The Link between Hypermetabolism and Hypernatremia in Severely Burned Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographics
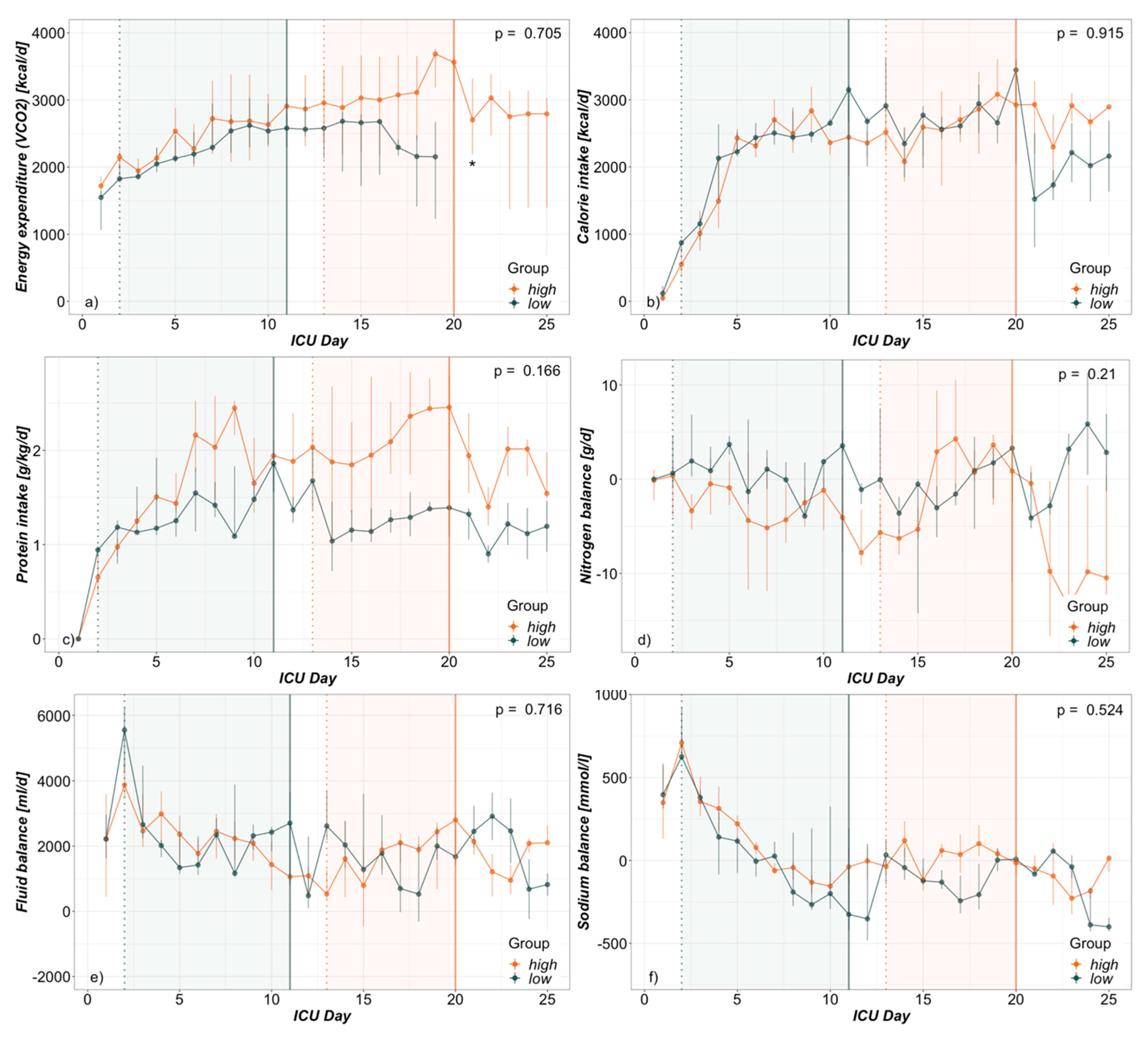
3.2. Hypermetabolism
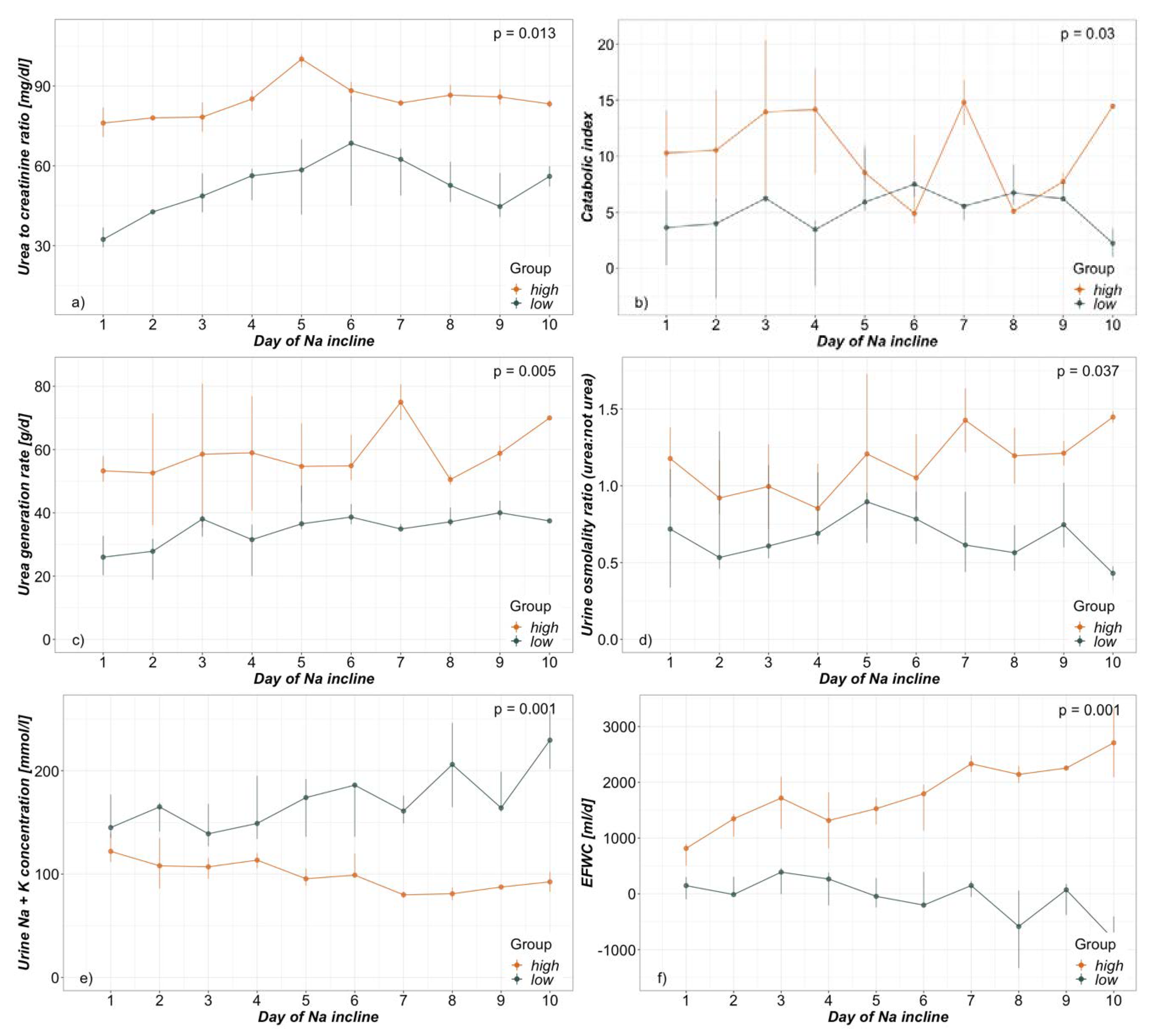
3.3. Electrolyte-Free Water Clearance
3.4. Hypernatremia
4. Discussion
4.1. Demographics
4.2. Hypermetabolism
4.3. Electrolyte-Free Water Clearance
4.4. Hypernatremia
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CrCl | creatinine clearance [ml/min] |
| CRP | serum c-reactive-protein concentration [mg/dl] |
| CreaS | serum creatinine concentration [mg/dl] |
| CreaU | urine creatinine concentration [mg/dl] |
| EEVCO2 | energy expenditure derived from VCO2 [kcal/d] |
| EFWC | electrolyte-free water clearance [ml/d] |
| FWC | free water clearance [ml/d] |
| GlucS | serum glucose concentration [mg/dl] |
| ICU | intensive care unit |
| INN | total nitrogen intake [g/d] |
| KUr | urine potassium concentration [mmol/l] |
| LOS | length of stay [d] |
| NaS | serum sodium concentration [mmol/l] |
| NaUr | urine sodium concentration [mmol/l] |
| OsmoPl | plasma osmolality [mosmol/kg] |
| OsmoUr | urine osmolality [mosmol/kg] |
| OsmoUrea:OsmoRest | ratio of urine osmolality due to urea and not due to urea |
| OUTUr | total urine output [ml/d] |
| PDMS | patient data management system |
| TBSA | total body surface area [%] |
| UreaS | serum urea concentration [mg/dl] |
| UreaUr | urine urea concentration [mg/dl] |
| UreaGR | urea generation rate [g/d] |
| Urea:Crea | serum urea to creatinine ratio [mg/dl:mg/dl] |
References
- Lindner, G.; Funk, G.-C. Hypernatremia in critically ill patients. J. Crit. Care 2013, 28, 216.e11–216.e20. [Google Scholar] [CrossRef] [PubMed]
- Namdar, T.; Siemers, F.; Stollwerck, P.L.; Stang, F.H.; Mailänder, P.; Lange, T. Increased mortality in hypernatremic burned patients. Ger. Med. Sci. 2010, 8. [Google Scholar] [CrossRef]
- Lindner, G.; Funk, G.-C.; Schwarz, C.; Kneidinger, N.; Kaider, A.; Schneeweiss, B.; Kramer, L.; Druml, W. Hypernatremia in the Critically Ill Is an Independent Risk Factor for Mortality. Am. J. Kidney Dis. 2007, 50, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, M.K.; George, A.; Bang, R.L. Only some septicaemic patients develop hypernatremia in the burn intensive care unit: Why? Burns 2002, 28, 543–547. [Google Scholar] [CrossRef]
- Lam, N.N.; Minh, N.T.N. Risk factors and outcome of Hypernatremia amongst severe adult burn patients. Ann. Burns Fire Disasters 2018, 31, 271–277. [Google Scholar] [PubMed]
- Stewart, I.J.; Morrow, B.D.; Tilley, M.A.; Snow, B.D.; Gisler, C.; Kramer, K.W.; Aden, J.K.; Renz, E.M.; Chung, K.K. Dysnatremias and Survival in Adult Burn Patients: A Retrospective Analysis. Am. J. Nephrol. 2013, 37, 59–64. [Google Scholar] [CrossRef]
- Sen, S.; Tran, N.; Chan, B.; Palmieri, T.L.; Greenhalgh, D.G.; Cho, K. Sodium variability is associated with increased mortality in severe burn injury. Burns Trauma 2017, 5, 34. [Google Scholar] [CrossRef]
- Adrogué, H.J.; Madias, N.E. Hypernatremia. N. Engl. J. Med. 2000, 342, 1493–1499. [Google Scholar] [CrossRef]
- Lindner, G.; Schwarz, C.; Funk, G.-C. Osmotic diuresis due to urea as the cause of hypernatraemia in critically ill patients. Nephrol. Dial. Transplant. 2012, 27, 962–967. [Google Scholar] [CrossRef]
- Distenhreft, J.I.Q.; Vianna, J.G.P.; Scopel, G.S.; Ramos, J.M.; Seguro, A.C.; Luchi, W.M. The role of urea-induced osmotic diuresis and hypernatremia in a critically ill patient: Case report and literature review. J. Bras. Nefrol. 2019. [Google Scholar] [CrossRef]
- Vadi, S.; Yim, K. Hypernatremia due to urea-induced osmotic diuresis: Physiology at the bedside. Indian J. Crit. Care Med. 2018, 22, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Barrett, E.J. Severe hypernatremia from a urea-induced diuresis due to body protein wasting in an insulin-resistant type 2 diabetic patient. J. Clin. Endocrinol. Metab. 2013, 98, 1800–1802. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bodonyi-Kovacs, G.; Lecker, S.H. Electrolyte-free water clearance: A key to the diagnosis of hypernatremia in resolving acute renal failure. Clin. Exp. Nephrol. 2008, 12, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.L. The role of the musculoskeletal system in post-burn hypermetabolism. Metabolism 2019, 97, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.K.; Hromatka, K.A.; Miller, K.R. Energy Expenditure and Protein Requirements Following Burn Injury. Nutr. Clin. Pract. 2019, 34, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Herndon, D.N.; Tompkins, R.G. Support of the metabolic response to burn injury. Lancet 2004, 363, 1895–1902. [Google Scholar] [CrossRef]
- Hart, D.W.; Wolf, S.E.; Mlcak, R.; Chinkes, D.L.; Ramzy, P.I.; Obeng, M.K.; Ferrando, A.A.; Wolfe, R.R.; Herndon, D.N. Persistence of muscle catabolism after severe burn. Surgery 2000, 128, 312–319. [Google Scholar] [CrossRef]
- Yu, Y.; Tompkins, R.G.; Ryan, C.M.; Young, V.R. The Metabolic Basis of the Increase in Energy Expenditure in Severely Burned Patients. JPEN J. Parenter. Enter. Nutr. 1999, 23, 160–168. [Google Scholar] [CrossRef]
- Bistrian, B.R. A simple technique to estimate severity of stress. Surg. Gynecol. Obstet. 1979, 148, 675–678. [Google Scholar]
- Stapel, S.N.; de Grooth, H.-J.S.; Alimohamad, H.; Elbers, P.W.; Girbes, A.R.; Weijs, P.J.; Straaten, H.M. Ventilator-derived carbon dioxide production to assess energy expenditure in critically ill patients: Proof of concept. Crit. Care 2015, 19, 370. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Kurtz, I. Derivation of a new formula for calculating urinary electrolyte-free water clearance based on the Edelman equation. Am. J. Physiol. Renal Physiol. 2005, 288, F1–F7. [Google Scholar] [CrossRef]
- Haines, R.W.; Zolfaghari, P.; Wan, Y.; Pearse, R.M.; Puthucheary, Z.; Prowle, J.R. Elevated urea-to-creatinine ratio provides a biochemical signature of muscle catabolism and persistent critical illness after major trauma. Intensive Care Med. 2019, 45, 1718–1731. [Google Scholar] [CrossRef]
- Noguchi, K.; Gel, Y.R.; Brunner, E.; Konietschke, F. nparLD: An R Software Package for the Nonparametric Analysis of Longitudinal Data in Factorial Experiments. J. Stat. Softw. 2012, 50, 1–23. [Google Scholar] [CrossRef]
- Bakdash, J.Z.; Marusich, L.R. Repeated Measures Correlation. Front. Psychol. 2017, 8, 456. [Google Scholar] [CrossRef]
- Balogh, D.; Benzer, A.; Hackl, J.M.; Bauer, M. Sodium balance and osmolarity in burn patients. Intensive Care Med. 1986, 12, 100–103. [Google Scholar] [CrossRef]
- Singer, P. Simple equations for complex physiology: Can we use VCO2 for calculating energy expenditure? Crit. Care 2016, 20, 72. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Przkora, R.; Suman, O.E.; Finnerty, C.C.; Mlcak, R.P.; Pereira, C.T.; Sanford, A.P.; Herndon, D.N. Sex differences in the long-term outcome after a severe thermal injury. Shock 2007, 27, 461–465. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Mlcak, R.P.; Finnerty, C.C.; Norbury, W.B.; Przkora, R.; Kulp, G.A.; Gauglitz, G.G.; Zhang, X.-J.; Herndon, D.N. Gender Differences in Pediatric Burn Patients. Ann. Surg. 2008, 248, 126–136. [Google Scholar] [CrossRef]
- Gunst, J.; Vanhorebeek, I.; Casaer, M.P.; Hermans, G.; Wouters, P.J.; Dubois, J.; Claes, K.; Schetz, M.; den Berghe, G.V. Impact of Early Parenteral Nutrition on Metabolism and Kidney Injury. J. Am. Soc. Nephrol. 2013, 24, 995–1005. [Google Scholar] [CrossRef]
- Gunst, J.; Vanhorebeek, I.; Thiessen, S.E.; den Berghe, G.V. Amino acid supplements in critically ill patients. Pharmacol. Res. 2017, 130, 127–131. [Google Scholar] [CrossRef]
- Gunst, J.; Kashani, K.B.; Hermans, G. The urea-creatinine ratio as a novel biomarker of critical illness-associated catabolism. Intensive Care Med. 2019, 45, 1813–1815. [Google Scholar] [CrossRef]
- Warden, G.D.; Wilmore, D.W.; Rogers, P.W.; Mason, A.D.; Pruitt, B.A. Hypernatremic state in hypermetabolic burn patients. Arch. Surg. 1973, 106, 420–427. [Google Scholar] [CrossRef]
- Regenmortel, N.; Verbrugghe, W.; Roelant, E.; den Wyngaert, T.; Jorens, P.G. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: A retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018, 44, 409–417. [Google Scholar] [CrossRef]

| Formula | Units | Ref |
|---|---|---|
| [ml/d] | [21] | |
| [ml/d] | [21] | |
| [g/d] | ||
| [g/d] | [19] | |
| [kcal/d] | [20] |
| Urea:creatinine | High (n = 31) Median (Q1–Q3) | Low (n = 44) Median (Q1–Q3) | p |
|---|---|---|---|
| Urea:creatinine | 81.3 (78.4–89.0) | 48.5 (40.0–66.9) | 0.013 |
| Catabolic index | 9.3 (6.1–17.4) | 5.9 (3.6–6.8) | 0.030 |
| Urea generation rate [g/d] | 54.9 (43.7–72.6) | 35.8 (31.7–39.1) | 0.005 |
| Insulin requirements [IU/d] | 14.8 (0.0–45.6) | 0.0 (0.0–24.6) | 0.208 |
| CRP [mg/dl] | 25.8 (15.6–29.6) | 19.9 (8.4–24.0) | 0.220 |
| Leucocytes [G/l] | 10.1 (9.4–11.5) | 7.3 (6.5–12.2) | 0.376 |
| Temperature [°C] | 37.9 (37.2–38.3) | 37.7 (37.0–38.2) | 0.688 |
| Urine OsmoUrea:OsmoRest | 1.1 (0.8–1.5) | 0.6 (0.5–1.0) | 0.037 |
| Na+ concentration urine [mmol/l] | 62.0 (51.0–79.5) | 100.0 (62.8–147.5) | 0.026 |
| K+ concentration urine [mmol/l] | 35.0 (28.0–49.5) | 64.5 (51.8–82.8) | 0.014 |
| Electrolyte-free water clearance [ml/d] | 1645 (824–2060) | 27 (−239–316) | 0.001 |
| Free water clearance [ml/d] | −1358 (−2091–466) | −2133 (−2899–−1423) | 0.061 |
| Creatinine clearance [ml/min] | 98.0 (71.0–148.0) | 119.5 (88.0–164.3) | 0.487 |
| Urine output [ml/d] | 3840 (3120–4410) | 1995 (1380–2958) | 0.024 |
| Furosemide use [mg/d] | 0.0 (0.0–83.3) | 0.0 (0.0–92.4) | 0.766 |
| Canrenone use [mg/d] | 0 (0–400) | 0 (0–0) | 0.479 |
| Urea:creatinine | High (n = 4) Median (Q1–Q3) | Low (n = 5) Median (Q1–Q3) | p |
|---|---|---|---|
| Age [years] | 55.5 (52.5–59.3) | 42.0 (27.0–62.0) | 0.539 |
| Gender male female | 3 1 | 2 3 | 0.524 |
| Burn injury TBSA [%] full thickness burn TBSA [%] inhalation injury [n] | 26.0 (24.0–28.8) 2.5 (0–5.3) 3 | 35.0 (20.0–40.0) 5.0 (4.0–9.0) 1 | 0.711 0.321 0.206 |
| SAPS III admission score | 52.5 (48.0–57.5) | 52.0 (48.0 – 61.0) | 0.901 |
| ICU LOS [d] | 45.0 (31.8–59.0) | 31.0 (23.0 – 37.0) | 0.219 |
| ICU outcome dead alive | 0 4 | 0 5 | 1.000 |
| Duration of NaS-incline [d] | 8.0 (5.8–10.0) | 9.0 (8.0–10.0) | 0.701 |
| NaS peak [mmol/l] | 159.0 (154.8–163.3) | 157.0 (156.0–157.0) | 0.443 |
| LOS day of NaS peak | 20.0 (18.5–22.0) | 11.0 (10.0–15.0) | 0.027 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rugg, C.; Ströhle, M.; Schmid, S.; Kreutziger, J. The Link between Hypermetabolism and Hypernatremia in Severely Burned Patients. Nutrients 2020, 12, 774. https://doi.org/10.3390/nu12030774
Rugg C, Ströhle M, Schmid S, Kreutziger J. The Link between Hypermetabolism and Hypernatremia in Severely Burned Patients. Nutrients. 2020; 12(3):774. https://doi.org/10.3390/nu12030774
Chicago/Turabian StyleRugg, Christopher, Mathias Ströhle, Stefan Schmid, and Janett Kreutziger. 2020. "The Link between Hypermetabolism and Hypernatremia in Severely Burned Patients" Nutrients 12, no. 3: 774. https://doi.org/10.3390/nu12030774
APA StyleRugg, C., Ströhle, M., Schmid, S., & Kreutziger, J. (2020). The Link between Hypermetabolism and Hypernatremia in Severely Burned Patients. Nutrients, 12(3), 774. https://doi.org/10.3390/nu12030774





