A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models
Abstract
:1. Introduction
2. Zinc Homeostasis and Its Role in Human Health
3. Zinc Absorption
3.1. Intestinal Zinc Transporters
3.2. Enterocyte Zinc Homeostasis and Regulation of Intestinal Zinc Absorption
4. Zinc in Nutrition and Its Intestinal Bioavailability
4.1. Intestinal Zinc Bioavailability
4.2. Dietary Factors Recognized to Influence Zinc Absorption
4.3. Physiological Factors Affecting Zinc Absorption
5. In Vitro Studies on Intestinal Zinc Absorption
5.1. Investigation of Zinc Uptake and Transport Using In Vitro Cellular Intestinal Models
5.2. Buffer Composition of In Vitro Cellular Intestinal Models
5.3. Cellular Composition of In Vitro Cellular Intestinal Models
5.4. Comparison of In Vitro Cellular Intestinal Models with the In Vivo Situation
6. Analytical Approaches to Studying In Vitro Zinc Absorption and Bioavailability
7. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| 3D | three-dimensional |
| λem | emission wavelength |
| λex | excitation wavelength |
| BRET | bioluminescence resonance energy transfer |
| BSA | bovine serum albumin |
| DGE | German Society for Nutrition, ger. Deutsche Gesellschaft für Ernährung |
| DMEM | Dulbecco’s Modified Eagles Medium |
| DMT-1 | divalent metal transporter |
| EDTA | ethylene-diamine-tetra-acetic acid |
| EFSA | European Food Safety Authority |
| EHS | Engelbreth-Holm-Swarm cells |
| FAAS | flame atomic absorption spectrometry |
| FCS | fetal calf serum |
| FLIM | fluorescence lifetime imaging microscopy |
| FRET | Förster resonance energy transfer |
| HD | high density |
| HBSS | Hanks’ Balanced Salt Solution |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HSA | human serum albumin |
| ICP-MS | inductively-coupled plasma mass spectrometry |
| ICP-OES | inductively-coupled plasma optical emission spectrometry |
| IP | inositolphosphate |
| Km | half saturation constant |
| KHB | Krebs-Henseleit buffer |
| LIM | Lin-11, Isl-1, Mec-3 |
| LMW | low molecular weight |
| LSM | laser scanning microscope |
| mRNA | messenger ribonucleic acid |
| MEM | minimum essential medium |
| MT | metallothionein |
| MTF-1 | metal regulatory transcription factor 1 |
| n.a. | not available |
| PBMC | peripheral blood mononuclear cells |
| PBS | phosphate buffered saline |
| PC | polycarbonate |
| PE | polyethylene |
| PES | polyester |
| PET | photo-induced electron transfer |
| qPCR | quantitative real time polymerase chain reaction (PCR) |
| RING | really interesting new gene |
| SLC | solute carrier |
| TEER | transepithelial electrical resistance |
| TJ | tight junction |
| TPEN | N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine |
| WHO | World Health Organization |
| ZIP | Zrt-, Irt-like protein |
| Zn | zinc |
| ZnT | zinc transporter |
References
- Maret, W. Zinc in cellular regulation: The nature and significance of “zinc signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rink, L.; Gabriel, P. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, N.F. Overview of zinc absorption and excretion in the human gastrointestinal tract. J. Nutr. 2000, 130, 1374S–1377S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, D. Intestinal and placental zinc transport pathways. Proc. Nutr. Soc. 2004, 63, 21–29. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. Zinc: An essential but elusive nutrient. Am. J. Clin. Nutr. 2011, 94, 6795–6845. [Google Scholar] [CrossRef] [Green Version]
- Trame, S.; Wessels, I.; Haase, H.; Rink, L. A short 18 items food frequency questionnaire biochemically validated to estimate zinc status in humans. J. Trace Elem. Med. Biol. 2018, 49, 285–295. [Google Scholar] [CrossRef]
- Hunt, J.R.; Beiseigel, J.M.; Johnson, L.K. Adaptation in human zinc absorption as influenced by dietary zinc and bioavailability. Am. J. Clin. Nutr. 2008, 87, 1336–1345. [Google Scholar] [CrossRef] [Green Version]
- Hambidge, K.M.; Miller, L.V.; Westcott, J.E.; Sheng, X.; Krebs, N.F. Zinc bioavailability and homeostasis. Am. J. Clin. Nutr. 2010, 91, 1478S–1483S. [Google Scholar] [CrossRef] [Green Version]
- Dosh, R.H.; Essa, A.; Jordan-Mahy, N.; Sammon, C.; Le Maitre, C.L. Use of hydrogel scaffolds to develop an in vitro 3d culture model of human intestinal epithelium. Acta Biomater. 2017, 62, 128–143. [Google Scholar] [CrossRef]
- Langerholc, T.; Maragkoudakis, P.A.; Wollgast, J.; Gradisnik, L.; Cencic, A. Novel and established intestinal cell line models—An indispensable tool in food science and nutrition. Trends Food Sci. Technol. 2011, 22, S11–S20. [Google Scholar] [CrossRef]
- Maret, W. The metals in the biological periodic system of the elements: Concepts and conjectures. Int. J. Mol. Sci. 2016, 17, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, K.H.; Riddell, L.J.; Nowson, C.A.; Booth, A.O.; Szymlek-Gay, E.A. Iron and zinc nutrition in the economically-developed world: A review. Nutrients 2013, 5, 3184–3211. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2005, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Takeda, T.A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef]
- Matthews, J.M.; Bhati, M.; Lehtomaki, E.; Mansfield, R.E.; Cubeddu, L.; Mackay, J.P. It takes two to tango: The structure and function of lim, ring, phd and mynd domains. Curr. Pharm. Des. 2009, 15, 3681–3696. [Google Scholar] [CrossRef]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef]
- Fukada, T.; Yamasaki, S.; Nishida, K.; Murakami, M.; Hirano, T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J. Biol. Inorg. Chem. 2011, 16, 1123–1134. [Google Scholar] [CrossRef] [Green Version]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
- Jackson, M.J. Physiology of zinc: General aspects. In Zinc in Human Biology; Mills, C.F., Ed.; Springer: London, UK, 1989; pp. 1–14. [Google Scholar]
- Driessen, C.; Hirv, K.; Kirchner, H.; Rink, L. Zinc regulates cytokine induction by superantigens and lipopolysaccharide. Immunology 1995, 84, 272–277. [Google Scholar]
- Wessells, K.R.; Jorgensen, J.M.; Hess, S.Y.; Woodhouse, L.R.; Peerson, J.M.; Brown, K.H. Plasma zinc concentration responds rapidly to the initiation and discontinuation of short-term zinc supplementation in healthy men. J. Nutr. 2010, 140, 2128–2133. [Google Scholar] [CrossRef] [Green Version]
- Hess, S.Y.; Peerson, J.M.; King, J.C.; Brown, K.H. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr. Bull. 2007, 28, S403–S429. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.J.; Bradwell, A.R. Identification of the serum binding proteins for iron, zinc, cadmium, nickel, and calcium. Clin. Chem. 1983, 29, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, J.; Simon, T.-P.; Doemming, S.; Thiele, C.; Marx, G.; Schuerholz, T.; Haase, H. Alterations in zinc binding capacity, free zinc levels and total serum zinc in a porcine model of sepsis. BioMetals 2015, 28, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Bloxam, D.L.; Tan, J.C.; Parkinson, C.E. Non-protein bound zinc concentration in human plasma and amniotic fluid measured by ultrafiltration. Clin. Chim. Acta 1984, 144, 81–93. [Google Scholar] [CrossRef]
- Magneson, G.R.; Puvathingal, J.M.; Ray, W.J., Jr. The concentrations of free mg2+ and free zn2+ in equine blood plasma. J. Biol. Chem. 1987, 262, 11140–11148. [Google Scholar]
- Alker, W.; Schwerdtle, T.; Schomburg, L.; Haase, H. A zinpyr-1-based fluorimetric microassay for free zinc in human serum. Int. J. Mol. Sci. 2019, 20, 4006. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.H.; Wuehler, S.E.; Peerson, J.M. The importance of zinc in human nutrition and estimation of the global prevalence of zinc deficiency. Food Nutr. Bull. 2001, 22, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Gibson, R.S.; King, J.C.; Lowe, N. A review of dietary zinc recommendations. Food Nutr. Bull. 2016, 37, 443–460. [Google Scholar] [CrossRef] [Green Version]
- International Zinc Nutrition Consultative Group; Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lonnerdal, B.; Ruel, M.T.; Sandtrom, B.; Wasantwisut, E.; et al. International zinc nutrition consultative group (izincg) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, S99–S203. [Google Scholar]
- King, J.C.; Shames, D.M.; Lowe, N.M.; Woodhouse, L.R.; Sutherland, B.; Abrams, S.A.; Turnlund, J.R.; Jackson, M.J. Effect of acute zinc depletion on zinc homeostasis and plasma zinc kinetics in men. Am. J. Clin. Nutr. 2001, 74, 116–124. [Google Scholar] [CrossRef]
- Lowe, N.M.; Woodhouse, L.R.; Sutherland, B.; Shames, D.M.; Burri, B.J.; Abrams, S.A.; Turnlund, J.R.; Jackson, M.J.; King, J.C. Kinetic parameters and plasma zinc concentration correlate well with net loss and gain of zinc from men. J. Nutr. 2004, 134, 2178–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, J.R.; Matthys, L.A.; Johnson, L.K. Zinc absorption, mineral balance, and blood lipids in women consuming controlled lactoovovegetarian and omnivorous diets for 8 wk. Am. J. Clin. Nutr. 1998, 67, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.M.; Bacon, J.R.; Aggett, P.J.; Bremner, I. Homeostatic regulation of zinc absorption and endogenous losses in zinc-deprived men. Am. J. Clin. Nutr. 1991, 53, 755–763. [Google Scholar] [CrossRef]
- Johnson, P.E.; Hunt, C.D.; Milne, D.B.; Mullen, L.K. Homeostatic control of zinc metabolism in men: Zinc excretion and balance in men fed diets low in zinc. Am. J. Clin. Nutr. 1993, 57, 557–565. [Google Scholar] [CrossRef]
- Lönnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef]
- Jackson, M.J.; Jones, D.A.; Edwards, R.H.; Swainbank, I.G.; Coleman, M.L. Zinc homeostasis in man: Studies using a new stable isotope-dilution technique. Br. J. Nutr. 1984, 51, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Hoadley, J.E.; Leinart, A.S.; Cousins, R.J. Kinetic analysis of zinc uptake and serosal transfer by vascularly perfused rat intestine. Am. J. Physiol. 1987, 252, G825–G831. [Google Scholar] [CrossRef]
- Ziegler, E.E.; Serfass, R.E.; Nelson, S.E.; Figueroa-Colon, R.; Edwards, B.B.; Houk, R.S.; Thompson, J.J. Effect of low zinc intake on absorption and excretion of zinc by infants studied with 70zn as extrinsic tag. J. Nutr. 1989, 119, 1647–1653. [Google Scholar] [CrossRef]
- Baer, M.T.; King, J.C. Tissue zinc levels and zinc excretion during experimental zinc depletion in young men. Am. J. Clin. Nutr. 1984, 39, 556–570. [Google Scholar] [CrossRef]
- Jackson, M.J.; Jones, D.A.; Edwards, R.H. Tissue zinc levels as an index of body zinc status. Clin. Physiol. 1982, 2, 333–343. [Google Scholar] [CrossRef]
- Alker, W.; Haase, H. Zinc and sepsis. Nutrients 2018, 10, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadley, D.B.K.; Edward, J.M.J.; Ander, E.L.; Michael, J.W.; Scott, D.Y.; Sue, W.; Martin, R. Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci. Rep. 2015, 5, 10974. [Google Scholar]
- Turnlund, J.R.; Durkin, N.; Costa, F.; Margen, S. Stable isotope studies of zinc absorption and retention in young and elderly men. J. Nutr. 1986, 116, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.; Samman, S. Vegetarian diets across the lifecycle: Impact on zinc intake and status. Adv. Food Nutr. Res. 2015, 74, 93–131. [Google Scholar]
- McClain, C.J. Zinc metabolism in malabsorption syndromes. J. Am. Coll. Nutr. 1985, 4, 49–64. [Google Scholar] [CrossRef]
- Valberg, L.S.; Flanagan, P.R.; Kertesz, A.; Bondy, D.C. Zinc absorption in inflammatory bowel disease. Dig. Dis. Sci. 1986, 31, 724–731. [Google Scholar] [CrossRef]
- Solomons, N.W.; Rosenberg, I.H.; Sandstead, H.H.; Vo-Khactu, K.P. Zinc deficiency in crohn’s disease. Digestion 1977, 16, 87–95. [Google Scholar] [CrossRef]
- World Health Organization/Food and Agricultural Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of nutrition for development (bond)-zinc review. J. Nutr. 2016, 146, 858S–885S. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.S.; Miale, A., Jr.; Farid, Z.; Sandstead, H.H.; Schulert, A.R. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypognadism. J. Lab. Clin. Med. 1963, 61, 537–549. [Google Scholar]
- Walker, C.L.; Black, R.E. Zinc for the treatment of diarrhoea: Effect on diarrhoea morbidity, mortality and incidence of future episodes. Int. J. Epidemiol. 2010, 39 (Suppl. 1), i63–i69. [Google Scholar] [CrossRef] [Green Version]
- Carter, J.P.; Grivetti, L.E.; Davis, J.T.; Nasiff, S.; Mansour, A.; Mousa, W.A.; Atta, A.E.; Patwardhan, V.N.; Abdel Moneim, M.; Abdou, I.A.; et al. Growth and sexual development of adolescent egyptian village boys. Effects of zinc, iron, and placebo supplementation. Am. J. Clin. Nutr. 1969, 22, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Beck, F.W.; Bao, B.; Fitzgerald, J.T.; Snell, D.C.; Steinberg, J.D.; Cardozo, L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007, 85, 837–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haase, H.; Overbeck, S.; Rink, L. Zinc supplementation for the treatment or prevention of disease: Current status and future perspectives. Exp. Gerontol. 2008, 43, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Liberatoa, S.C.; Singha, G.; Mulhollanda, K. Zinc supplementation in young children: A review of the literature focusing on diarrhoea prevention and treatment. Clin. Nutr. 2015, 34, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Sandström, B. Dose dependence of zinc and manganese absorption in man. Proc. Nutr. Soc. 1992, 51, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.H.; Prasad, A.S.; Brewer, G.J.; Owyang, C. Zinc absorption in human small intestine. Am. J. Physiol. 1989, 256, G87–G91. [Google Scholar] [CrossRef]
- Davies, N.T. Studies on the absorption of zinc by rat intestine. Br. J. Nutr. 1980, 43, 189–203. [Google Scholar] [CrossRef] [Green Version]
- Seal, C.J.; Heaton, F.W. Chemical factors affecting the intestinal absorption of zinc in vitro and in vivo. Br. J. Nutr. 1983, 50, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Seal, C.J.; Mathers, J.C. Intestinal zinc transfer by everted gut sacs from rats given diets containing different amounts and types of dietary fibre. Br. J. Nutr. 1989, 62, 151–163. [Google Scholar] [CrossRef]
- Antonson, D.L.; Barak, A.J.; Vanderhoof, J.A. Determination of the site of zinc absorption in rat small intestine. J. Nutr. 1979, 109, 142–147. [Google Scholar] [CrossRef]
- Wang, S.C.; Chen, Y.S.; Chen, S.M.; Young, T.K. Possible site of decreased intestinal zinc absorption in chronic uremic rats. Nephron 2001, 89, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Steinhardt, H.J.; Adibi, S.A. Interaction between transport of zinc and other solutes in human intestine. Am. J. Physiol. 1984, 247, G176–G182. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.G. Zinc transport in mammalian cells. Am. J. Physiol. 1996, 270, C401–C410. [Google Scholar] [CrossRef] [PubMed]
- Raffaniello, R.D.; Lee, S.-Y.; Teichberc, S.; Wapnir, R.A. Distinct mechanisms of zinc uptake at the apical and basolateral membranes of caco-2 cells. J. Cell. Physiol. 1992, 152, 356–361. [Google Scholar] [CrossRef]
- Finley, J.W.; Briske-Anderson, M.; Reeves, P.G.; Johnson, L.K. Zinc uptake and transcellular movement by caco-2 cells: Studies with media containing fetal bovine serum. J. Nutr. Biochem. 1995, 6, 137–144. [Google Scholar] [CrossRef]
- Rossi, A.; PoveriniI, R.; Di Lullo, G.; Modesti, A.; Modica, A.; Scarinos, M.L. Heavy metal toxicity following apical and basolateral exposure in the human intestinal cell line caco-2. Toxicol. Vitr. 1996, 10, 27–36. [Google Scholar] [CrossRef]
- Lönnerdal, B. Intestinal absorption of zinc. In Zinc in Human Biology; Mills, C.F., Ed.; Springer: London, UK, 1989; pp. 33–55. [Google Scholar]
- Steel, L.; Cousins, R.J. Kinetics of zinc absorption by luminally and vascularly perfused rat intestine. Am. J. Physiol. 1985, 248, G46–G53. [Google Scholar] [CrossRef]
- Yasuno, T.; Okamoto, H.; Nagai, M.; Kimura, S.; Yamamoto, T.; Nagano, K.; Furubayashi, T.; Yoshikawa, Y.; Yasui, H.; Katsumi, H.; et al. In vitro study on the transport of zinc across intestinal epithelial cells using caco-2 monolayers and isolated rat intestinal membranes. Biol. Pharm. Bull. 2012, 35, 588–593. [Google Scholar] [CrossRef] [Green Version]
- Menard, M.P.; Cousins, R.J. Zinc transport by brush border membrane vesicles from rat intestine. J. Nutr. 1983, 113, 1434–1442. [Google Scholar] [CrossRef]
- Ghishan, F.K.; Sobo, G. Intestinal maturation: In vivo zinc transport. Pediatr. Res. 1983, 17, 148–151. [Google Scholar] [CrossRef]
- Cragg, R.A.; Christie, G.R.; Phillips, S.R.; Russi, R.M.; Kury, S.; Mathers, J.C.; Taylor, P.M.; Ford, D. A novel zinc-regulated human zinc transporter, hztl1, is localized to the enterocyte apical membrane. J. Biol. Chem. 2002, 277, 22789–22797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandström, B. Dietary pattern and zinc supply. In Zinc in Human Biology; Mills, C.F., Ed.; Springer: London, UK, 1989. [Google Scholar]
- Scholmerich, J.; Freudemann, A.; Kottgen, E.; Wietholtz, H.; Steiert, B.; Lohle, E.; Haussinger, D.; Gerok, W. Bioavailability of zinc from zinc-histidine complexes. I. Comparison with zinc sulfate in healthy men. Am. J. Clin. Nutr. 1987, 45, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Sandström, B.; Arvidsson, B.; Cederblad, A.; Björn-Rasmussen, E. Zinc absorption from composite meals. I. The significance of whest extraction rate, zinc, calcium, and protein content in meals based on bread. Am. J. Clin. Nutr. 1980, 33, 739–745. [Google Scholar]
- Wada, L.; Turnlund, J.R.; King, J.C. Zinc utilization in young men fed adequate and low zinc intakes. J. Nutr. 1985, 115, 1345–1354. [Google Scholar] [CrossRef]
- Yasuno, T.; Okamoto, H.; Nagai, M.; Kimura, S.; Yamamoto, T.; Nagano, K.; Furubayashi, T.; Yoshikawa, Y.; Yasui, H.; Katsumi, H.; et al. The disposition and intestinal absorption of zinc in rats. Eur. J. Pharm. Sci. 2011, 44, 410–415. [Google Scholar] [CrossRef]
- Istfan, N.W.; Janghorbani, M.; Young, V.R. Absorption of stable70 zn in healthy young men in relation to zinc intake. Am. J. Clin. Nutr. 1983, 38, 187–194. [Google Scholar] [CrossRef]
- Kury, S.; Dreno, B.; Bezieau, S.; Giraudet, S.; Kharfi, M.; Kamoun, R.; Moisan, J.P. Identification of slc39a4, a gene involved in acrodermatitis enteropathica. Nat. Genet. 2002, 31, 239–240. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, B.; Kuo, Y.M.; Zemansky, J.; Gitschier, J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am. J. Hum. Genet. 2002, 71, 66–73. [Google Scholar] [CrossRef] [Green Version]
- McMahon, R.J.; Cousins, R.J. Regulation of the zinc transporter znt-1 by dietary zinc. Proc. Natl. Acad. Sci. USA 1998, 95, 4841–4846. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Kim, B.E.; Petris, M.J.; Eide, D.J. The mammalian zip5 protein is a zinc transporter that localizes to the basolateral surface of polarized cells. J. Biol. Chem. 2004, 279, 51433–51441. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, G.J.; Aydemir, T.B.; Troche, C.; Martin, A.B.; Chang, S.M.; Cousins, R.J. Influence of zip14 (slc39a14) on intestinal zinc processing and barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G171–G178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cragg, R.A.; Phillips, S.R.; Piper, J.M.; Varma, J.S.; Campbell, F.C.; Mathers, J.C.; Ford, D. Homeostatic regulation of zinc transporters in the human small intestine by dietary zinc supplementation. Gut 2005, 54, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Valentine, R.A.; Jackson, K.A.; Christie, G.R.; Mathers, J.C.; Taylor, P.M.; Ford, D. Znt5 variant b is a bidirectional zinc transporter and mediates zinc uptake in human intestinal caco-2 cells. J. Biol. Chem. 2007, 282, 14389–14393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, K.A.; Helston, R.M.; McKay, J.A.; O'Neill, E.D.; Mathers, J.C.; Ford, D. Splice variants of the human zinc transporter znt5 (slc30a5) are differentially localized and regulated by zinc through transcription and mrna stability. J. Biol. Chem. 2007, 282, 10423–10431. [Google Scholar] [CrossRef] [Green Version]
- Gunshin, H.; Mackenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997, 388, 482–488. [Google Scholar] [CrossRef]
- Tandy, S.; Williams, M.; Leggett, A.; Lopez-Jimenez, M.; Dedes, M.; Ramesh, B.; Srai, S.K.; Sharp, P. Nramp2 expression is associated with ph-dependent iron uptake across the apical membrane of human intestinal caco-2 cells. J. Biol. Chem. 2000, 275, 1023–1029. [Google Scholar] [CrossRef] [Green Version]
- Kordas, K.; Stoltzfus, R.J. New evidence of iron and zinc interplay at the enterocyte and neural tissues. J. Nutr. 2004, 134, 1295–1298. [Google Scholar] [CrossRef]
- Ferguson, C.J.; Wareing, M.; Ward, D.T.; Green, R.; Smith, C.P.; Riccardi, D. Cellular localization of divalent metal transporter dmt-1 in rat kidney. Am. J. Physiol. Ren. Physiol. 2001, 280, F803–F814. [Google Scholar] [CrossRef]
- Garrick, M.D.; Dolan, K.G.; Horbinski, C.; Ghio, A.J.; Higgins, D.; Porubcin, M.; Moore, E.G.; Hainsworth, L.N.; Umbreit, J.N.; Conrad, M.E.; et al. Dmt1: A mammalian transporter for multiple metals. BioMetals 2003, 16, 41–54. [Google Scholar] [CrossRef]
- Yamaji, S.; Tennant, J.; Tandy, S.; Williams, M.; Singh Srai, S.K.; Sharp, P. Zinc regulates the function and expression of the iron transporters dmt1 and ireg1 in human intestinal caco-2 cells. Febs Lett. 2001, 507, 137–141. [Google Scholar] [CrossRef]
- Fukada, T.; Kambe, T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Met. Integr. Biometal Sci. 2011, 3, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.K.; Pfaender, S.; Hagmeyer, S.; Tarana, L.; Mattes, A.K.; Briel, F.; Kury, S.; Boeckers, T.M.; Grabrucker, A.M. Characterization of zinc amino acid complexes for zinc delivery in vitro using caco-2 cells and enterocytes from hipsc. BioMetals 2017, 30, 643–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maret, W. Analyzing free zinc(ii) ion concentrations in cell biology with fluorescent chelating molecules. Met. Integr. Biometal Sci. 2015, 7, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Bozym, R.A.; Chimienti, F.; Giblin, L.J.; Gross, G.W.; Korichneva, I.; Li, Y.; Libert, S.; Maret, W.; Parviz, M.; Frederickson, C.J.; et al. Free zinc ions outside a narrow concentration range are toxic to a variety of cells in vitro. Exp. Biol. Med. 2010, 235, 741–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maret, W. Molecular aspects of human cellular zinc homeostasis: Redox control of zinc potentials and zinc signals. BioMetals 2009, 22, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Colvin, R.A.; Holmes, W.R.; Fontaine, C.P.; Maret, W. Cytosolic zinc buffering and muffling: Their role in intracellular zinc homeostasis. Met. Integr. Biometal Sci. 2010, 2, 306–317. [Google Scholar] [CrossRef]
- Maares, M.; Keil, C.; Thomsen, S.; Gunzel, D.; Wiesner, B.; Haase, H. Characterization of caco-2 cells stably expressing the protein-based zinc probe ecalwy-5 as a model system for investigating intestinal zinc transport. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2018, 49, 296–304. [Google Scholar] [CrossRef]
- Maares, M.; Duman, A.; Keil, C.; Schwerdtle, T.; Haase, H. The impact of apical and basolateral albumin on intestinal zinc resorption in the caco-2/ht-29-mtx co-culture model. Met. Integr. Biometal Sci. 2018, 10, 979–991. [Google Scholar] [CrossRef]
- Maares, M.; Keil, C.; Koza, J.; Straubing, S.; Schwerdtle, T.; Haase, H. In vitro studies on zinc binding and buffering by intestinal mucins. Int. J. Mol. Sci. 2018, 19, 2662. [Google Scholar] [CrossRef] [Green Version]
- Wellenreuther, G.; Cianci, M.; Tucoulou, R.; Meyer-Klaucke, W.; Haase, H. The ligand environment of zinc stored in vesicles. Biochem. Biophys. Res. Commun. 2009, 380, 198–203. [Google Scholar] [CrossRef]
- Maares, M. Investigations on Zinc Resorption Using In Vitro Intestinal Models; Berlin Institute of Technology: Berlin, Germany, 2019. [Google Scholar]
- Maret, W.; Li, Y. Coordination dynamics of zinc in proteins. Chem. Rev. 2009, 109, 4682–4707. [Google Scholar] [CrossRef] [PubMed]
- Krezel, A.; Hao, Q.; Maret, W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch. Biochem. Biophys. 2007, 463, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kambe, T. The functions of metallothionein and zip and znt transporters: An overview and perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapiero, H.; Tew, K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003, 57, 399–411. [Google Scholar] [CrossRef]
- Cousins, R.J.; Liuzzi, J.P.; Lichten, L.A. Mammalian zinc transport, trafficking, and signals. J. Biol. Chem. 2006, 281, 24085–24089. [Google Scholar] [CrossRef] [Green Version]
- Andrews, G.K. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 1999, 59, 95–104. [Google Scholar] [CrossRef]
- Richards, M.P.; Cousins, R.J. Mammalian zinc homeostasis: Requirement for rna and metallothionein synthesis. Biochem. Biophys. Res. Commun. 1975, 64, 1215–1223. [Google Scholar] [CrossRef]
- Menard, M.P.; McCormick, C.C.; Cousins, R.J. Regulation of intestinal metallothionein biosynthesis in rats by dietary zinc. J. Nutr. 1981, 111, 1353–1361. [Google Scholar] [CrossRef]
- Reeves, P.G. Adaptation responses in rats to long-term feeding of high-zinc diets: Emphasis on intestinal metallothionein. J. Nutr. Biochem. 1995, 6, 48–54. [Google Scholar] [CrossRef]
- Smith, K.T.; Cousins, R.J. Quantitative aspects of zinc absorption by isolated, vascularly perfused rat intestine. J. Nutr. 1980, 110, 316–323. [Google Scholar] [CrossRef]
- Hinskens, B.; Philcox, J.C.; Coyle, P.; Rofe, A.M. Increased zinc absorption but not secretion in the small intestine of metallothionein-null mice. Biol. Trace Elem. Res. 2000, 78, 231–240. [Google Scholar] [CrossRef]
- Hoadley, J.E.; Leinart, A.S.; Cousins, R.J. Relationship of 65zn absorption kinetics to intestinal metallothionein in rats: Effects of zinc depletion and fasting. J. Nutr. 1988, 118, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; McMahon, R.J.; Cousins, R.J. Metallothionein knockout and transgenic mice exhibit altered intestinal processing of zinc with uniform zinc-dependent zinc transporter-1 expression. J. Nutr. 1998, 128, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Coppen, D.E.; Davies, N.T. Studies on the effects of dietary zinc dose on 65zn absorption in vivo and on the effects of zn status on 65zn absorption and body loss in young rats. Br. J. Nutr. 1987, 57, 35–44. [Google Scholar] [CrossRef]
- Moltedo, O.; Verde, C.; Capasso, A.; Parisi, E.; Remondelli, P.; Bonatti, S.; Alvarez-Hernandez, X.; Glass, J.; Alvino, C.G.; Leone, A. Zinc transport and metallothionein secretion in the intestinal human cell line caco-2. J. Biol. Chem. 2000, 275, 31819–31825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, E.J.; Quaife, C.J.; Froelick, G.J.; Palmiter, R.D. Metallothionein i and ii protect against zinc deficiency and zinc toxicity in mice. J. Nutr. 1996, 126, 1782–1790. [Google Scholar] [PubMed]
- Colvin, R.A.; Bush, A.I.; Volitakis, I.; Fontaine, C.P.; Thomas, D.; Kikuchi, K.; Holmes, W.R. Insights into zn2+ homeostasis in neurons from experimental and modeling studies. Am. J. Physiol. Cell Physiol. 2008, 294, C726–C742. [Google Scholar] [CrossRef]
- Hempe, J.M.; Cousins, R.J. Cysteine-rich intestinal protein and intestinal metallothionein: An inverse relationship as a conceptual model for zinc absorption in rats. J. Nutr. 1992, 122, 89–95. [Google Scholar] [CrossRef]
- Lanningham-Foster, L.; Green, C.L.; Langkamp-Henken, B.; Davis, B.A.; Nguyen, K.T.; Bender, B.S.; Cousins, R.J. Overexpression of crip in transgenic mice alters cytokine patterns and the immune response. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1197–E1203. [Google Scholar] [CrossRef] [Green Version]
- Nishito, Y.; Kambe, T. Absorption mechanisms of iron, copper, and zinc: An overview. J. Nutr. Sci. Vitaminol. 2018, 64, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, G.K. Regulation and function of zip4, the acrodermatitis enteropathica gene. Biochem. Soc. Trans. 2008, 36, 1242–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufner-Beattie, J.; Wang, F.; Kuo, Y.-M.; Gitschier, J.; Eide, D.; Andrews, G.K. The acrodermatitis enteropathica gene zip4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J. Biol. Chem. 2003, 278, 33474–33481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasana, S.; Din, J.; Maret, W. Genetic causes and gene-nutrient interactions in mammalian zinc deficiencies: Acrodermatitis enteropathica and transient neonatal zinc deficiency as examples. J. Trace Elem. Med. Biol. 2015, 29, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Wang, F.; Dufner-Beattie, J.; Andrews, G.K.; Eide, D.J.; Petris, M.J. Zn2+-stimulated endocytosis of the mzip4 zinc transporter regulates its location at the plasma membrane. J. Biol. Chem. 2004, 279, 4523–4530. [Google Scholar] [CrossRef] [Green Version]
- Weaver, B.P.; Dufner-Beattie, J.; Kambe, T.; Andrews, G.K. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse slc39a4 and slc39a5 zinc transporters (zip4 and zip5). Biol. Chem. 2007, 388, 1301–1312. [Google Scholar] [CrossRef] [Green Version]
- Dufner-Beattie, J.; Kuo, Y.M.; Gitschier, J.; Andrews, G.K. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters zip4 and zip5. J. Biol. Chem. 2004, 279, 49082–49090. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, A.; Nakagawa, M.; Tsujimura, N.; Miyazaki, S.; Kizu, K.; Goto, T.; Komatsu, Y.; Matsunaga, A.; Shirakawa, H.; Narita, H.; et al. Properties of zip4 accumulation during zinc deficiency and its usefulness to evaluate zinc status: A study of the effects of zinc deficiency during lactation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R459–R468. [Google Scholar] [CrossRef]
- Mao, X.; Kim, B.E.; Wang, F.; Eide, D.J.; Petris, M.J. A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hzip4, and protects against zinc cytotoxicity. J. Biol. Chem. 2007, 282, 6992–7000. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.L.; Dufner-Beattie, J.; Andrews, G.K. Expression and regulation of slc39a family zinc transporters in the developing mouse intestine. Dev. Biol. 2006, 295, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Lichten, L.A.; Cousins, R.J. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Bobo, J.A.; Lichten, L.A.; Samuelson, D.A.; Cousins, R.J. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc. Natl. Acad. Sci. USA 2004, 101, 14355–14360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liuzzi, J.P.; Blanchard, R.K.; Cousins, R.J. Differential regulation of zinc transporter 1, 2, and 4 mrna expression by dietary zinc in rats. J. Nutr. 2001, 131, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Nishito, Y.; Kambe, T. Zinc transporter 1 (znt1) expression on the cell surface is elaborately controlled by cellular zinc levels. J. Biol. Chem. 2019, 294, 15686–15697. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.Y.; Hall, A.G.; Philipps, A.F.; Kelleher, S.L.; Lonnerdal, B. Tissue-specific alterations in zinc transporter expression in intestine and liver reflect a threshold for homeostatic compensation during dietary zinc deficiency in weanling rats. J. Nutr. 2009, 139, 835–841. [Google Scholar] [CrossRef] [Green Version]
- Andrews, G.K.; Wang, H.; Dey, S.K.; Palmiter, R.D. Mouse zinc transporter 1 gene provides an essential function during early embryonic development. Genesis 2004, 40, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Kirschke, C.P.; Huang, L. Immunohistochemical analysis of znt1, 4, 5, 6, and 7 in the mouse gastrointestinal tract. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2007, 55, 223–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, K.; Matsuda, K.; Itoh, M.; Kawaguchi, H.; Tomoike, H.; Aoyagi, T.; Nagai, R.; Hori, M.; Nakamura, Y.; Tanaka, T. Osteopenia and male-specific sudden cardiac death in mice lacking a zinc transporter gene, znt5. Hum. Mol. Genet. 2002, 11, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Cole, T.B.; Finley, S.D. Znt-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J. 1996, 15, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.Y.; Philipps, A.F.; Kelleher, S.L.; Lonnerdal, B. Effects of zinc exposure on zinc transporter expression in human intestinal cells of varying maturity. J. Pediatric Gastroenterol. Nutr. 2010, 50, 587–595. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Bobo, J.A.; Cui, L.; McMahon, R.J.; Cousins, R.J. Zinc transporters 1, 2 and 4 are differentially expressed and localized in rats during pregnancy and lactation. J. Nutr. 2003, 133, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Podany, A.B.; Wright, J.; Lamendella, R.; Soybel, D.I.; Kelleher, S.L. Znt2-mediated zinc import into paneth cell granules is necessary for coordinated secretion and paneth cell function in mice. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 369–383. [Google Scholar] [CrossRef] [Green Version]
- Murgia, C.; Vespignani, I.; Cerase, J.; Nobili, F.; Perozzi, G. Cloning, expression, and vesicular localization of zinc transporter dri 27/znt4 in intestinal tissue and cells. Am. J. Physiol. 1999, 277, G1231–G1239. [Google Scholar] [CrossRef] [PubMed]
- Kirschke, C.P.; Huang, L. Znt7, a novel mammalian zinc transporter, accumulates zinc in the golgi apparatus. J. Biol. Chem. 2003, 278, 4096–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Kirschke, C.P.; Gitschier, J. Functional characterization of a novel mammalian zinc transporter, znt6. J. Biol. Chem. 2002, 277, 26389–26395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennigar, S.R.; McClung, J.P. Zinc transport in the mammalian intestine. Compr. Physiol. 2018, 9, 59–74. [Google Scholar] [PubMed]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Lönnerdal, B. Phytic acid–trace element (zn, cu, mn) interactions. Int. J. Food Sci. Technol. 2002, 37, 749–758. [Google Scholar] [CrossRef]
- Wapnir, R.A. Zinc deficiency, malnutrition and the gastrointestinal tract. J. Nutr. 2000, 130, 13885–13925. [Google Scholar] [CrossRef]
- Raffaniello, R.D.; Wapnir, R.A. Zinc uptake by isolated rat enterocytes: Effect of low molecular weight ligands. Proc. Soc. Exp. Biol. Med. 1989, 192, 219–224. [Google Scholar] [CrossRef]
- Hennigar, S.R.; McClung, J.P. Hepcidin attenuates zinc efflux in caco-2 cells. J. Nutr. 2016, 146, 2167–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandström, B.; Sandberg, A.S. Inhibitory effects of isolated inositol phosphates on zinc absorption in humans. J. Trace Elem. Electrolytes Health Dis. 1992, 6, 99–103. [Google Scholar] [PubMed]
- Lönnerdal, B.; Sandberg, A.-S.; Sandström, B.; Kunz, C. Inhibitory effects of phytic acid and other inositol phosphates on zinc and calcium absorption in suckling rats. J. Nutr. 1989, 119, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, A.; Andlid, T. Phytogenic and microbial phytases in human nutrition. Int. J. Food Sci. Technol. 2002, 37, 823–833. [Google Scholar] [CrossRef]
- Nävert, B.; Sandström, B.; Cederblad, A. Reduction of the phytate content of bran by leavening in bread and its effect on zinc absorption in man. Br. J. Nutr. 1985, 53, 47–53. [Google Scholar]
- Kemme, P.A.; Schlemmer, U.; Mroz, Z.; Jongbloed, A.W. Monitoring the stepwise phytate degradation in the upper gastrointestinal tract of pigs. J. Sci. Food Agric. 2006, 86, 612–622. [Google Scholar] [CrossRef]
- Iqbal, T.H.; Lewis, K.O.; Cooper, B.T. Phytase activity in the human and rat small intestine. Gut 1994, 35, 1233–1236. [Google Scholar] [CrossRef] [Green Version]
- Turk, M.; Sandberg, A.S. Phytate degradation during breadmaking: Effect of phytase addition. J. Cereal Sci. 1992, 15, 281–294. [Google Scholar] [CrossRef]
- Vasca, E.; Materazzi, S.; Caruso, T.; Milano, O.; Fontanella, C.; Manfredi, C. Complex formation between phytic acid and divalent metal ions: A solution equilibria and solid state investigation. Anal. Bioanal. Chem. 2002, 374, 173–178. [Google Scholar] [CrossRef]
- Tang, N.; Skibsted, L.H. Zinc bioavailability from phytate-rich foods and zinc supplements. Modeling the effects of food components with oxygen, nitrogen, and sulfur donor ligands. J. Agric. Food Chem. 2017, 65, 8727–8743. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Cederblad, A.; Davidson, L.; Sandström, B. The effect of individual components of soy formula and cows’ milk formula on zinc bioavailability. Am. J. Clin. Nutr. 1984, 40, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Fallingborg, J. Intraluminal ph of the human gastrointestinal tract. Dan. Med. Bull. 1999, 46, 183–196. [Google Scholar] [PubMed]
- Khouzam, R.B.; Pohl, P.; Lobinski, R. Bioaccessibility of essential elements from white cheese, bread, fruit and vegetables. Talanta 2011, 86, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.J.; Wise, A. Binding of zinc and calcium to inositol phosphates (phytate) in vitro. Br. J. Nutr. 1990, 64, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Turnlund, J.R.; King, J.C.; Keyes, W.R.; Gong, B.; Michel, M.C. A stable isotope study of zinc absorption in young men: Effects of phytate and alpha-cellulose. Am. J. Clin. Nutr. 1984, 40, 1071–1077. [Google Scholar] [CrossRef]
- Oberleas, D.; Harland, B.F. Phytate content of foods: Effect on dietary zinc bioavailability. J. Am. Diet. Assoc. 1981, 79, 433–436. [Google Scholar]
- Davies, N.T.; Olpin, S.E. Studies on the phytate: Zinc molar contents in diets as a determinant of zn availability to young rats. Br. J. Nutr. 1979, 41, 590–603. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, M.B.; Hels, O.; Morberg, C.M.; Marving, J.; Bugel, S.; Tetens, I. Total zinc absorption in young women, but not fractional zinc absorption, differs between vegetarian and meat-based diets with equal phytic acid content. Br. J. Nutr. 2006, 95, 963–967. [Google Scholar] [CrossRef]
- Fredlund, K.; Isaksson, M.; Rossander-Hulthén, L.; Almgren, A.; Sandberg, A.-S. Absorption of zinc and retention of calcium: Dose-dependent inhibition by phytate. J. Trace Elem. Med. Biol. 2006, 20, 49–57. [Google Scholar] [CrossRef]
- Hunt, J.R.; Beiseigel, J.M. Dietary calcium does not exacerbate phytate inhibition of zinc absorption by women from conventional diets. Am. J. Clin. Nutr. 2009, 89, 839–843. [Google Scholar] [CrossRef] [Green Version]
- Abebe, Y.; Bogale, A.; Hambidge, K.M.; Stoecker, B.J.; Bailey, K.; Gibson, R.S. Phytate, zinc, iron and calcium content of selected raw and prepared foods consumed in rural sidama, southern ethiopia, and implications for bioavailability. J. Food Compos. Anal. 2007, 20, 161–168. [Google Scholar] [CrossRef]
- Lazarte, C.E.; Carlsson, N.-G.; Almgren, A.; Sandberg, A.-S.; Granfeldt, Y. Phytate, zinc, iron and calcium content of common bolivian food, and implications for mineral bioavailability. J. Food Compos. Anal. 2015, 39, 111–119. [Google Scholar] [CrossRef]
- Magallanes-López, A.M.; Hernandez-Espinosa, N.; Velu, G.; Posadas-Romano, G.; Ordoñez-Villegas, V.M.G.; Crossa, J.; Ammar, K.; Guzmán, C. Variability in iron, zinc and phytic acid content in a worldwide collection of commercial durum wheat cultivars and the effect of reduced irrigation on these traits. Food Chem. 2017, 237, 499–505. [Google Scholar] [CrossRef]
- Umeta, M.; West, C.E.; Fufa, H. Content of zinc, iron, calcium and their absorption inhibitors in foods commonly consumed in ethiopia. J. Food Compos. Anal. 2005, 18, 803–817. [Google Scholar] [CrossRef]
- Ma, G.; Jin, Y.; Piao, J.; Kok, F.; Guusje, B.; Jacobsen, E. Phytate, calcium, iron, and zinc contents and their molar ratios in foods commonly consumed in china. J. Agric. Food Chem. 2005, 53, 10285–10290. [Google Scholar] [CrossRef] [PubMed]
- Sandström, B.; Cederblad, A. Zinc absorption from composite meals. Ii. Influence of the main protein source. Am. J. Clin. Nutr. 1980, 33, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Sandström, B.; Almgren, A.; Kivisto, B.; Cederblad, A. Effect of protein level and protein source on zinc absorption in humans. J. Nutr. 1989, 119, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, L.; Almgren, A.; SandströM, B.; Juillerat, M.E.-A.; Hurrell, R.F. Zinc absorption in adult humans: The effect of protein sources added to liquid test meals. Br. J. Nutr. 1996, 75, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Kiela, P.R.; Ghishan, F.K. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Sandström, B.; Davidsson, L.; Cederblad, A.; Lönnerdal, B. Oral iron, dietary ligands and zinc absorption. J. Nutr. 1985, 115, 411–414. [Google Scholar] [CrossRef]
- Wapnir, R.A.; Stiel, L. Zinc intestinal absorption in rats: Specificity of amino acids as ligands. J. Nutr. 1986, 116, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Hempe, J.M.; Cousins, R.J. Effect of edta and zinc-methionine complex on zinc absorption by rat intestine. J. Nutr. 1989, 119, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Sandstead, H.H.; Smith, J.C., Jr. Deliberations and evaluations of approaches, endpoints and paradigms for determining zinc dietary recommendations. J. Nutr. 1996, 126, 2410s–2418s. [Google Scholar] [CrossRef]
- Davidsson, L.; Kastenmayer, P.; Hurrell, R.F. Sodium iron edta [nafe(iii)edta] as a food fortificant: The effect on the absorption and retention of zinc and calcium in women. Am. J. Clin. Nutr. 1994, 60, 231–237. [Google Scholar] [CrossRef]
- Davidsson, L.; Almgren, A.; Sandström, B.; Hurrell, R.F. Zinc absorption in adult humans: The effect of iron fortification. Br. J. Nutr. 1995, 74, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Turnlund, J.R.; Keyes, W.R.; Hudson, C.A.; Betschart, A.A.; Kretsch, M.J.; Sauberlich, H.E. A stable-isotope study of zinc, copper, and iron absorption and retention by young women fed vitamin b-6-deficient diets. Am. J. Clin. Nutr. 1991, 54, 1059–1064. [Google Scholar] [CrossRef]
- Solomons, N.W.; Jacob, R.A. Studies on the bioavailability of zinc in humans: Effects of heme and nonheme iron on the absorption of zinc. Am. J. Clin. Nutr. 1981, 34, 475–482. [Google Scholar] [CrossRef]
- Sandström, B. Micronutrient interactions: Effects on absorption and bioavailability. Br. J. Nutr. 2001, 85 (Suppl. 2), S181–S185. [Google Scholar]
- August, D.; Janghorbani, M.; Young, V.R. Determination of zinc and copper absorption at three dietary zn-cu ratios by using stable isotope methods in young adult and elderly subjects. Am. J. Clin. Nutr. 1989, 50, 1457–1463. [Google Scholar] [CrossRef]
- Sandstead, H.H. Requirements and toxicity of essential trace elements, illustrated by zinc and copper. Am. J. Clin. Nutr. 1995, 61, 621s–624s. [Google Scholar] [CrossRef]
- Blakeborough, P.; Salter, D.N. The intestinal transport of zinc studied using brush-border-membrane vesicles from the piglet. Br. J. Nutr. 1987, 57, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valberg, L.S.; Flanagan, P.R.; Chamberlain, M.J. Effects of iron, tin, and copper on zinc absorption in humans. Am. J. Clin. Nutr. 1984, 40, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Baier, M.J.; Greger, J.L. Effects of dietary tin on zinc, copper, iron, manganese, and magnesium metabolism of adult males. Am. J. Clin. Nutr. 1982, 35, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Solomons, N.W.; Jacob, R.A.; Pineda, O.; Viteri, F.E. Studies on the bioavailability of zinc in man. Iii. Effects of ascorbic acid on zinc absorption. Am. J. Clin. Nutr. 1979, 32, 2495–2499. [Google Scholar] [CrossRef]
- Nair, K.M.; Brahmam, G.N.; Radhika, M.S.; Dripta, R.C.; Ravinder, P.; Balakrishna, N.; Chen, Z.; Hawthorne, K.M.; Abrams, S.A. Inclusion of guava enhances non-heme iron bioavailability but not fractional zinc absorption from a rice-based meal in adolescents. J. Nutr. 2013, 143, 852–858. [Google Scholar] [CrossRef] [Green Version]
- Wegmuller, R.; Tay, F.; Zeder, C.; Brnic, M.; Hurrell, R.F. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J. Nutr. 2014, 144, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Pabón, M.L.; Lönnerdal, B. Effect of citrate on zinc bioavailability from milk, milk fractions and infant formulas. Nutr. Res. 1993, 13, 103–111. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Stanislowski, A.G.; Hurley, L.S. Isolation of a low molecular weight zinc binding ligand from human milk. J. Inorg. Biochem. 1980, 12, 71–78. [Google Scholar] [CrossRef]
- Sandström, B.; Cederblad, A.; Lonnerdal, B. Zinc absorption from human milk, cow’s milk, and infant formulas. Am. J. Dis. Child. 1983, 137, 726–729. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Furniss, D.E.; Vuichoud, J.; Finot, P.A.; Hurrell, R.F. The effect of maillard reaction products on zinc metabolism in the rat. Br. J. Nutr. 1989, 62, 739–749. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Morrissey, P.A. Metal ion complexation by products of the maillard reaction. Food Chem. 1997, 58, 17–27. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Quarterman, J. Metal absorption and the intestinal mucus layer. Digestion 1987, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Krezel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, M.W.; Thompson, R.P.; Powell, J.J. Regulation of metal absorption in the gastrointestinal tract. Gut 1996, 39, 625–628. [Google Scholar] [CrossRef] [Green Version]
- Powell, J.J.; Jugdaohsingh, R.; Thompson, R.P. The regulation of mineral absorption in the gastrointestinal tract. Proc. Nutr. Soc. 1999, 58, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Glover, C.N.; Hogstrand, C. In vivo characterisation of intestinal zinc uptake in freshwater rainbow trout. J. Exp. Biol. 2002, 205, 141–150. [Google Scholar]
- Leal, J.; Smyth, H.D.C.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Linden, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Rudzki, Z.; Baker, R.J.; Deller, D.J. The iron-binding glycoprotein of human gastric juice. Ii. Nature of the interaction of the glycoprotein with iron. Digestion 1973, 8, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Crowther, R.S.; Marriott, C. Counter-ion binding to mucus glycoproteins. J. Pharm. Pharmacol. 1984, 36, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.E.; Umbreit, J.N.; Moore, E.G. A role for mucin in the absorption of inorganic iron and other metal cations. A study in rats. Gastroenterology 1991, 100, 129–136. [Google Scholar] [CrossRef]
- De Domenico, I.; Ward, D.M.; Kaplan, J. Hepcidin regulation: Ironing out the details. J. Clin. Investig. 2007, 117, 1755–1758. [Google Scholar] [CrossRef] [Green Version]
- Haase, H.; Hebel, S.; Engelhardt, G.; Rink, L. The biochemical effects of extracellular zn(2+) and other metal ions are severely affected by their speciation in cell culture media. Met. Integr. Biometal Sci. 2015, 7, 102–111. [Google Scholar] [CrossRef]
- Bal, W.; Sokołowska, M.; Kurowskaa, E.; Faller, P. Binding of transition metal ions to albumin: Sites, affinities and rates. Biochim. Biophys. Acta 2013, 1830, 5444–5455. [Google Scholar] [CrossRef]
- Meloun, B.; Morávek, L.; Kostka, V. Complete amino acid sequence of human serum albumin. FEBS Lett. 1975, 58, 134–137. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.T.; Failla, M.L.; Cousins, R.J. Identification of albumin as the plasma carrier for zinc absorption by perfused rat intestine. Biochem. J. 1979, 184, 627–633. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, B. Dietary zinc absorption: A play of zips and znts in the gut. Iubmb Life 2010, 62, 176–182. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Song, M.K. The intestinal zinc transport in aged rats. Mech. Ageing Dev. 1987, 41, 189–197. [Google Scholar] [CrossRef]
- Song, M.K.; Mooradian, A.D. Intestinal zinc transport: Influence of streptozotocin-induced diabetes, insulin and arachidonic acid. Life Sci. 1988, 42, 687–694. [Google Scholar] [CrossRef]
- Song, M.K.; Lee, D.B.; Adham, N.F. Influence of prostaglandins on unidirectional zinc fluxes across the small intestine of the rat. Br. J. Nutr. 1988, 59, 417–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.K.; Kim, Y.Y.; Heng, M.C.; Adham, N.F.; Ament, M.E. Prostaglandin interacts with steroid sex hormones in the regulation of intestinal zinc transport. Comp. Biochem. Physiol. Comp. Physiol. 1992, 101, 477–481. [Google Scholar] [CrossRef]
- Bzik, V.A.; Medani, M.; Baird, A.W.; Winter, D.C.; Brayden, D.J. Mechanisms of action of zinc on rat intestinal epithelial electrogenic ion secretion: Insights into its antidiarrhoeal actions. J. Pharm. Pharmacol. 2012, 64, 644–653. [Google Scholar] [CrossRef]
- Carlson, D.; Sehested, J.; Poulsen, H.D. Zinc reduces the electrophysiological responses in vitro to basolateral receptor mediated secretagogues in piglet small intestinal epithelium. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 514–519. [Google Scholar] [CrossRef]
- Carlson, D.; Poulsen, H.D.; Sehested, J. Influence of weaning and effect of post weaning dietary zinc and copper on electrophysiological response to glucose, theophylline and 5-ht in piglet small intestinal mucosa. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 137, 757–765. [Google Scholar] [CrossRef]
- Tacnet, F.; Watkins, D.W.; Ripoche, P. Studies of zinc transport into brush-border membrane-vesicles isolated from pig small-intestine. Biochim. Biophys. Acta 1990, 1024, 323–330. [Google Scholar] [CrossRef]
- Tacnet, F.; Watkins, D.W.; Ripoche, P. Zinc binding in intestinal brush-border membrane isolated from pig. Biochim. Biophys. Acta 1991, 1063, 51–59. [Google Scholar] [CrossRef]
- Smith, K.T.; cousins, R.J.; Silbon, B.L.; Failla, M.L. Zinc absorption and metabolism by isolated, vascularly perfused rat intestine. J. Nutr. 1978, 108, 1849–1857. [Google Scholar] [CrossRef]
- Russel, M.W.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Flecknell, P. Replacement, reduction and refinement. Altex 2002, 19, 73–78. [Google Scholar]
- Westerhout, J.; Wortelboer, H.; Verhoeckx, K. Ussing chamber. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 263–273. [Google Scholar]
- Fleet, J.C.; Turnbull, A.J.; Bourcier, M.; Wood, R.J. Vitamine d-sensitive and quinacrine-sensitive zinc transport in human intestinal cell line caco-2. Am. J. Physiol. 1993, 264, G1037–G1045. [Google Scholar] [PubMed]
- Reeves, P.G.; Briske-Anderson, M.; Johnson, L. Pre-treatment of caco-2 cells with zinc during the differentiation phase alters the kinetics of zinc uptake and transport. J. Nutr. Biochem. 2001, 12, 674–684. [Google Scholar] [CrossRef]
- Huang, C.; Cui, X.; Sun, X.; Yang, J.; Li, M. Zinc transporters are differentially expressed in human non-small cell lung cancer. Oncotarget 2016, 7, 66935–66943. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhuo, Z.; Fang, S.; Yue, M.; Feng, J. Different zinc sources have diverse impacts on gene expression of zinc absorption related transporters in intestinal porcine epithelial cells. Biol. Trace Elem. Res. 2016, 173, 325–332. [Google Scholar] [CrossRef]
- Gefeller, E.M.; Bondzio, A.; Aschenbach, J.R.; Martens, H.; Einspanier, R.; Scharfen, F.; Zentek, J.; Pieper, R.; Lodemann, U. Regulation of intracellular zn homeostasis in two intestinal epithelial cell models at various maturation time points. J. Physiol. Sci. 2015, 65, 317–328. [Google Scholar] [CrossRef]
- Jou, M.Y.; Du, X.; Hotz, C.; Lonnerdal, B. Biofortification of rice with zinc: Assessment of the relative bioavailability of zinc in a caco-2 cell model and suckling rat pups. J. Agric. Food Chem. 2012, 60, 3650–3657. [Google Scholar] [CrossRef]
- Shah, P.; Jogani, V.; Bagchi, T.; Misra, A. Role of caco-2 cell monolayers in prediction of intestinal drug absorption. Biotechnol. Prog. 2006, 22, 186–198. [Google Scholar] [CrossRef]
- Laparra, J.M.; Barbera, R.; Alegria, A.; Glahn, R.P.; Miller, D.D. Purified glycosaminoglycans from cooked haddock may enhance fe uptake via endocytosis in a caco-2 cell culture model. J. Food Sci. 2009, 74, H168–H173. [Google Scholar] [CrossRef]
- Reeves, P.G.; Briske-Anderson, M.; Johnson, L. Physiologic concentrations of zinc affect the kinetics of copper uptake and transport in the human intestinal cell model, caco-2. J. Nutr. 1998, 1794–1801. [Google Scholar] [CrossRef]
- Jovani, M.; Barbera, R.; Farre, R.; Aguilera, E.M.D. Calcium, iron, and zinc uptake from digests of infant formulas by caco-2 cells. J. Agric. Food Chem. 2001, 49, 3480–3485. [Google Scholar] [CrossRef]
- Corti, G.; Maestrelli, F.; Cirri, M.; Zerrouk, N.; Mura, P. Development and evaluation of an in vitro method for prediction of human drug absorption ii. Demonstration of the method suitability. Eur. J. Pharm. Sci. 2006, 27, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Delie, F.; Werner, R. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: Advantages and limitations of the caco-2 model. Crit. Rev. Ther. Drug Carr. Syst. 1997, 14, 221–286. [Google Scholar] [CrossRef]
- Pinto, M.; Robineleon, S.; Appay, M.D.; Kedinger, M.; Triadou, N.; Dussaulx, E.; Lacroix, B.; Simonassmann, P.; Haffen, K.; Fogh, J.; et al. Enterocyte-like differentiation and polarization of the human colon carcinoma cell-line caco-2 in culture. Biol. Cell 1983, 47, 323–330. [Google Scholar]
- Sambuy, Y.; de Angelis, M.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The caco-2 cell line as a model for the intestinal barrier: Influence of cell and culture-related factors on caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Hayeshi, R.; Hilgendorf, C.; Artursson, P.; Augustijns, P.; Brodin, B.; Dehertogh, P.; Fisher, K.; Fossati, L.; Hovenkamp, E.; Korjamo, T.; et al. Comparison of drug transporter gene expression and functionality in caco-2 cells from 10 different laboratories. Eur. J. Pharm. Sci. 2008, 35, 383–396. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Ebmeyer, J.; Knappe, P.; Juling, S.; Bohmert, L.; Selve, S.; Niemann, B.; Braeuning, A.; Thunemann, A.F.; Lampen, A. Impact of food components during in vitro digestion of silver nanoparticles on cellular uptake and cytotoxicity in intestinal cells. Biol. Chem. 2015, 396, 1255–1264. [Google Scholar] [CrossRef]
- Mahler, G.J.; Shuler, M.L.; Glahn, R.P. Characterization of caco-2 and ht29-mtx cocultures in an in vitro digestion/cell culture model used to predict iron bioavailability. J. Nutr. Biochem. 2009, 20, 494–502. [Google Scholar] [CrossRef]
- Welcome, M.O. Gastrointestinal Physiology—Development, Principles and Mechanisms of Regulation; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Lozoya-Agullo, I.; Araújo, F.; González-Álvarez, I.; Merino-Sanjuán, M.; González-Álvarez, M.; Bermejo, M.; Sarmento, B. Usefulness of caco-2/ht29-mtx and caco-2/ht29-mtx/raji b coculture models to predict intestinal and colonic permeability compared to caco-2 monoculture. Mol. Pharm. 2017, 14, 1264–1270. [Google Scholar] [CrossRef]
- Van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, L.; Du, F.; Chen, T.; Hou, H.; Li, B. The chelating peptide (gpagphgppg) derived from alaska pollock skin enhances calcium, zinc and iron transport in caco-2 cells. Int. J. Food Sci. Technol. 2017, 52, 1283–1290. [Google Scholar] [CrossRef]
- Sreenivasulu, K.; Raghu, P.; Nair, K.M. Polyphenol-rich beverages enhance zinc uptake and metallothionein expression in caco-2 cells. J. Food Sci. 2010, 75, H123–H128. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, K.; Raghu, P.; Ravinder, P.; Nair, K.M. Effect of dietary ligands and food matrices on zinc uptake in caco-2 cells: Implications in assessing zinc bioavailability. J. Agric. Food Chem. 2008, 56, 10967–10972. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Tako, E.; Yeung, A.; Welch, R.M.; Glahn, R.P. Evaluation of metallothionein formation as a proxy for zinc absorption in an in vitro digestion/caco-2 cell culture model. Food Funct. 2012, 3, 732–736. [Google Scholar] [CrossRef] [PubMed]
- García-Nebot, M.J.; Alegría, A.; Barberá, R.; Clemente, G.; Romero, F. Does the addition of caseinophosphopeptides or milk improve zinc in vitro bioavailability in fruit beverages? Food Res. Int. 2009, 42, 1475–1482. [Google Scholar] [CrossRef]
- Cámara, F.; Barberá, R.; Amaro, M.A.; Farré, R. Calcium, iron, zinc and copper transport and uptake by caco-2 cells in school meals: Influence of protein and mineral interactions. Food Chem. 2007, 100, 1085–1092. [Google Scholar] [CrossRef]
- Iyengar, V.; Pullakhandam, R.; Nair, K.M. Dietary ligands as determinants of iron-zinc interactions at the absorptive enterocyte. J. Food Sci. 2010, 75, H260–H264. [Google Scholar] [CrossRef]
- Tupe, R.S.; Agte, V.V. Effect of water soluble vitamins on zn transport of caco-2 cells and their implications under oxidative stress conditions. Eur. J. Nutr. 2009, 49, 53–61. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Pai, T.-K.; Han, O. Effect of bioactive dietary polyphenols on zinc transport across the intestinal caco-2 cell monolayers. J. Agric. Food Chem. 2011, 59, 3606–3612. [Google Scholar] [CrossRef] [Green Version]
- Viadel, B.; Barberá, R.; Farré, R. Uptake and retention of calcium, iron, and zinc from raw legumes and the effect of cooking on lentils in caco-2 cells. Nutr. Res. 2006, 26, 591–596. [Google Scholar] [CrossRef]
- Salunke, R.; Rawat, N.; Tiwari, V.K.; Neelam, K.; Randhawa, G.S.; Dhaliwal, H.S.; Roy, P. Determination of bioavailable-zinc from biofortified wheat using a coupled in vitro digestion/caco-2 reporter-gene based assay. J. Food Compos. Anal. 2012, 25, 149–159. [Google Scholar] [CrossRef]
- Kruger, J.; Taylor, J.R.N.; Du, X.; De Moura, F.F.; Lönnerdal, B.; Oelofse, A. Effect of phytate reduction of sorghum, through genetic modification, on iron and zinc availability as assessed by an in vitro dialysability bioaccessibility assay, caco-2 cell uptake assay, and suckling rat pup absorption model. Food Chem. 2013, 141, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Han, O.; Failla, M.L.; Hill, A.D.; Morris, E.R.; Smith, J.C., Jr. Inositol phosphates inhibit uptake and transport of iron and zinc by a human intestinal cell line. J. Nutr. 1994, 124, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Frontela, C. Effect of dephytinization on bioavailability of iron, calcium and zinc from infant cereals assessed in the caco-2 cell model. World J. Gastroenterol. 2009, 15, 1977. [Google Scholar] [CrossRef] [PubMed]
- Frontela, C.; Ros, G.; Martínez, C. Phytic acid content and “in vitro” iron, calcium and zinc bioavailability in bakery products: The effect of processing. J. Cereal Sci. 2011, 54, 173–179. [Google Scholar] [CrossRef]
- Zemann, N.; Zemann, A.; Klein, P.; Elmadfa, I.; Huettinger, M. Differentiation- and polarization-dependent zinc tolerance in caco-2 cells. Eur. J. Nutr. 2011, 50, 379–386. [Google Scholar] [CrossRef]
- Jackson, K.A.; Valentine, R.A.; McKay, J.A.; Swan, D.C.; Mathers, J.C.; Ford, D. Analysis of differential gene-regulatory responses to zinc in human intestinal and placental cell lines. Br. J. Nutr. 2009, 101, 1474–1483. [Google Scholar] [CrossRef] [Green Version]
- Lodemann, U.; Gefeller, E.M.; Aschenbach, J.R.; Martens, H.; Einspanier, R.; Bondzio, A. Dose effects of apical versus basolateral zinc supplementation on epithelial resistance, viability, and metallothionein expression in two intestinal epithelial cell lines. J. Biochem. Mol. Toxicol. 2015, 29, 410–417. [Google Scholar] [CrossRef]
- Shen, H.; Qin, H.; Guo, J. Cooperation of metallothionein and zinc transporters for regulating zinc homeostasis in human intestinal caco-2 cells. Nutr. Res. 2008, 28, 406–413. [Google Scholar] [CrossRef]
- Wang, X.; Valenzano, M.C.; Mercado, J.M.; Zurbach, E.P.; Mullin, J.M. Zinc supplementation modifies tight junctions and alters barrier function of caco-2 human intestinal epithelial layers. Dig. Dis. Sci. 2012, 58, 77–87. [Google Scholar] [CrossRef]
- Pieri, M.; Christian, H.C.; Wilkins, R.J.; Boyd, C.A.; Meredith, D. The apical (hpept1) and basolateral peptide transport systems of caco-2 cells are regulated by amp-activated protein kinase. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G136–G143. [Google Scholar] [CrossRef] [Green Version]
- Hardyman, J.E.; Tyson, J.; Jackson, K.A.; Aldridge, C.; Cockell, S.J.; Wakeling, L.A.; Valentine, R.A.; Ford, D. Zinc sensing by metal-responsive transcription factor 1 (mtf1) controls metallothionein and znt1 expression to buffer the sensitivity of the transcriptome response to zinc. Met. Integr. Biometal Sci. 2016, 8, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Michalczyk, A.A.; Ackland, M.L. Hzip1 (hslc39a1) regulates zinc homoeostasis in gut epithelial cells. Genes Nutr. 2013, 8, 475–486. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Hu, Q.; Fang, S.; Feng, J. Dosage effect of zinc glycine chelate on zinc metabolism and gene expression of zinc transporter in intestinal segments on rat. Biol. Trace Elem. Res. 2016, 171, 363–370. [Google Scholar] [CrossRef]
- Lodemann, U.; Einspanier, R.; Scharfen, F.; Martens, H.; Bondzio, A. Effects of zinc on epithelial barrier properties and viability in a human and a porcine intestinal cell culture model. Toxicol. Vitr. Int. J. Publ. Assoc. Bibra 2013, 27, 834–843. [Google Scholar] [CrossRef]
- Martin, L.; Lodemann, U.; Bondzio, A.; Gefeller, E.M.; Vahjen, W.; Aschenbach, J.R.; Zentek, J.; Pieper, R. A high amount of dietary zinc changes the expression of zinc transporters and metallothionein in jejunal epithelial cells in vitro and in vivo but does not prevent zinc accumulation in jejunal tissue of piglets. J. Nutr. 2013, 143, 1205–1210. [Google Scholar] [CrossRef] [Green Version]
- Zakrzewski, S.S.; Richter, J.F.; Krug, S.M.; Jebautzke, B.; Lee, I.-F.M.; Rieger, J.; Sachtleben, M.; Bondzio, A.; Schulzke, J.D.; Fromm, M.; et al. Improved cell line ipec-j2, characterized as a model for porcine jejunal epithelium. PLoS ONE 2013, 8, e79643. [Google Scholar] [CrossRef] [Green Version]
- Nossol, C.; Barta-Boszormenyi, A.; Kahlert, S.; Zuschratter, W.; Faber-Zuschratter, H.; Reinhardt, N.; Ponsuksili, S.; Wimmers, K.; Diesing, A.K.; Rothkotter, H.J. Comparing two intestinal porcine epithelial cell lines (ipecs): Morphological differentiation, function and metabolism. PLoS ONE 2015, 10, e0132323. [Google Scholar] [CrossRef] [Green Version]
- Vergauwen, H. The ipec-j2 cell line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 125–134. [Google Scholar]
- Ollig, J.; Kloubert, V.; Weßels, I.; Haase, H.; Rink, L. Parameters influencing zinc in experimental systems in vivo and in vitro. Metals 2016, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Kenneth, V.H.; John, A.S.; Walter, C. Fetal bovine serum: A multivariate standard. Proc. Soc. Exp. Biol. Med. 1975, 149, 344–347. [Google Scholar]
- Zheng, X.; Baker, H.; Hancock, W.S.; Fawaz, F.; McCaman, M.; Pungor, E. Proteomic analysis for the assessment of different lots of fetal bovine serum as a raw material for cell culture. Part iv. Application of proteomics to the manufacture of biological drugs. Biotechnol. Prog. 2006, 22, 1294–1300. [Google Scholar] [CrossRef]
- Gstraunthaler, G. Alternatives to the use of fetal bovine serum: Serum-free cell culture. Altex 2003, 20, 275–281. [Google Scholar] [PubMed]
- Pattison, S.E.; Cousins, R.J. Kinetics of zinc uptake and exchange by primary cultures of rat hepatocytes. Am. J. Physiol. Endocrinol. Metab. 1986, 250, E677–E685. [Google Scholar] [CrossRef] [PubMed]
- Galvez, M.; Moreno, J.A.; Elosegui, L.M.; Escanero, J.F. Zinc uptake by human erythrocytes with and without serum albumins in the medium. Biol. Trace Elem. Res. 2001, 84, 45–56. [Google Scholar] [CrossRef]
- Messer, H.H.; Murray, E.J.; Goebel, N.K. Removal of trace metals from culture media and sera for in vitro deficiency studies. J. Nutr. 1982, 112, 652–657. [Google Scholar] [CrossRef]
- Finamore, A.; Massimi, M.; Conti Devirgiliis, L.; Mengheri, E. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in caco-2 cells. J. Nutr. 2008, 138, 1664–1670. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.E.; Sjovall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Jin, F.; Welch, R.; Glahn, R. Moving toward a more physiological model: Application of mucin to refine the in vitro digestion/caco-2 cell culture system. J. Agric. Food Chem. 2006, 54, 8962–8967. [Google Scholar] [CrossRef]
- Schomig, V.J.; Kasdorf, B.T.; Scholz, C.; Bidmon, K.; Lieleg, O.; Berensmeier, S. An optimized purification process for porcine gastric mucin with preservation of its native functional properties. Rsc Adv. 2016, 6, 44932–44943. [Google Scholar] [CrossRef] [Green Version]
- Svensson, O.; Arnebrant, T. Mucin layers and multilayers—Physicochemical properties and applications. Curr. Opin. Colloid Interface Sci. 2010, 15, 395–405. [Google Scholar] [CrossRef]
- Hilgendorf, C.; Spahn-Langguth, H.; Regardh, C.G.; Lipka, E.; Amidon, G.L.; Langguth, P. Caco-2 versus caco-2/ht29-mtx co-cultured cell lines: Permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J. Pharm. Sci. 2000, 89, 63–75. [Google Scholar] [CrossRef]
- Beduneau, A.; Tempesta, C.; Fimbel, S.; Pellequer, Y.; Jannin, V.; Demarne, F.; Lamprecht, A. A tunable caco-2/ht29-mtx co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure. Eur. J. Pharm. Biopharm. 2014, 87, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Araujo, F.; Sarmento, B. Towards the characterization of an in vitro triple co-culture intestine cell model for permeability studies. Int. J. Pharm. 2013, 458, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.; Janich, S.; Roessler, B.J.; Hilfiger, J.M.; Amidon, G.L. Ht29-mtx/caco-2 cocultures as an in vitro model for the intestinal epithelium: In vitro−in vivo correlation with permeability data from rats and humans. J. Pharm. Sci. 1996, 85, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Pontier, C.; Pachot, J.; Botham, R.; Lenfant, B.; Arnaud, P. Ht29-mtx and caco-2/tc7 monolayers as predictive models for human intestinal absorption: Role of the mucus layer. J. Pharm. Sci. 2001, 90, 1608–1619. [Google Scholar] [CrossRef]
- Ferraretto, A.; Bottani, M.; De Luca, P.; Cornaghi, L.; Arnaboldi, F.; Maggioni, M.; Fiorilli, A.; Donetti, E. Morphofunctional properties of a differentiated caco2/ht-29 co-culture as an in vitro model of human intestinal epithelium. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Calatayud, M.; Vazquez, M.; Devesa, V.; Velez, D. In vitro study of intestinal transport of inorganic and methylated arsenic species by caco-2/ht29-mtx cocultures. Chem. Res. Toxicol. 2012, 25, 2654–2662. [Google Scholar] [CrossRef]
- Vazquez, M.; Calatayud, M.; Velez, D.; Devesa, V. Intestinal transport of methylmercury and inorganic mercury in various models of caco-2 and ht-29-mtx cells. Toxicology 2013, 311, 147–153. [Google Scholar] [CrossRef]
- Laparra, J.M.; Glahn, R.P.; Miller, D.D. Different responses of fe transporters in caco-2/ht29-mtx cocultures than in independent caco-2 cell cultures. Cell Biol. Int. 2009, 33, 971–977. [Google Scholar] [CrossRef]
- Moreno-Olivas, F.; Tako, E.; Mahler, G.J. Zno nanoparticles affect nutrient transport in an in vitro model of the small intestine. Food Chem. Toxicol. 2018, 124, 112–117. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Comparison of in vitro models to study bacterial adhesion to the intestinal epithelium. Lett. Appl. Microbiol. 2009, 49, 695–701. [Google Scholar] [CrossRef] [Green Version]
- Ponce de Leon-Rodriguez, M.D.C.; Guyot, J.P.; Laurent-Babot, C. Intestinal in vitro cell culture models and their potential to study the effect of food components on intestinal inflammation. Crit. Rev. Food Sci. Nutr. 2018, 59, 3648–3666. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; Gimeno-Alcaniz, J.V.; Devesa, V.; Velez, D. Proinflammatory effect of trivalent arsenical species in a co-culture of caco-2 cells and peripheral blood mononuclear cells. Arch. Toxicol. 2015, 89, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.; Gagnon, M.; Chassard, C.; Zimmermann, M.B.; O'Mahony, L.; Lacroix, C. Salmonella adhesion, invasion and cellular immune responses are differentially affected by iron concentrations in a combined in vitro gut fermentation-cell model. PLoS ONE 2014, 9, e93549. [Google Scholar] [CrossRef]
- Oestreicher, P.; Cousins, R.J. Zinc uptake by basolateral membrane vesicles from rat small intestine. J. Nutr. 1989, 119, 639–646. [Google Scholar] [CrossRef]
- Helander, H.F.; Fandriks, L. Surface area of the digestive tract—Revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef]
- Krebs, N.F.; Hambidge, K.M. Zinc metabolism and homeostasis: The application of tracer techniques. BioMetals 2001, 14, 397–412. [Google Scholar] [CrossRef]
- Wastney, M.E.; Aamodt, R.L.; Rumble, W.F.; Henkin, R.I. Kinetic analysis of zinc metabolism and its regulation in normal humans. Am. J. Physiol. 1986, 251, R398–R408. [Google Scholar] [CrossRef]
- Sandström, B.; Lönnerdal, B. Promoters and antagonists of zinc absorption. In Zinc in Human Biology; Mills, C.F., Ed.; Springer: London, UK, 1989; pp. 57–78. [Google Scholar]
- Tran, C.D.; Gopalsamy, G.L.; Mortimer, E.K.; Young, G.P. The potential for zinc stable isotope techniques and modelling to determine optimal zinc supplementation. Nutrients 2015, 7, 4271–4295. [Google Scholar] [CrossRef]
- Cerchiaro, G.; Manieri, T.M.; Bertuchi, F.R. Analytical methods for copper, zinc and iron quantification in mammalian cells. Met. Integr. Biometal Sci. 2013, 5, 1336–1345. [Google Scholar] [CrossRef]
- Richardson, C.E.R.; Nolan, E.M.; Shoulders, M.D.; Lippard, S.J. A sensitive, nonradioactive assay for zn(ii) uptake into metazoan cells. Biochemistry 2018, 57, 6807–6815. [Google Scholar] [CrossRef] [Green Version]
- Chabosseau, P.; Woodier, J.; Cheung, R.; Rutter, G.A. Sensors for measuring subcellular zinc pools. Met. Integr. Biometal Sci. 2018, 10, 229–239. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Ackerman, C.M.; Lee, S.; Chang, C.J. Analytical methods for imaging metals in biology: From transition metal metabolism to transition metal signaling. Anal. Chem. 2017, 89, 22–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hessels, A.M.; Chabosseau, P.; Bakker, M.H.; Engelen, W.; Rutter, G.A.; Taylor, K.M.; Merkx, M. Ezinch-2: A versatile, genetically encoded fret sensor for cytosolic and intraorganelle zn(2+) imaging. Acs Chem. Biol. 2015, 10, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Vinkenborg, J.L.; Nicolson, T.J.; Bellomo, E.A.; Koay, M.S.; Rutter, G.A.; Merkx, M. Genetically encoded fret sensors to monitor intracellular zn2+ homeostasis. Nat. Methods 2009, 6, 737–740. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Miranda, J.G.; Stoddard, C.I.; Dean, K.M.; Galati, D.F.; Palmer, A.E. Direct comparison of a genetically encoded sensor and small molecule indicator: Implications for quantification of cytosolic zn2+. ACS Chem. Biol. 2013, 8, 2366–2371. [Google Scholar] [CrossRef] [Green Version]
- Park, J.G.; Qin, Y.; Galati, D.F.; Palmer, A.E. New sensors for quantitative measurement of mitochondrial zn2+. ACS Chem. Biol. 2012, 7, 1636–1640. [Google Scholar] [CrossRef] [Green Version]
- Aper, S.J.; Dierickx, P.; Merkx, M. Dual readout bret/fret sensors for measuring intracellular zinc. ACS Chem. Biol. 2016, 11, 2854–2864. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Sammond, D.W.; Braselmann, E.; Carpenter, M.C.; Palmer, A.E. Development of an optical zn(2+) probe based on a single fluorescent protein. ACS Chem. Biol. 2016, 11, 2744–2751. [Google Scholar] [CrossRef] [Green Version]
- Wallrabe, H.; Periasamy, A. Imaging protein molecules using fret and flim microscopy. Curr. Opin. Biotechnol. 2005, 16, 19–27. [Google Scholar] [CrossRef]
- Boute, N.; Jockers, R.; Issad, T. The use of resonance energy transfer in high-throughput screening: Bret versus fret. Trends Pharmacol. Sci. 2002, 23, 351–354. [Google Scholar] [CrossRef]
- Pfleger, K.D.G.; Seeber, R.M.; Eidne, K.A. Bioluminescence resonance energy transfer (bret) for the real-time detection of protein-protein interactions. Nat. Protoc. 2006, 1, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, P.J.; Miranda, J.G.; Gorski, J.A.; Palmer, A.E. Genetically encoded sensors to elucidate spatial distribution of cellular zinc. J. Biol. Chem. 2009, 284, 16289–16297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabosseau, P.; Tuncay, E.; Meur, G.; Bellomo, E.A.; Hessels, A.; Hughes, S.; Johnson, P.R.; Bugliani, M.; Marchetti, P.; Turan, B.; et al. Mitochondrial and er-targeted ecalwy probes reveal high levels of free zn2+. ACS Chem. Biol. 2014, 9, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Dittmer, P.J.; Park, J.G.; Jansen, K.B.; Palmer, A.E. Measuring steady-state and dynamic endoplasmic reticulum and golgi zn(2+) with genetically encoded sensors. Proc. Natl. Acad. Sci. USA 2011, 108, 7351–7356. [Google Scholar] [CrossRef] [Green Version]
- Pancholi, J.; Hodson, D.J.; Jobe, K.; Rutter, G.A.; Goldup, S.M.; Watkinson, M. Biologically targeted probes for zn2+: A diversity oriented modular “click-snar-click” approach. Chem. Sci. 2014, 5, 3528–3535. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Chen, S.; Bellomo, E.A.; Tarasov, A.I.; Kaut, C.; Rutter, G.A.; Li, W.-H. Imaging dynamic insulin release using a fluorescent zinc indicator for monitoring induced exocytotic release (zimir). Proc. Natl. Acad. Sci. USA 2011, 108, 21063–21068. [Google Scholar] [CrossRef] [Green Version]
- Radford, R.J.; Chyan, W.; Lippard, S.J. Peptide-based targeting of fluorescent zinc sensors to the plasma membrane of live cells. Chem. Sci. 2013, 4, 3080–3084. [Google Scholar] [CrossRef] [Green Version]
- Ranaldi, G.; Ferruzza, S.; Canali, R.; Leoni, G.; Zalewski, P.D.; Sambuy, Y.; Perozzi, G.; Murgia, C. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine tnf-alpha. J. Nutr. Biochem. 2013, 24, 967–976. [Google Scholar] [CrossRef]
- Krezel, A.; Maret, W. Zinc-buffering capacity of a eukaryotic cell at physiological pzn. J. Biol. Inorg. Chem. 2006, 11, 1049–1062. [Google Scholar] [CrossRef]
- Kindermann, B.; Döring, F.; Fuchs, D.; Pfaffl, M.W.; Daniel, H. Effects of increased cellular zinc levels on gene and protein expression in ht-29 cells. BioMetals 2005, 18, 243–253. [Google Scholar] [CrossRef] [PubMed]




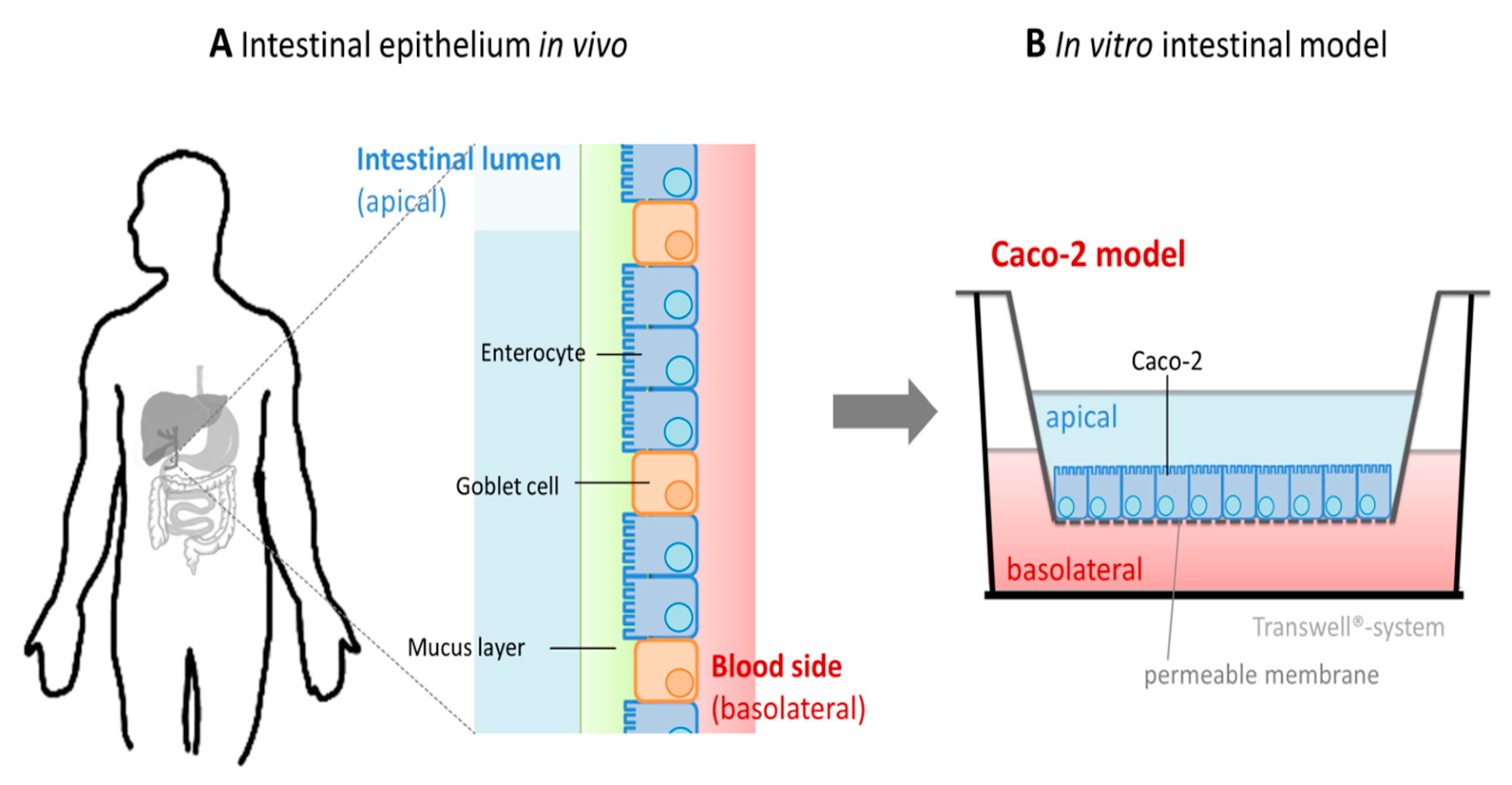

| Food Group | Food | Zinc Content (mg/100g) | Phytate Content (mg/100g) | Phytate: Zinc Molar Ratio | Reference |
|---|---|---|---|---|---|
| Seeds and nuts | Sesame seeds | 2.48 | 1525 | 60.9 | [176] |
| Beans and lentils | Lentils | 3.03–4.02 | 747–961 | 18.5–27.8 | [177] |
| Whole grain cereals | Durum wheat | 2.4–4.8 | 460–952 | 16.9–23.6 | [178] |
| Vegetables | Sweet potato (boiled) | 0.30 | 31–37 | 12.3–15.2 | [179] |
| Fruit | Passion fruit | 0.41–0.48 | 77.2–86.8 | 15.3–20.6 | [179] |
| Refined cereals | Refined wheat flour | 0.52 | 37 | 6.47 | [180] |
| Intestinal Model | Method | Main Outcome | Advantage | Disadvantage | References |
|---|---|---|---|---|---|
| Ussing chamber |
|
|
|
| [225,226,227,228,229,230,231] |
| Everted gut sac |
|
|
|
| [60,61] |
| Perfused intestine |
|
|
|
| [38,70,115,234] |
| Brush border membrane vesicles |
|
|
|
| [72,232,233] |
| In vitro intestinal cell model |
|
|
|
| [66,67,68,71,96,102,103,120,156,238,239,240,241,242,243] |
| Cell Model | Incubation Parameter | Type of Zinc | Main Outcome | Reference |
|---|---|---|---|---|
| Caco-2 cells Cultivation time: 14 d 3D Transwell (PC membrane) 14 d | ZnCl2 20 µM (Kinetic 0–50 min) 0–100 µM (10 min) (in salt buffer on apical and basolateral side) Inhibitor: ouabain, vanadate, dinitrophenol, sodium cyanide, ammonium vanadate Potential zinc ligands: histidine, cysteine, proline, glutathione | radioactive zinc (65Zn) |
| [66] |
| Caco-2 cells Cultivation time: 18–21 d 3D Transwell | ZnSO4 10–1000 µM (for 90 min) 10 nM 1α,25-dihydroxyvitamin D3 (preincubation for 72 h) + 100 µM ZnSO4 (for 90 min) Apical: MES-buffer with NaCl, KCl, MgSO4, CaCl2, glutamine, glucose, Basolateral: 2.5 mg/mL BSA in Hepes with NaCl, KCl, MgSO4, CaCl2, glutamine, glucose, | radioactive zinc (65Zn) |
| [238] |
| Caco-2 cells Cultivation time: 21 d 2D, 3D Transwell (PE membrane) | zinc species: n.a. 1–200 µM (in DMEM + 10% FCS on apical and basolateral side) for 0–30 h | radioactive zinc (65Zn) |
| [67] |
| Caco-2 cells Cultivation time: 14–16 days of 3D Transwell (Polyethylene terephthalate membrane) | ZnSO4 0–1000 µM (in DMEM + 10% FCS on apical) and 0–450 µM (in DMEM + 10% FCS on basolateral side) for 24 h | total Zn |
| [68] |
| Caco-2 cells Cultivation time: 18–21 days of 3D Transwell (PC) | ZnCl2 50–200 µM (in serum free medium on apical and basolateral side) for 6 h, 12 h, and 24 h | radioactive zinc (65Zn) |
| [120] |
| Caco-2 cells Cultivation time: 21 d 3D (PES-HD membranes) | ZnSO4 5 µM or 25 µM (in DMEM + 10% FCS on apical and basolateral) (preincubation for 7 d) | radioactive zinc (65Zn) |
| [239] |
| Caco-2 cells Cultivation time: 21 d 3D Transwell (PC) | ZnSO4 15.6–500 µM (apical: KHB buffer, basolateral: KHB-buffer + 5% BSA) | total Zn |
| [71] |
| Caco-2 cells Cultivation time: 17 days 3D Transwell (Polytetrafluoroethylene) | ZnSO4 100 µM (serum free medium on apical and basolateral side) for 3–24 h 1 µM hepcidin | stable zinc isotope (67Zn) |
| [156] |
| Caco-2/HT-29-MTX co-culture Cultivation time: 21 days 3D Transwell (PC) | ZnSO4 0–100 µM (apical: serum-free transport buffer, basolateral: DMEM +10% FCS + 0 or 30 mg mL−1 BSA) for 8 h | total Zn |
| [102] |
| Caco-2/HT-29-MTX co-culture and Caco-2 monoculture Cultivation time: 21 days 3D Transwell (PC) | ZnSO4 0–100 µM (apical: serum-free transport buffer, basolateral: DMEM + 10% FCS + 30 mg mL−1 BSA) for 4 h | total Zn |
| [103] |
| In Vitro Caco-2/HT-29-MTX [102] (Absorption Area = 1.12 cm2, Volume: 500 µL) | |||
| Apical Zinc | Fractional Absorption (%) | Absorbed Zinc (µg/Total Absorption Area) | Absorbed Zinc (µg cm−2) |
| 100 µM = 3.23 µg/1.12 cm2 | 2.9 | 0.09 | 0.08 |
| 25 µM = 0.82 µg/1.12 cm2 | 5.8 | 0.05 | 0.04 |
| In vivo [7] (Absorption Area = ~30 m2 [314], Volume: ~3 L [255]) | |||
| Apical Zinc | Fractional Absorption (%) | Absorbed Zinc (µg/Total Absorption Area) | Absorbed Zinc (µg cm−2) |
| 17 mg/30 m2 = 86 µM | 24 | 4080 | 0.14 |
| 4.3 mg/30 m2 = 21 µM | 49 | 2100 | 0.07 |
| Cell Model | Sensor | Incubation Parameter | Main Outcome | Reference |
|---|---|---|---|---|
HT-29 Cultivation time:
| FluoZin-3 (Kd = 8.9 nM) Newport Green (Kd = 30 µM) (low molecular weight sensors) |
|
| [340] |
| Caco-2/TC7 Cultivation time: 15-17 days; 2D | FluoZin-3 (Kd = 15 nM) Zinquin (low molecular weight sensors) |
|
| [339] |
| HT-29 Cultivation time: n.a.; 2D | Newport Green (low molecular weight sensor) |
|
| [341] |
| Caco-2 Cultivation time: 17 days; 2D | FluoZin-3 (Kd = 15 nM) (LMW sensor) |
|
| [156] |
| Caco-2 Cultivation time: 10 days; 2D | Zinypr-1 (Kd = 0.7 nM) (low molecular weight sensor) |
|
| [96] |
| Caco-2-eCalwy Cultivation time: resting state; 2D | eCalwy-5 (Kd = 1.85 nM) (Genetically encoded protein-based sensor) | FRET and FLIM-FRET measurements using low molecular weight (LSM) in assay buffer b |
| [101] |
| Caco-2 Cultivation time: 21 days; 2D | Zinpyr-1 (Kd = 0.7 nM) (low molecular weight sensor) |
|
| [102] |
| Caco-2 Cultivation time: 21 dHT-29, HT-29-MTX Cultivation time:7 days; 2D | Zinypr-1 (Kd = 0.7 nM) (LMW sensor) |
|
| [103] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maares, M.; Haase, H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients 2020, 12, 762. https://doi.org/10.3390/nu12030762
Maares M, Haase H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients. 2020; 12(3):762. https://doi.org/10.3390/nu12030762
Chicago/Turabian StyleMaares, Maria, and Hajo Haase. 2020. "A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models" Nutrients 12, no. 3: 762. https://doi.org/10.3390/nu12030762
APA StyleMaares, M., & Haase, H. (2020). A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients, 12(3), 762. https://doi.org/10.3390/nu12030762






