Exploring the Comparative Efficacy of Metformin and Resveratrol in the Management of Diabetes-Associated Complications: A Systematic Review of Preclinical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Assessment of Quality
3. Results
3.1. Characteristic Features of Included Studies
3.2. Risk of Bias and Quality of the Studies
3.3. In vitro Evidence on the Comparative Effects of Metformin with Resveratrol Against Diabetes-Associated Complications
3.4. In Vivo Evidence on the Comparative Effects of Metformin with Resveratrol Against Diabetes-Associated Complications
3.5. Experimental Evidence on the Combined Effects of Metformin with Resveratrol Against Diabetes-Associated Complications
4. Discussion
5. Conclusions
Abbreviations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poretsky, L. Principles of Diabetes Mellitus; Springer: Boston, MA, USA, 2010; Volume 21, Available online: https://www.springer.com/gp/book/9780387098418 (accessed on 8 October 2019).
- International Diabetes Federation. IDF Diabetes Atlas Eighth Edition. 2017. Available online: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/134-idf-diabetes-atlas-8th-edition.html (accessed on 3 November 2019).
- World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 2 November 2019).
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 3 November 2019).
- Trikkalinou, A.; Papazafiropoulou, A.K.; Melidonis, A. Type 2 diabetes and quality of life. World J. Diabetes 2017, 8, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Backholer, K.; Gearon, E.; Harding, J.; Freak-Poli, R.; Stevenson, C.; Peeters, A. Diabetes and risk of physical disability in adults: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013, 1, 106–114. [Google Scholar] [CrossRef]
- Kirkman, M.S.; Briscoe, V.J.; Clark, N.; Florez, H.; Haas, L.B.; Halter, J.B.; Huang, E.S.; Korytkowski, M.T.; Munshi, M.N.; Odegard, P.S.; et al. Diabetes in older adults: A consensus report. J. Am. Geriatr. Soc. 2012, 60, 2342–2356. [Google Scholar] [CrossRef] [PubMed]
- Assah, F.K.; Ekelund, U.; Brage, S.; Mbanya, J.C.; Wareham, N.J. Urbanization, physical activity, and metabolic health in sub-Saharan Africa. Diabetes Care 2011, 34, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Pitsavos, C.; Panagiotakos, D.; Weinem, M.; Stefanadis, C. Diet, exercise and the metabolic syndrome. Rev. Diabet. Stud. 2006, 3, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Conget, I. Diagnosis, classification and cathogenesis of diabetes mellitus. Rev. Esp. Cardiol. 2002, 55, 528–535. [Google Scholar] [CrossRef]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.H.; Orci, L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet (Lond. Engl.) 1975, 1, 14–16. [Google Scholar] [CrossRef]
- Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; Grobbee, D.; et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.L.; Williams, D.M.; O’Neil, L.K.; Hayes, J.; Stephens, J.W.; Bracken, R.M. Physical exercise and non-insulin glucose-lowering therapies in the management of Type 2 diabetes mellitus: A clinical review. Diabet. Med. 2019, 36, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Calvano, A.; Izuora, K.; Oh, E.C.; Ebersole, J.L.; Lyons, T.J.; Basu, A. Dietary berries, insulin resistance and type 2 diabetes: An overview of human feeding trials. Food Funct. 2019, 10, 6227–6243. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Joubert, E.; Muller, C.J.F.; Louw, J.; Johnson, R. Hyperglycemia-induced oxidative stress and heart disease-cardioprotective effects of rooibos flavonoids and phenylpyruvic acid-2-O-β-D-glucoside. Nutr. Metab. (Lond.) 2017, 14, 45. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, C.; Qiu, S.; Yuan, X.; Li, L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: Systematic review and meta-analysis. Nutr. Metab. (Lond.) 2017, 14, 60. [Google Scholar] [CrossRef]
- Park, E.J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta 2015, 1852, 1071–1113. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem CID: 445154. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/445154#section=Top (accessed on 5 November 2019).
- Duarte-Vázquez, M.A.; Gomez-Solis, M.A.; Gómez-Cansino, R.; Reyes-Esparza, J.; Jorge, L.R.; Rodríguez-Fragoso, L. Effects of combined resveratrol plus metformin therapy in db/db diabetic mice. J. Metab. Synd. 2016, 5, 217. [Google Scholar]
- Frendo-Cumbo, S.; MacPherson, R.E.; Wright, D.C. Beneficial effects of combined resveratrol and metformin therapy in treating diet-induced insulin resistance. Physiol. Rep. 2016, 4, e12877. [Google Scholar] [CrossRef]
- Jorgensen, P.G.; Jensen, M.T.; Mensberg, P.; Storgaard, H.; Nyby, S.; Jensen, J.S.; Knop, F.K.; Vilsbøll, T. Effect of exercise combined with glucagon-like peptide-1 receptor agonist treatment on cardiac function: A randomized double-blind placebo-controlled clinical trial. Diabetes Obes. Metab. 2017, 19, 1040–1044. [Google Scholar] [CrossRef]
- Li, A.; Zhang, S.; Li, J.; Liu, K.; Huang, F.; Liu, B. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol. Cell. Endocrinol. 2016, 434, 36–47. [Google Scholar] [CrossRef]
- Mehdi, F.; Keihan, G.S.; Asadollah, A.S.; Effat, F. The effects of resveratrol, metformin, cold and strength training on the level of perilipin 5 in the heart, skeletal muscle and brown adipose tissues in mouse. Cell Biochem. Biophys. 2018, 76, 471–476. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem CID: 4091. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Metformin (accessed on 19 November 2019).
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Mahlangu, T.; Dludla, P.V.; Nyambuya, T.M.; Mxinwa, V.; Mazibuko-Mbeje, S.E.; Cirilli, I.; Marcheggiani, F.; Tiano, L.; Louw, J.; Nkambule, B.B. A systematic review on the functional role of Th1/Th2 cytokines in type 2 diabetes and related metabolic complications. Cytokine 2019, 126, 154892. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Hattangady, N.G.; Rajadhyaksha, M.S. A brief review of in vitro models of diabetic neuropathy. Int. J. Diabetes Dev. Ctries. 2009, 29, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Pérez, M.Á.; Sanz, M.B.; Sanjuan, V.M.; González-Álvarez, M.; Álvarez, I.G. Importance and Applications of Cell-And tisSue-Based In Vitro Models for Drug Permeability Screening in Early Stages of Drug Development; Concepts and models for drug permeability Studies; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2016; pp. 3–29. [Google Scholar]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 2006, 55, 2180–2191. [Google Scholar] [CrossRef] [PubMed]
- Vetterli, L.; Brun, T.; Giovannoni, L.; Bosco, D.; Maechler, P. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E beta-cells and human islets through a SIRT1-dependent mechanism. J. Biol. Chem. 2011, 286, 6049–6060. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, H.; Li, J.; Li, T.; Zheng, B.; Zheng, Y.; Jin, H.; He, Y.; Gu, Q.; Xu, X. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes 2012, 61, 217–228. [Google Scholar] [CrossRef]
- Choi, K.M.; Lee, H.L.; Kwon, Y.Y.; Kang, M.S.; Lee, S.K.; Lee, C.K. Enhancement of mitochondrial function correlates with the extension of lifespan by caloric restriction and caloric restriction mimetics in yeast. Biochem. Biophys. Res. Commun. 2013, 441, 236–242. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lim, J.H.; Youn, H.H.; Hong, Y.A.; Yang, K.S.; Park, H.S.; Chung, S.; Ko, S.H.; Shin, S.J.; Choi, B.S.; et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1alpha axis in db/db mice. Diabetologia 2013, 56, 204–217. [Google Scholar] [CrossRef]
- Zhang, E.; Guo, Q.; Gao, H.; Xu, R.; Teng, S.; Wu, Y. Metformin and resveratrol inhibited high glucose-induced metabolic memory of endothelial senescence through SIRT1/p300/p53/p21 Pathway. PLoS ONE 2015, 10, e0143814. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Wang, L.; Li, A.; Qiu, Z.; Qi, L.W.; Kou, J.; Liu, K.; Liu, B.; Huang, F. The role of metformin and resveratrol in the prevention of hypoxia-inducible factor 1alpha accumulation and fibrosis in hypoxic adipose tissue. Br. J. Pharmacol. 2016, 173, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Vasamsetti, S.B.; Karnewar, S.; Gopoju, R.; Gollavilli, P.N.; Narra, S.R.; Kumar, J.M.; Kotamraju, S. Resveratrol attenuates monocyte-to-macrophage differentiation and associated inflammation via modulation of intracellular GSH homeostasis: Relevance in atherosclerosis. Free Radic. Biol. Med. 2016, 96, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Marti, N.; Bouchoucha, N.; Sauter, K.S.; Fluck, C.E. Resveratrol inhibits androgen production of human adrenocortical H295R cells by lowering CYP17 and CYP21 expression and activities. PLoS ONE 2017, 12, e0174224. [Google Scholar] [CrossRef]
- Zhao, W.; Li, A.; Feng, X.; Hou, T.; Liu, K.; Liu, B.; Zhang, N. Metformin and resveratrol ameliorate muscle insulin resistance through preventing lipolysis and inflammation in hypoxic adipose tissue. Cell. Signal. 2016, 28, 1401–1411. [Google Scholar] [CrossRef]
- Cuyas, E.; Verdura, S.; Llorach-Pares, L.; Fernandez-Arroyo, S.; Joven, J.; Martin-Castillo, B.; Bosch-Barrera, J.; Brunet, J.; Nonell-Canals, A.; Sanchez-Martinez, M.; et al. Metformin is a direct SIRT1-activating compound: Computational modeling and experimental validation. Front. Endocrinol. (Lausanne) 2018, 9, 657. [Google Scholar] [CrossRef]
- Zhao, W.; Li, A.; Feng, X.; Hou, T.; Liu, K.; Liu, B.; Zhang, N. Data on biochemical indexes of HFD-fed mice treatment with metformin or resveratrol. Data Brief 2016, 8, 1190–1193. [Google Scholar] [CrossRef][Green Version]
- Nowak, W.N.; Taha, H.; Markiewicz, J.; Kachamakova-Trojanowska, N.; Stepniewski, J.; Kloska, D.; Florczyk-Soluch, U.; Niżankowski, R.; Frołow, M.; Walter, Z.; et al. Atorvastatin and conditioned media from atorvastatin-treated human hematopoietic stem/progenitor-derived cells show proangiogenic activity in vitro but not in vivo. Mediat. Inflamm. 2019, 2019, 1868170. [Google Scholar] [CrossRef]
- Chang, G.W.; Kam, P.C. The physiological and pharmacological roles of cytochrome P450 isoenzymes. Anaesthesia 1999, 54, 42–50. [Google Scholar] [CrossRef]
- Al-Awar, A.; Kupai, K.; Veszelka, M.; Szucs, G.; Attieh, Z.; Murlasits, Z.; Török, S.; Pósa, A.; Varga, C. Experimental diabetes mellitus in different animal models. J. Diabetes Res. 2016, 2016, 9051426. [Google Scholar] [CrossRef]
- Chi, T.C.; Chen, W.P.; Chi, T.L.; Kuo, T.F.; Lee, S.S.; Cheng, J.T.; Su, M.J. Phosphatidylinositol-3-kinase is involved in the antihyperglycemic effect induced by resveratrol in streptozotocin-induced diabetic rats. Life Sci. 2007, 80, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Middela, H.; Matapally, S.; Padiya, R.; Bastia, T.; Madhusudana, K.; Reddy, B.R.; Chakravarty, S.; Banerjee, S.K. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacol. Res. 2012, 66, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, J.; Xiao, N.; Wang, M.; Kou, J.; Qi, L.; Huang, F.; Liu, B.; Liu, K. Pharmacological activation of AMPK ameliorates perivascular adipose/endothelial dysfunction in a manner interdependent on AMPK and SIRT1. Pharmacol. Res. 2014, 89, 19–28. [Google Scholar] [CrossRef]
- Deng, W.; Cha, J.; Yuan, J.; Haraguchi, H.; Bartos, A.; Leishman, E.; Viollet, B.; Bradshaw, H.B.; Hirota, Y.; Dey, S.K. p53 coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern parturition timing. J. Clin. Investig. 2016, 126, 2941–2954. [Google Scholar] [CrossRef]
- Kaur, G.; Padiya, R.; Adela, R.; Putcha, U.K.; Reddy, G.S.; Reddy, B.R.; Kumar, K.P.; Chakravarty, S.; Banerjee, S.K. Garlic and resveratrol attenuate diabetic complications, loss of beta-cells, pancreatic and hepatic oxidative stress in streptozotocin-induced diabetic rats. Front. Pharmacol. 2016, 7, 360. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.R.; Maitra, S.; Jhelum, P.; Kumar, K.P.; Bagul, P.K.; Kaur, G.; Banerjee, S.K.; Kumar, A.; Chakravarty, S. Sirtuin 1 and 7 mediate resveratrol-induced recovery from hyper-anxiety in high-fructose-fed prediabetic rats. J. Biosci. 2016, 41, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Pereira, G.; Carneiro, V.C.; Mata-Santos, H.; Vicentino, A.R.; Ramos, I.P.; Giarola, N.L.; Feijó, D.F.; Meyer-Fernandes, J.R.; Paula-Neto, H.A.; Medei, E.; et al. Resveratrol reverses functional chagas heart disease in mice. PLoS Pathog. 2016, 12, e1005947. [Google Scholar] [CrossRef]
- Barger, J.L.; Vann, J.M.; Cray, N.L.; Pugh, T.D.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Newton, M.A.; Weindruch, R.; Prolla, T.A. Identification of tissue-specific transcriptional markers of caloric restriction in the mouse and their use to evaluate caloric restriction mimetics. Aging Cell 2017, 16, 750–760. [Google Scholar] [CrossRef]
- Stockinger, J.; Maxwell, N.; Shapiro, D.; deCabo, R.; Valdez, G. Caloric Restriction Mimetics Slow Aging of Neuromuscular Synapses and Muscle Fibers. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 21–28. [Google Scholar] [CrossRef]
- Rehman, K.; Saeed, K.; Munawar, S.M.; Akash, M.S.H. Resveratrol regulates hyperglycemia-induced modulations in experimental diabetic animal model. Biomed. Pharmacother. 2018, 102, 140–146. [Google Scholar] [CrossRef]
- Bruckbauer, A.; Zemel, M.B. Synergistic effects of metformin, resveratrol, and hydroxymethylbutyrate on insulin sensitivity. Diabetes Metab. Syndr. Obes. Targets Ther. 2013, 6, 93–102. [Google Scholar]
- Fu, L.; Bruckbauer, A.; Li, F.; Cao, Q.; Cui, X.; Wu, R.; Shi, H.; Zemel, M.B.; Xue, B. Leucine amplifies the effects of metformin on insulin sensitivity and glycemic control in diet-induced obese mice. Metabolism 2015, 64, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Furat Rencber, S.; Kurnaz Ozbek, S.; Eraldemir, C.; Sezer, Z.; Kum, T.; Ceylan, S.; Guzel, E. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: An experimental study. J. Ovarian Res. 2018, 11, 55. [Google Scholar] [CrossRef]
- Das, S.; Beehera, J.P.; Rojaramani, Y.; Mohanty, R.R. Effects of resveratrol on oxidative stress in high fat diet /streptozocin induced diabetic wistar albino rats. Int. J. Basic Clin. Pharmacol. 2019, 8, 482. [Google Scholar] [CrossRef]
- Yang, A.J.T.; Frendo-Cumbo, S.; MacPherson, R.E.K. Resveratrol and metformin recover prefrontal cortex AMPK activation in diet-induced obese mice but reduce BDNF and synaptophysin protein content. J. Alzheimers Dis. 2019, 71, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Madsen, K.S.; Chi, Y.; Metzendorf, M.I.; Richter, B.; Hemmingsen, B. Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2019, 12, Cd008558. [Google Scholar] [CrossRef]
- Halimi, S.; Schweizer, A.; Minic, B.; Foley, J.; Dejager, S. Combination treatment in the management of type 2 diabetes: Focus on vildagliptin and metformin as a single tablet. Vasc. Health Risk Manag. 2008, 4, 481–492. [Google Scholar]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Aditya, R.; Kiran, A.R.; Varma, D.S.; Vemuri, R.; Gundamaraju, R. A Review on SIRtuins in Diabetes. Curr. Pharm. Des. 2017, 23, 2299–2307. [Google Scholar] [CrossRef]
- Bartoli-Leonard, F.; Wilkinson, F.L.; Schiro, A.; Inglott, F.S.; Alexander, M.Y.; Weston, R. Suppression of SIRT1 in diabetic conditions induces osteogenic differentiation of human vascular smooth muscle cells via RUNX2 signalling. Sci. Rep. 2019, 9, 878. [Google Scholar] [CrossRef]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Roux, C.; Johnson, R.; Ghoor, S.; Joubert, E.; Louw, J.; Opoku, A.R.; Muller, C.J.F. Aspalathin-enriched green rooibos extract reduces hepatic insulin resistance by modulating PI3K/AKT and AMPK pathways. Int. J. Mol. Sci. 2019, 20, 633. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. (N.Y.) 2012, 32, 537–541. [Google Scholar] [CrossRef]
- Movahed, A.; Nabipour, I.; Lieben Louis, X.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid. Based Complement. Alternat. Med. 2013, 2013, 851267. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.F.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; de Ligt, M.; Phielix, E.; van de Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.; Schrauwen-Hinderling, V.B.; et al. Resveratrol as add-on therapy in subjects with well-controlled type 2 diabetes: A randomized controlled trial. Diabetes Care 2016, 39, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
- Durham, J.T.; Herman, I.M. Microvascular modifications in diabetic retinopathy. Curr Diab Rep. 2011, 11, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients 2018, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Dludla, P.; Joubert, E.; February, F.; Mazibuko, S.; Ghoor, S.; Muller, C.; Louw, J. Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose induced shifts in substrate preference and apoptosis. Mol. Nutr. Food Res. 2016, 60, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.G.; Francis, N.; Hill, R.; Waters, D.; Blanchard, C.; Santhakumar, A.B. Dietary polyphenols and gene expression in molecular pathways associated with type 2 diabetes mellitus: A Review. Int. J. Mol. Sci. 2019, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Mxinwa, V.; Tiano, L.; Marcheggiani, F.; Cirilli, I.; Louw, J.; Nkambule, B.B. The beneficial effects of N-acetyl cysteine (NAC) against obesity associated complications: A systematic review of pre-clinical studies. Pharmacol. Res. 2019, 146, 104332. [Google Scholar] [CrossRef] [PubMed]

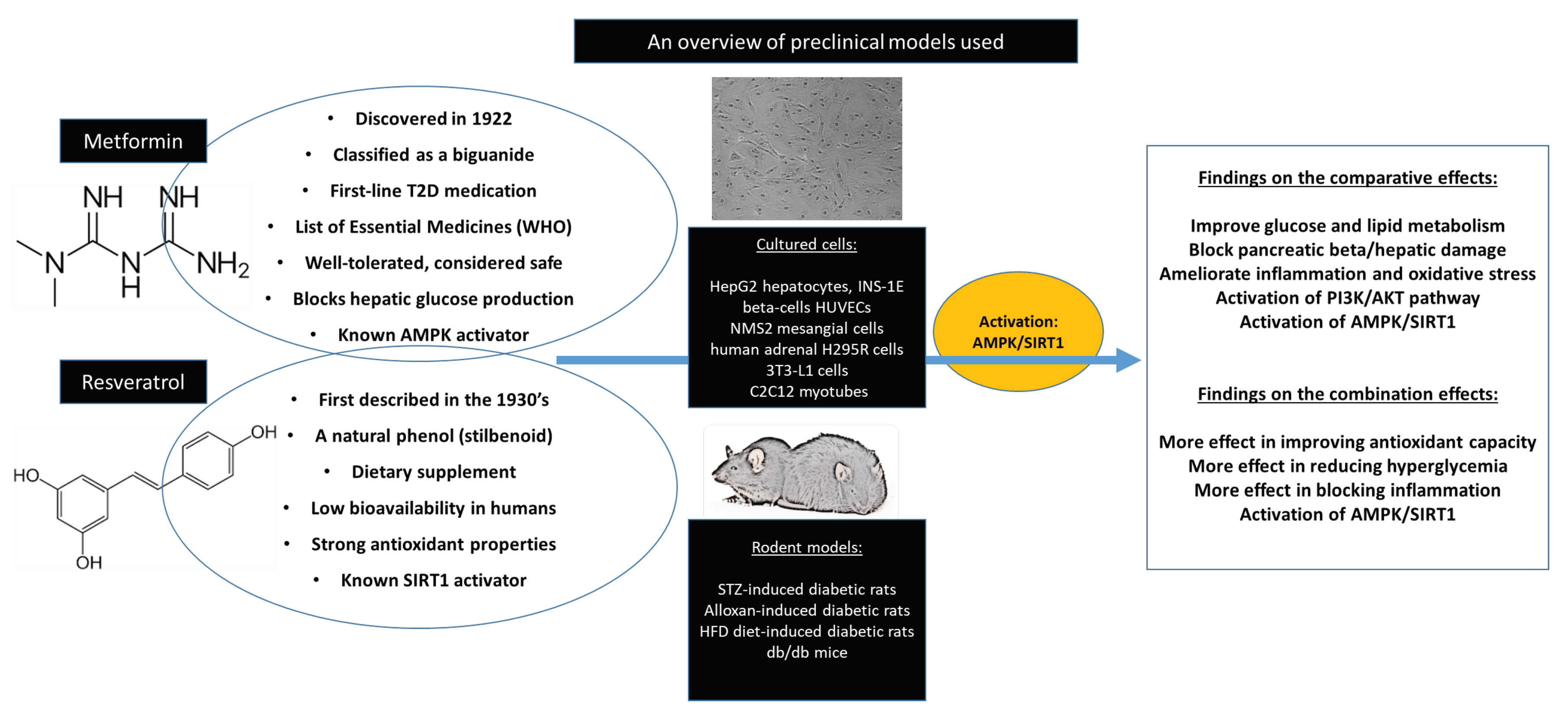
| Author, Year | Experimental Model, Dose Used, and Intervention Period | Experimental Outcome and Proposed Mechanism |
|---|---|---|
| Zang et al. 2006 [33] | HepG2 hepatocytes exposed to high glucose before treated with metformin (2 mmol/L) or resveratrol (10 μmol/L) for 1 h | Metformin and resveratrol significantly stimulated 5’ AMP-activated protein kinase (AMPK) phosphorylation and inhibited intracellular levels of triglycerides |
| Vetterli et al. 2011 [34] | INS-1E beta-cells and human islets were cultured with 25 μM resveratrol or 5 mM metformin for 24 h | Resveratrol improved glucose-stimulated insulin secretion. This effect was associated with elevated glycolytic flux, resulting in increased glucose oxidation, ATP generation, and mitochondrial oxygen consumption. Such changes correlated with up-regulation of key genes for β-cell function, i.e., glucose transporter (GLUT)2, glucokinase, pancreatic and duodenal homeobox 1 (PDX-1). In human islets, chronic resveratrol treatment similarly increased both the glucose secretory response and expression of the same set of genes, eventually restoring the glucose response in islets obtained from one type 2 diabetic donor. Overexpression of Sirt1 (silent mating type information regulation 2 homolog 1) in INS-1E cells potentiated resveratrol effects on insulin secretion. Conversely, inhibition of SIRT1 abolished resveratrol effects on glucose responses |
| Zheng et al. 2012 [35] | Bovine retinal capillary endothelial cells (BRECs) exposed to high glucose before treatment with metformin (1 mmol/L) or resveratrol (100 mmol/L) for 1 week | Metformin inhibited the increase of mitochondrial reactive oxygen species-mediated glyceraldehyde-3-phosphate dehydrogenase by poly (ADP-ribose) polymerase (PARP) activity through the upregulation of liver kinase B1/AMP-activated protein kinase (LKB1/AMPK). Metformin also performed similarly to resveratrol in suppressing nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and bcl-2-like protein 4 (BAX) expression. Furthermore, metformin suppressed hyperglycemia stress in the diabetic retinas, which may be involved in the SIRT1/LKB1/AMPK pathway |
| Choi et al. 2013 [36] | Compared seven identified caloric restriction mimetics (CRMs) which extend the lifespans of various organisms including caffeine, curcumin, dapsone, metformin, rapamycin, and resveratrol at concentrations ranging from 1 to 100 μM for different culture times in a single model, Saccharomyces cerevisiae | Rapamycin extended chronological lifespan (CLS), but other CRMs failed to extend CLS. Rapamycin enhanced mitochondrial function like caloric restriction did, but other CRMs did not. Both caloric restriction and rapamycin worked on mitochondrial function, but they worked at different windows of time during the chronological aging process |
| Kim et al. 2013 [37] | NMS2 mesangial cells exposed to high glucose before treatment with resveratrol (1, 10 or 50 ng/mL), or metformin (1 mmol/L) for 48 h | Resveratrol prevented high-glucose-induced oxidative stress and apoptosis in cultured mesangial cells through the phosphorylation of AMPK and activation of SIRT1/ peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1)α signalling and the downstream effectors, peroxisome proliferator-activated receptor (PPARα)/estrogen-related receptor (ERR)-1α/sterol regulatory element-binding protein (SREBP)1 |
| Zhang et al. 2015 [38] | Human umbilical vascular endothelial cells (HUVECs) were exposed to high glucose for 6 days, or 3 days followed by 3 days of normal glucose treatment with or without resveratrol (0, 1.25, 2.5, 5, or 10 μM) or metformin (0, 1, 10, 50, 100, 250 μM) | Resveratrol and metformin treatment prevented senescent "memory" by modulating SIRT1/p300/p53/p21 pathway. Notably, early and continuous treatment of metformin, but not resveratrol, was particularly important for preventing senescent "memory |
| Li et al. 2016 [39] | Differentiated 3T3-L1 cells were incubated with metformin (1 mM) or resveratrol (10 μM) under normoxia for 4 h | Metformin and resveratrol reduced ATP production and prevented the reduction in oxygen tension in 3T3-L1 cells, suggesting that it prevented hypoxia by limiting oxygen consumption, whereas resveratrol reduced HIF-1α accumulation by promoting its proteasomal degradation via the regulation of AMPK/SIRT1 |
| Li et al. 2016 [25] | 3T3-L1 adipocytes with high glucose (33 mM) for 24 h were incubated with metformin (1 mM) or resveratrol (10 μM) under normoxia for 4 h | Metformin and resveratrol inhibited inflammation and reduced cell apoptosis in adipose tissue or adipocytes exposed to high glucose |
| Vasamsetti et al. 2016 [40] | THP-1 monocytes treated with metformin (0.5–2 mM) or resveratrol (0-100 μmmol/L) for 24 h | Similar to resveratrol, metformin dose-dependently increased glutathione (GSH) levels. Furthermore, incubation of cells with buthionine sulfoximine (BSO) did not affect either metformin-or resveratrol-mediated AMPK activation |
| Zhao et al. 2016 [42,44] | 3T3-L1 cells and C2C12 myotubes were treated with metformin (1mmol/L) or resveratrol (10 μmol/L) under hypoxia conditions for 16 h | Metformin or resveratrol ameliorated insulin resistance in muscle cells by blocking free fatty acid trafficking |
| Marti et al. 2017 [41] | Human adrenal H295R cells were treated with metformin and resveratrol at 1 mM. for 72 h | Metformin and resveratrol was found to inhibit protein expression and enzyme activities of cytochrome enzymes (CYP17 and CYP21). It did not alter CYP17 and CYP21 mRNA expression, nor protein degradation. Only SIRT3 mRNA expression was found to be altered by resveratrol, but SIRT1, 3 and 5 overexpression did not result in a change in the steroid profile of H295R cells |
| Cuyàs et al. 2018 [43] | In silico analysis focusing on the molecular docking and dynamic simulation of the putative interactions between metformin and SIRT1. Using eight different crystal structures of human SIRT1 protein In vitro enzymatic assays | Metformin was predicted to interact with the very same allosteric site occupied by resveratrol and other sirtuin-activating compounds (STATCs) at the amino-terminal activation domain of SIRT1. Second, metformin was predicted to interact with the NAD+ binding site in a manner slightly different to that of SIRT1 inhibitors containing an indole ring. Third, metformin was predicted to interact with the C-terminal regulatory segment of SIRT1 bound to the NAD+ hydrolysis product ADP-ribose, a "C-pocket"-related mechanism that appears to be essential for mechanism-based activation of SIRT1 Enzymatic assays confirmed that the net biochemical effect of metformin and other biguanides such as a phenformin was to improve the catalytic efficiency of SIRT1 operating in conditions of low NAD+ in vitro |
| Nowak et al. 2019 [45] | Human peripheral blood CD34+ cells were isolated from peripheral blood mononuclear cells (PBMCs) obtained from a healthy volunteer cultured on day 6 with 7.5, 15, or 30 μmol/L resveratrol or 5.0 mmol/L metformin for 48 h | Failed to stabilize tubes and or enhance the paracrine angiogenic activity of human myeloid angiogenic cells |
| Author, Year | Experimental Model, Dose Used, and Intervention Period | Experimental Outcome and Proposed Mechanism |
|---|---|---|
| Chi et al. 2007 [48] | Streptozotocin (STZ)-induced diabetic Wistar rats treated with resveratrol (3 mg/kg) or metformin (100 mg/kg) for 7 days | Resveratrol produced a hypoglycemic effect in a dose-dependent manner and increased insulin levels in diabetic rats. This was followed by reduced plasma glucose and activation of phosphatidyl-3-kinase (PI3K). Resveratrol normalized hepatic phosphoenolpyruvate carboxykinase (PEPCK) expression and increased glucose transporter (GLUT)4 expression in the soleus muscle of STZ-diabetic rats. In STZ-diabetic rats, resveratrol lowered plasma glucose to a similar level as compared to the effect exerted by metformin |
| Bagul et al. 2012 [49] | Fructose-fed male Sprague Dawley rats administered metformin (300 mg/kg/day) or resveratrol (10 mg/kg/day) orally for 8 weeks | Although both drugs normalized altered metabolic parameters, resveratrol showed more potency in improving insulin sensitivity. Similarly, while metformin administration failed to normalize the increased thiobarbituric acid reactive substances (TBARS) levels and decreased superoxide dismutase (SOD) activity, resveratrol showed an enhanced effect in attenuating oxidative stress parameters, partially through the upregulation of nuclear factor erythroid 2-related factor 2 (NRF2) |
| Sun et al. 2014 [50] | Fructose-fed Sprague-Dawley rats simultaneously received resveratrol (20 mg/kg) or metformin (100 mg/kg) by oral gavage every day for 8 weeks | Long-term fructose-feeding in rats induced dysregulation of adipocytokine expression in perivascular adipose tissue (PVAT) and the loss of endothelium-dependent vasodilation, whereas oral administration of resveratrol and metformin reversed these alterations |
| Deng et al. 2016 [51] | Uterine-specific deletion of transformation-related protein 53 (p53d/d mice) treated with metformin orally (1 mg/kg BW per dose) on days 8, 10, and 12. Resveratrol was given (30 mg/kg BW per dose) on days 8, 10, 12, and 14 | Treatment of pregnant p53d/d mice with either the antidiabetic drug metformin or the antioxidant resveratrol activated AMPK signaling and inhibited mammalian target of rapamycin complex 1 (mTORC1) signaling in decidual cells. Both metformin and resveratrol protected against spontaneous and inflammation-induced preterm birth in p53d/d mice |
| Kaur et al. 2016 [52] | STZ-induced diabetic rats were fed raw garlic homogenate (250 mg/kg/day), resveratrol (25 mg/kg/day), and metformin (500 mg/kg/day) orally for 4 weeks | Administration of garlic, resveratrol, and metformin significantly normalized altered metabolic and oxidative stress parameters as well as histopathological changes. Treatment with these compounds also ameliorated pancreatic β-cell damage and hepatic injury |
| Li et al. 2016 [39] | ICR male mice fed high-fat diet before treatment with metformin (200 mg/kg), resveratrol (50 mg/kg) or TUDCA (50 mg/kg) by gavage every day for 7 days | Metformin or resveratrol comparably prevented hypoxia and reduced hypoxia-inducible factor 1 (HIF-1)α accumulation with dephosphorylation of inositol-requiring enzyme 1α and eukaryotic initiation factor 2α, indicative of suppression of hypoxic HIF-1α activation and endoplasmic reticulum stress. Metformin and resveratrol down-regulated the expression genes related collagen deposition such as elastin and lysyl oxidase. The increased gene expressions of tumor necrosis factor (TNF)-α, interleukin (IL)-6, monocyte chemoattractant protein 1 and F4/80 were also down-regulated by metformin and resveratrol |
| Li et al. 2016 [25] | STZ-diabetic ICR male mice and followed by oral administration of metformin (200 mg/kg), resveratrol (50 mg/kg) or ER stress inhibitor TUDCA (50 mg/kg) for 7 days | Metformin and resveratrol inhibited reactive oxygen species (ROS)-associated mitochondrial fission by upregulating Drp1 phosphorylation (Ser 637) in an AMPK-dependent manner, and then suppressed endoplasmic reticulum (ER) stress indicated by dephosphorylation of endoribonuclease 1α and eukaryotic initiation factor 2 in the adipose tissue |
| Reddy et al. 2016 [53] | Sprague-Dawley rats on high-fructose diet received resveratrol and metformin together with fructose diet at a single dose of 10 mg/kg/day of resveratrol orally or 300 mg/kg/day of metformin orally, for 8 weeks | Resveratrol was more effective in protecting both the metabolic (prediabetic) and affective (anxiety) disorders than metformin. Molecular studies showed that recovery was associated with the upregulation of few nuclear sirtuins that act epigenetically—SIRT1 and 7 |
| Vilar-Pereira et al. 2016 [54] | BALB/c mice infected type I Colombian strain of T. cruzi before treatment with 15 mg/kg trans-resveratrol, 40 mg/kg resveratrol and 500 mg.kg-1 metformin | Resveratrol increased heart rates and reversed sinus arrhythmia, atrial and atrioventricular conduction disorders; restored a normal left ventricular ejection fraction, improved stroke volume and cardiac output. Resveratrol activated the AMPK-pathway and reduced both ROS production and heart parasite burden, without interfering with vascularization or myocarditis intensity. Metformin and tempol mimicked the beneficial effects of resveratrol on heart function and decreased lipid peroxidation, but did not alter parasite burden |
| Zhao et al. 2016 [42,44] | High fat diet-fed ICR mice received metformin (200 mg/kg), or resveratrol (50 mg/kg), every day for 10 days | Metformin and resveratrol attenuated adipose hypoxia, inhibited HIF-1α expression and inflammation in the adipose tissue. Metformin and resveratrol inhibited lipolysis. Metformin and resveratrol reduced free fatty acid influx and diacylglycerol accumulation and thus improved insulin signaling in the muscle by inhibiting protein kinase C (PKC)θ translocation |
| Barger et al. 2017 [55] | White adipose tissue, gastrocnemius muscle, heart, and brain neocortex from seven mouse strains (C3H/HeJ, CBA/J, DBA/2J, B6C3F1/J, 129S1/SvImJ, C57BL/6J, and BALB/cJ) treated with trans-resveratrol (510 mg/kg) or metformin (1909 mg/kg) supplemented in diet for 3 months | Metformin and resveratrol were effective as the caloric restriction in reducing plasma interferon-gamma levels |
| Stockinger et al. 2017 [56] | Male C57BL/6J wild-type mice fed 40% less fat calories starting at 4 months of age containing resveratrol at 400 mg/kg or metformin at 1,000 mg/kg starting at 1 year of age. Mice were sacrificed at 2 years of age to examine NMJs and muscle fibers | Resveratrol significantly slowed aging of neuromuscular junctions (NMJs) in the extensor digitorum longus muscle of 2-year-old mice. Resveratrol also preserved the morphology of muscle fibers in old mice. Although metformin slowed the rate of muscle fiber aging, it did not significantly affect aging of NMJs. Resveratrol also increased the number of postsynaptic sites on myotubes exhibiting a youthful architecture, suggesting that resveratrol directly affects the NMJ |
| Mehdi et al. 2018 [26] | C57BL/6 J male mice received 400 mg/kg of resveratrol or metformin 250 mg/kg, via gavage for 45 days | In brown adipose tissue, resveratrol significantly reduced the lipid droplet-associated (PLIN5) protein level and gene expression. In heart tissue, resveratrol, and strength training, decreased the plin5 expression, but metformin increased the gene expression. In skeletal muscle, resveratrol, strength training, cold and metformin significantly increased the plin5 expression at the gene and protein level |
| Rehman et al. 2018 [57] | Alloxan-induced albino rats received metformin (500 mg/kg/day) or resveratrol (30 mg/kg/day) for 30 days | Resveratrol alone and/or in combination with vitamin E exhibited a highly significant therapeutic potential by ameliorating the glycemia-induced modulations. Moreover, resveratrol in combination with vitamin E also exhibited a better therapeutic effects when compared with that of metformin |
| Author, Year | Experimental Model, Dose Used, and Intervention Period | Experimental Outcome and Proposed Mechanism |
|---|---|---|
| Bruckbauer et al. 2013 [58] | C2C12 skeletal myotubes and 3T3-L1 adipocytes treated with resveratrol 0.2 μM, hydroxymethylbutyrate (HMB) 5 μM, and metformin 0.1 mM alone or in combination. Type 2 diabetic (db/db) mice treated for 2 weeks with high (1.5 g/kg diet), low (0.75 g/kg diet), or very low (0.25 g/kg diet) doses of metformin | The combination of metformin-resveratrol-HMB significantly increased fat oxidation, AMP-activated protein kinase (AMPK), and SIRT1 activity in muscle cells compared with metformin or resveratrol-HMB alone. A similar trend was found in 3T3L1 adipocytes In mice, the two lower doses of metformin exerted no independent effect but, when combined with resveratrol-HMB, both low-dose and very low-dose metformin improved insulin sensitivity (HOMA(IR)), plasma insulin levels, and insulin tolerance test response to a level comparable with that found for high-dose metformin. Additionally, the metformin-resveratrol-HMB combination decreased visceral fat and liver weight in mice |
| Fu et al. 2015 [59] | High fat diet-fed (HFD) male C57BL/6 mice received a combination of leucine (24 g/kg diet), resveratrol (12.5 mg/kg/diet) and metformin (0.05–0.5 g/kg diet) for 6 weeks | The combination of leucine, metformin, and resveratrol was more effective than the use of metformin as a monotherapy in improving glucose tolerance |
| Duarte-Vázquez et al. 2016 [22] | Type 2 diabetic (db/db) mice received resveratrol (20 mg/kg/day), metformin (150 mg/kg/day) and combined metformin/resveratrol therapy for 5 weeks | Data clearly showed that combined metformin/resveratrol treatment reduced obesity, glucose, and triglyceride levels, as well as improving renal function and partially improving liver function in diabetic mice |
| Frendo-Cumbo et al. 2016 [23] | Male C57BL6 mice fed HFD were given metformin (231 mg/kg/day), resveratrol (93 mg/kg/day), or combined (metformin 232.01 ± 17.12 mg/kg/day and resveratrol 92 mg/kg/day) treatment groups for 4 weeks | Treatment with each compound alone did not have beneficial effects on glucose tolerance, although metformin significantly improved insulin tolerance. Glucose and insulin tolerance were significantly improved in the combination treatment. This was mirrored by enhanced insulin-stimulated protein kinase B (AKT) phosphorylation in triceps muscle and inguinal subcutaneous adipose tissue. However, improvements with combination treatment did not affect body weight, adiposity, or markers of adipose tissue inflammation |
| Furat et al. 2018 [60] | Dehydroepiandrosterone (DHEA)-induced Wistar rats were given resveratrol (20 mg/kg/day), metformin (300 mg/kg/day) and combined therapy for 28 days | Metformin and combined treatments reduced the body and ovary weights. All the treatment groups decreased luteinizing hormone, follicle-stimulating hormone, tumor necrosis factor (TNF)-α and tissue anti-mullerian hormone(AMH) levels, whereas metformin was unable to improve the increased malondialdehyde (MDA) and plasma AMH levels. Resveratrol and metformin increased SIRT1 and AMPK immunoreactivity |
| Das et al. 2019 [61] | Wistar fed HFD received metformin (0.5 gm/kg), resveratrol (5, 10, and 20 mg/kg), or the combination of a half dose of metformin and resveratrol (10 mg/kg) for 2 weeks | The combination treatment significantly decreased fasting blood glucose. This effect was consistent with significant improvement in dyslipidemia compared to their baseline values. There was also a significant change in the serum MDA level and superoxide dismutase activity |
| Yang et al. 2019 [62] | Male C57BL/6J mice fed HFD treated with/without metformin (250 mg/kg/day) and resveratrol (100 mg/kg/day), or the combination of metformin (250 mg/kg/day and 100 mg/kg/day, respectively) for 5 weeks | Resveratrol alone or when combined to metformin promoted phosphorylation of cortex AMPK and raptor (the regulatory subunit of mTORC1). Furthermore, all treatment reduced p62 content; while activating mTOR and Unc-51 like autophagy activating kinase (ULK1) in the cortex and hippocampus. Brain-derived neurotropic factor (BDNF) was significantly decreased resveratrol or combination treatment |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dludla, P.V.; Silvestri, S.; Orlando, P.; Gabuza, K.B.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Mxinwa, V.; Mokgalaboni, K.; Johnson, R.; Muller, C.J.F.; et al. Exploring the Comparative Efficacy of Metformin and Resveratrol in the Management of Diabetes-Associated Complications: A Systematic Review of Preclinical Studies. Nutrients 2020, 12, 739. https://doi.org/10.3390/nu12030739
Dludla PV, Silvestri S, Orlando P, Gabuza KB, Mazibuko-Mbeje SE, Nyambuya TM, Mxinwa V, Mokgalaboni K, Johnson R, Muller CJF, et al. Exploring the Comparative Efficacy of Metformin and Resveratrol in the Management of Diabetes-Associated Complications: A Systematic Review of Preclinical Studies. Nutrients. 2020; 12(3):739. https://doi.org/10.3390/nu12030739
Chicago/Turabian StyleDludla, Phiwayinkosi V., Sonia Silvestri, Patrick Orlando, Kwazi B. Gabuza, Sithandiwe E. Mazibuko-Mbeje, Tawanda M. Nyambuya, Vuyolwethu Mxinwa, Kabelo Mokgalaboni, Rabia Johnson, Christo J. F. Muller, and et al. 2020. "Exploring the Comparative Efficacy of Metformin and Resveratrol in the Management of Diabetes-Associated Complications: A Systematic Review of Preclinical Studies" Nutrients 12, no. 3: 739. https://doi.org/10.3390/nu12030739
APA StyleDludla, P. V., Silvestri, S., Orlando, P., Gabuza, K. B., Mazibuko-Mbeje, S. E., Nyambuya, T. M., Mxinwa, V., Mokgalaboni, K., Johnson, R., Muller, C. J. F., Tiano, L., Louw, J., & Nkambule, B. B. (2020). Exploring the Comparative Efficacy of Metformin and Resveratrol in the Management of Diabetes-Associated Complications: A Systematic Review of Preclinical Studies. Nutrients, 12(3), 739. https://doi.org/10.3390/nu12030739








