43] | Healthy older adults | 60–74 | Muscle mass and PWV | Correlation (r = −0.24; p < 0.05) | In older adults, arterial stiffness is negatively correlated with muscle mass |
| Lee 2014 [44] | Healthy older adults | 52–95 | Muscle mass and augmentation index | Beta coefficient (p < 0.05) | In older adults, arterial stiffness is negatively associated with muscle mass |
| Lima-Junior 2018 # [57] | Hypertensive older adults | 48–68 | Muscular strength and augmentation index | Beta coefficient (−0.49; p < 0.05) | In older adults with hypertension, arterial stiffness is negatively associated with muscular strength |
| Ochi 2010 [46] | Healthy adults | n/r | Muscle CSA and PWV | Correlation (r = −0.34; p < 0.05) | In men, arterial stiffness is negatively associated with muscle mass |
| Phillips 2012 * [34] | Healthy adults—resistance exercise | 21–72 | Muscle mass/strength and leg blood flow | Difference between groups (p < 0.05) † | Following resistance exercise training, adults experience increases in leg blood flow, muscle mass and strength regardless of age in response to feeding |
| Sampaio 2014 # [58] | Healthy and hypertensive older adults | 70–77 | Muscle mass and PWV | Odds ratio (1.82; p < 0.05) | In healthy and hypertensive older adults, arterial stiffness is negatively associated with muscle mass |
| Sanada 2010 [48] | Healthy adults | 41–71 | Muscle mass and PWV | Difference between groups (p < 0.05) † | Women with sarcopenia have higher arterial stiffness compared to healthy controls |
| Shimizu 2017 # [59] | Hypertensive older adults | 60–89 | Muscular strength and CIMT | Difference between groups (p < 0.05) † | In older adults with hypertension, CIMT is negatively associated with muscular strength |
| Shiotsu 2018 * [49] | Healthy older men—resistance exercise | 63–85 | Muscular strength/function and PWV | Difference between groups (p < 0.05) † | Following resistance exercise training, older men experience a decrease in arterial stiffness and an increase in muscular strength/function |
| Suwa 2018 [50] | Healthy adult men | 35–59 | Muscular function and CIMT | Beta coefficient (−0.189; p < 0.05) | In middle-aged adults, CIMT is negatively associated with arm flexibility |
| Wong 2018 *# [60] | Hypertensive older women—stair climbing exercise | 49–67 | Muscular strength and PWV | Correlation (r = −0.47; p < 0.05) | Following stair climbing training, hypertensive older women experience a decrease in arterial stiffness and an increase in muscular strength |
| Yamamoto 2009 [51] | Healthy adults | 40–83 | Muscular function and PWV | Correlation (r = 0.17/0.45; p < 0.05) | In middle-aged and older adults, arterial stiffness is negatively correlated with flexibility |
| Yoo 2018 [53] | Older women | 67–79 | Muscular strength and endothelial function | Correlation (r = 0.176; p < 0.05) | After adjusting for comorbidities, in older women, endothelial function is positively correlated with muscular strength |
| Yoshizawa 2009 * [52] | Healthy women—aerobic exercise | 32–59 | Muscular function and PWV | Difference between groups (p < 0.05) † | Following aerobic training, middle-aged women experience a decrease in arterial stiffness and an increase in muscular function |
| Zhang 2019 [54] | Older adults | 65–81 | Muscle mass and PWV | Odds ratio (1.11; p < 0.05) | After adjusting for comorbidities, in older adults, arterial stiffness is negatively associated with muscle mass |
| Study & Year | Sample | Age | Muscle and Vascular Association | Type of Association | Finding |
|---|---|---|---|---|---|
| Barnouin 2017 [33] | Healthy adults | 22–74 | Muscle fiber CSA and capillary density | Correlation (R2 = 0.46; p < 0.05) | In young and older adults, capillary-to-fiber ratio is positively correlated with muscle mass |
| Brightwell 2018 * [26] | Healthy older adults—aerobic exercise | 65–82 | Muscular strength and capillary density | Difference between groups (p < 0.05) † | Following aerobic training, older adults experience an increase in capillary density and an increase in muscular strength |
| Groen 2014 [61] | Healthy adult men | 23–71 | Muscle fiber CSA and capillary density | Difference between groups (p < 0.05) † | Older adults have reduced capillary-to-fiber ratio and muscle mass compared to young controls |
| Gueugneau 2016 # [56] | Healthy and hypertensive older men | 72–74 | Muscle fiber CSA and capillary density | Difference between groups (p < 0.05) † | Older adults with hypertension have lower capillary-to-fiber ratio and muscle mass compared to healthy older controls |
| Mitchell 2013 [25] | Healthy adult men | 18–75 | Muscle mass and microvascular blood flow | Difference between groups (p < 0.05) † | Young adults have higher muscle mass and have higher microvascular blood flow in response to feeding compared to healthy older adults |
| Prior 2016 [47] | Healthy adults | 45–80 | Muscle mass and capillary density | Correlation (r = 0.30–0.37; p < 0.05) | In adults, capillary-to-fiber ratio is positively correlated with muscle mass |
| Timmerman 2012 [20] | Healthy older adults—aerobic exercise | 67–73 | Muscle protein synthesis and microvascular blood flow | Difference between groups (p < 0.05) † | Following aerobic exercise, older adults experience improved microvascular flow and muscle protein synthesis |
| Verdijk 2016 * [27] | Healthy older adults—resistance exercise | 65–83 | Muscle fiber CSA/strength and capillary density | Difference between groups (p < 0.05) † | Following resistance training, older adults experience an increase in capillary-to-fiber ratio and an increase in muscle mass and strength |
| Wüst 2009 [62] | Adults | n/r | Muscle fiber CSA and capillary density | Correlation (r = 0.62; p < 0.05) | In adults, capillary-to-fiber ratio is positively correlated with muscle mass |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvoretskiy, S.; Lieblein-Boff, J.C.; Jonnalagadda, S.; Atherton, P.J.; Phillips, B.E.; Pereira, S.L. Exploring the Association between Vascular Dysfunction and Skeletal Muscle Mass, Strength and Function in Healthy Adults: A Systematic Review. Nutrients 2020, 12, 715. https://doi.org/10.3390/nu12030715
Dvoretskiy S, Lieblein-Boff JC, Jonnalagadda S, Atherton PJ, Phillips BE, Pereira SL. Exploring the Association between Vascular Dysfunction and Skeletal Muscle Mass, Strength and Function in Healthy Adults: A Systematic Review. Nutrients. 2020; 12(3):715. https://doi.org/10.3390/nu12030715
Chicago/Turabian StyleDvoretskiy, Svyatoslav, Jacqueline C. Lieblein-Boff, Satya Jonnalagadda, Philip J. Atherton, Bethan E. Phillips, and Suzette L. Pereira. 2020. "Exploring the Association between Vascular Dysfunction and Skeletal Muscle Mass, Strength and Function in Healthy Adults: A Systematic Review" Nutrients 12, no. 3: 715. https://doi.org/10.3390/nu12030715
APA StyleDvoretskiy, S., Lieblein-Boff, J. C., Jonnalagadda, S., Atherton, P. J., Phillips, B. E., & Pereira, S. L. (2020). Exploring the Association between Vascular Dysfunction and Skeletal Muscle Mass, Strength and Function in Healthy Adults: A Systematic Review. Nutrients, 12(3), 715. https://doi.org/10.3390/nu12030715







