Cytidine 5?-Diphosphocholine (Citicoline): Evidence for a Neuroprotective Role in Glaucoma
Abstract
:1. Introduction
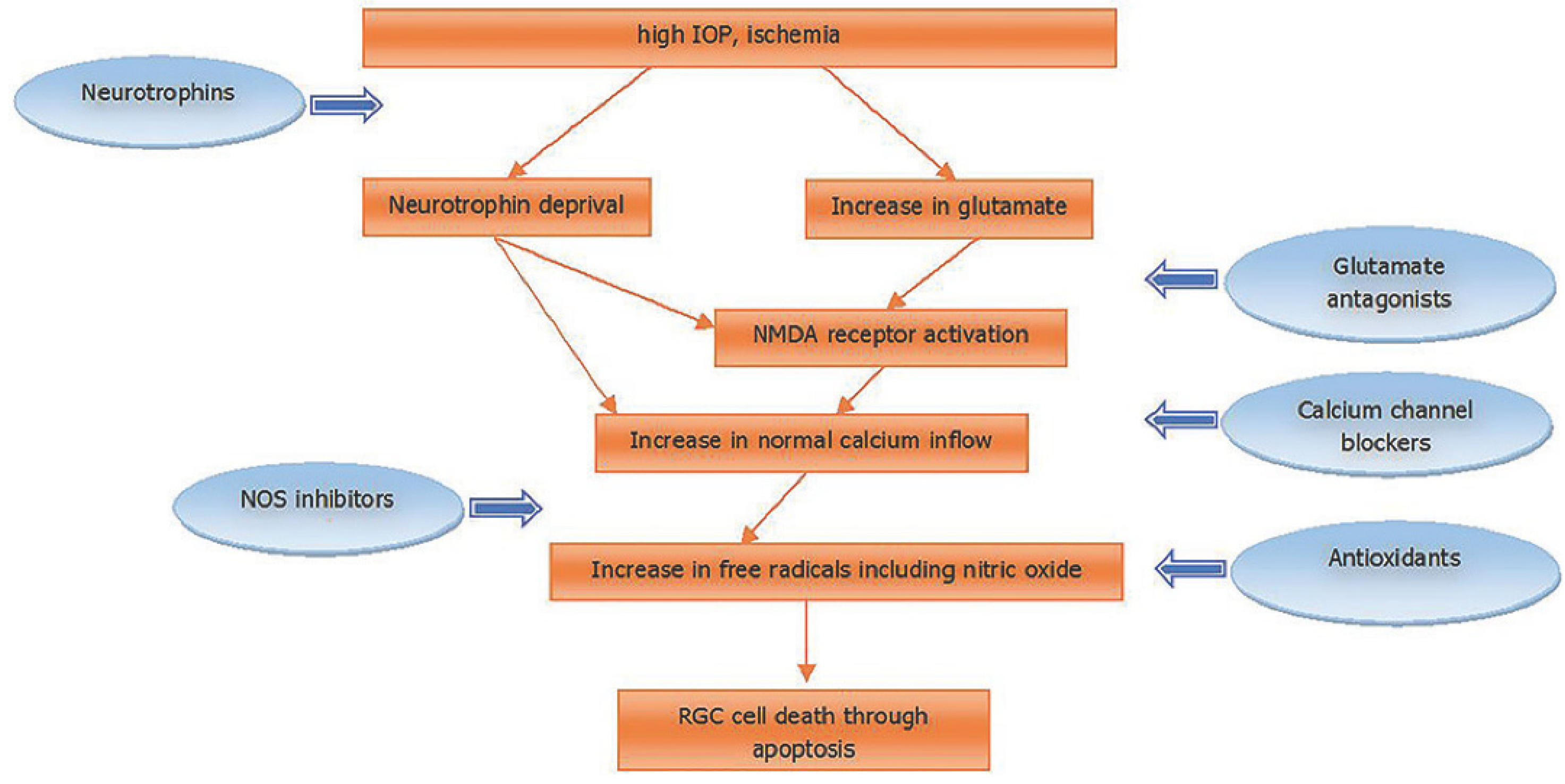
2. Pathophysiology of Glaucoma
3. Neuroprotection in Glaucoma
4. Role of Citicoline as a Neuroprotective Agent
4.1. Role of Choline in Retinal Disorders
4.2. Neuroprotective Properties of Citicoline
4.3. Experimental Evidence
4.4. Evidence from Clinical Studies
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [Green Version]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [Green Version]
- Bourne, R.R.; Taylor, H.R.; Flaxman, S.R.; Keeffe, J.; Leasher, J.; Naidoo, K.; Pesudovs, K.; White, R.A.; Wong, T.Y.; Resnikoff, S.; et al. Number of people blind or visually impaired by glaucoma worldwide and in world regions 1990–2010: A meta-analysis. PLoS ONE 2016, 11, e0162229. [Google Scholar] [CrossRef] [PubMed]
- Grieb, P.; Junemann, A.; Rekas, M.; Rejdak, R. Citicoline: A food beneficial for patients suffering from or threated with glaucoma. Front. Aging Neurosci. 2016, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Rusciano, D.; Pezzino, S.; Mutolo, M.G.; Giannotti, R.; Librando, A.; Pescosolido, N. Neuroprotection in glaucoma: Old and new promising treatments. Adv. Pharmacol. Sci. 2017, 2017, 4320408. [Google Scholar] [CrossRef]
- Peters, D.; Bengtsson, B.; Heijl, A. Lifetime risk of blindness in open-angle glaucoma. Am. J. Ophthalmol. 2013, 156, 724–730. [Google Scholar] [CrossRef] [Green Version]
- Malihi, M.; Moura Filho, E.R.; Hodge, D.O.; Sit, A.J. Long-term trends in glaucoma-related blindness in Olmsted County, Minnesota. Ophthalmology 2014, 121, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, Y.; Yu, F.; Coleman, A.L. Burden of undetected and untreated glaucoma in the United States. Am. J. Ophthalmol. 2014, 158, 1121–1129. [Google Scholar] [CrossRef]
- Roberti, G.; Tanga, L.; Michelessi, M.; Quaranta, L.; Parisi, V.; Manni, G.; Oddone, F. Cytidine 5′-Diphosphocholine (Citicoline) in glaucoma: Rationale of its use, current evidence and future perspectives. Int. J. Mol. Sci. 2015, 16, 28401–28417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucolo, C.; Platania, C.B.M.; Drago, F.; Bonfiglio, V.; Reibaldi, M.; Avitabile, T.; Uva, M. Novel therapeutics in glaucoma management. Curr. Neuropharmacol. 2018, 16, 978–992. [Google Scholar] [CrossRef] [PubMed]
- Doozandeh, A.; Yazdani, S. Neuroprotection in glaucoma. J. Ophthalmic Vis. Res. 2016, 11, 209–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medline Clark, W.M.; Warach, S.J.; Pettigrew, L.C. Citicoline Monograph. Altern. Med. Rev. 2008, 13, 50–57. [Google Scholar]
- Yücel, Y.H.; Zhang, Q.; Gupta, N.; Kaufman, P.L.; Weinreb, R.N. Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch. Ophthalmol. 2000, 118, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Yücel, Y.H.; Zhang, Q.; Weinreb, R.N.; Kaufman, P.L.; Gupta, N. Atrophy of relay neurons in magno- and parvocellular layers in the lateral geniculate nucleus in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3216–3222. [Google Scholar]
- Parisi, V.; Miglior, S.; Manni, G.; Centofanti, M.; Bucci, M.G. Clinical ability of pattern electroretinograms and visual evoked potentials in detecting visual dysfunction in ocular hypertension and glaucoma. Ophthalmology 2006, 113, 216–228. [Google Scholar] [CrossRef]
- Weber, A.J.; Chen, H.; Hubbard, W.C.; Kaufman, P.L. Experimental glaucoma and cell size, density, and number in the primate lateral geniculate nucleus. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1370–1379. [Google Scholar]
- Yücel, Y.H.; Zhang, Q.; Weinreb, R.N.; Kaufman, P.L.; Gupta, N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog. Retin. Eye Res. 2003, 22, 465–481. [Google Scholar] [CrossRef]
- Gupta, N.; Yücel, Y.H. Brain changes in glaucoma. Eur. J. Ophthalmol. 2003, 13, S32–S35. [Google Scholar] [CrossRef]
- Gupta, N.; Ang, L.C.; Noel de Tilly, L.; Bidaisee, L.; Yücel, Y.H. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br. J. Ophthalmol. 2006, 90, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Crish, S.D.; Sappington, R.M.; Inman, D.M.; Horner, P.J.; Calkins, D.J. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 5196–5201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crish, S.D.; Calkins, D.J. Neurodegeneration in glaucoma: Progression and calcium-dependent intracellular mechanisms. Neuroscience 2011, 176, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerrigan-Baumrind, L.A.; Quigley, H.A.; Pease, M.E.; Kerrigan, D.F.; Mitchell, R.S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investig. Ophthalmol. Vis. Sci. 2000, 41, 741–748. [Google Scholar] [PubMed]
- Grieb, P.; Rejdak, R. Pharmacodynamics of citicoline relevant to the treatment of glaucoma. J. Neurosci. Res. 2002, 67, 143–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iulia, C.; Ruxandra, T.; Costin, L.B.; Liliana-Mary, V. Citicoline—A neuroprotector with proven effects on glaucomatous disease. Rom. J. Ophthalmol. 2017, 61, 152–158. [Google Scholar]
- Nucci, C.; Martucci, A.; Giannini, C.; Morrone, L.A.; Bagetta, G.; Mancino, R. Neuroprotective agents in the management of glaucoma. Eye 2018, 32, 938–945. [Google Scholar] [CrossRef]
- Adornetto, A.; Russo, R.; Parisi, V. Neuroinflammation as a target for glaucoma therapy. Neural Regen. Res. 2019, 14, 391–394. [Google Scholar] [CrossRef]
- Murphy, M.C.; Conner, I.P.; Teng, C.Y.; Lawrence, J.D.; Safiullah, Z.; Wang, B.; Bilonick, R.A.; Kim, S.G.; Wollstein, G.; Schuman, J.S.; et al. Retinal structures and visual cortex activity are impaired prior to clinical vision loss in glaucoma. Sci. Rep. 2016, 6, 31464. [Google Scholar] [CrossRef]
- Sanders, L.M.; Zeisel, S.H. Choline: Dietary requirements and role in brain development. Nutr. Today 2007, 42, 181–186. [Google Scholar] [CrossRef]
- Faiq, M.A.; Wollstein, G.; Schuman, J.S.; Chan, K.C. Cholinergic nervous system and glaucoma: From basic science to clinical applications. Prog. Retin. Eye Res. 2019, 72, 100767. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.C.; So, K.F.; Wu, E.X. Proton magnetic resonance spectroscopy revealed choline reduction in the visual cortex in an experimental model of chronic glaucoma. Exp. Eye Res. 2009, 88, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Wen, G.; Wu, G.; Zhang, X. Proton magnetic resonance spectroscopy (1H-MRS) reveals geniculocalcarine and striate area degeneration in primary glaucoma. PLoS ONE 2013, 8, e73197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bubella, R.M.; Carità, S.; Badalamenti, R.; Bubella, D.M. Neuroprotezione del paziente con glaucoma cronico ad an golo aperto: Ruolo della citicolina in soluzione orale. Ottica Fisiopatologica 2011, 16, 171–177. [Google Scholar]
- Ottobelli, L.; Manni, G.L.; Centofanti, M.; Iester, M.; Allevena, F.; Rossetti, L. Citicoline oral solution in glaucoma: Is there a role in slowing disease progression? Ophthalmologica 2013, 229, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V. Electrophysiological assessment of glaucomatous visual dysfunction during treatment with cytidine-5′-diphosphocholine (citicoline): A study of 8 years of follow-up. Doc. Ophthalmol. 2005, 110, 91–102. [Google Scholar] [CrossRef]
- Parisi, V.; Centofanti, M.; Ziccardi, L.; Tanga, L.; Michelessi, M.; Roberti, G.; Manni, G. Treatment with citicoline eye drops enhances retinal function and neural conduction along the visual pathways in open angle glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1327–1340. [Google Scholar] [CrossRef]
- Parisi, V.; Coppola, G.; Centofanti, M.; Oddone, F.; Angrisani, A.M.; Ziccardi, L.; Ricci, B.; Quaranta, L.; Manni, G. Evidence of the neuroprotective role of citicoline in glaucoma patients. Prog. Brain Res. 2008, 173, 541–554. [Google Scholar]
- Parisi, V.; Manni, G.; Colacino, G.; Bucci, M.G. Cytidine-5′-diphosphocholine (citicoline) improves retinal and cortical responses in patients with glaucoma. Ophthalmology 1999, 106, 1126–1134. [Google Scholar] [CrossRef]
- Pecori Giraldi, J.; Virno, M.; Covelli, G.; Grechi, G.; De Gregorio, F. Therapeutic value of citicoline in the treatment of glaucoma (computerized and automated perimetric investigation). Int. Ophthalmol. 1989, 13, 109–112. [Google Scholar] [CrossRef]
- Rejdak, R.; Toczolowski, J.; Kurkowski, J.; Kaminski, M.L.; Rejdak, K.; Stelmasiak, Z.; Grieb, P. Oral citicoline treatment improves visual pathway function in glaucoma. Med. Sci. Monit. 2003, 9, PI24–PI28. [Google Scholar] [PubMed]
- Roberti, G.; Tanga, L.; Parisi, V.; Sampalmieri, M.; Centofanti, M.; Manni, G. A preliminary study of the neuroprotective role of citicoline eye drops in glaucomatous optic neuropathy. Indian J. Ophthalmol. 2014, 62, 549–553. [Google Scholar] [CrossRef]
- Virno, M.; Pecori-Giraldi, J.; Liguori, A.; De Gregorio, F. The protective effect of citicoline on the progression of the perimetric defects in glaucomatous patients (perimetric study with a 10-year follow-up). Acta Ophthalmol. Scand. Suppl. 2000, 78, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S.H. Choline: Clinical nutrigenetic/nutrigenomic approaches for identification of functions and dietary requirements. World Rev. Nutr. Diet. 2010, 101, 73–83. [Google Scholar]
- Zeisel, S.H. Nutritional genomics: Defining the dietary requirement and effects of choline. J. Nutr. 2011, 141, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Vennemann, F.B.; Ioannidou, S.; Valsta, L.M.; Dumas, C.; Ocke, M.C.; Mensink, G.B.; Lindtner, O.; Virtanen, S.M.; Tlustos, C.; D’Addezio, L.; et al. Dietary intake and food sources of choline in European populations. Br. J. Nutr. 2015, 114, 2046–2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, T.C.; Fulgoni, V.L., III. Assessment of total choline intakes in the United States. J. Am. Coll. Nutr. 2016, 35, 108–112. [Google Scholar] [CrossRef]
- Wallace, T.C.; Fulgoni, V.L. Usual choline intakes are associated with egg and protein food consumption in the United States. Nutrients 2017, 9, 839. [Google Scholar] [CrossRef] [Green Version]
- Synoradzki, K.; Grieb, P. Citicoline: A superior form of choline? Nutrients 2019, 11, 1569. [Google Scholar] [CrossRef] [Green Version]
- Agut, J.; Font, E.; Sacristan, A.; Ortiz, J.A. Bioavailability of methyl-14C CDP-choline by oral route. Arzneimittelforschung 1983, 33, 1045–1047. [Google Scholar]
- Parisi, V.; Oddone, F.; Ziccardi, L.; Roberti, G.; Coppola, G.; Manni, G. Citicoline and retinal ganglion cells: Effects on morphology and function. Curr. Neuropharmacol. 2018, 16, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Grieb, P. Citicoline and Eye Health. In Handbook of Nutrition, Diet, and the Eye, 2nd ed.; Preedy, V., Watson, R., Eds.; Academic Press: London, UK, 2019; pp. 585–603. [Google Scholar]
- Grieb, P. Neuroprotective properties of citicoline: Facts, doubts and unresolved issues. CNS Drugs 2014, 28, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberhardt, R.; Birbamer, G.; Gerstenbrand, F.; Rainer, E.; Traegner, H. Citicoline in the treatment of Parkinson’s disease. Clin. Ther. 1990, 12, 489–495. [Google Scholar] [PubMed]
- Agnoli, A.; Ruggieri, S.; Denaro, A.; Bruno, G. New strategies in the management of Parkinson’s disease: A biological approach using a phospholipid precursor (CDP-choline). Neuropsychobiology 1982, 8, 289–296. [Google Scholar] [CrossRef]
- Cacabelos, R.; Caamano, J.; Gomez, M.J.; Fernandez-Novoa, L.; Franco-Maside, A.; Alvarez, X.A. Therapeutic effects of CDP-choline in Alzheimer’s disease. Cognition, brain mapping, cerebrovascular hemodynamics, and immune factors. Ann. N. Y. Acad. Sci. 1996, 777, 399–403. [Google Scholar] [CrossRef]
- Alvarez, X.A.; Mouzo, R.; Pichel, V.; Perez, P.; Laredo, M.; Fernandez-Novoa, L.; Corzo, L.; Zas, R.; Alcaraz, M.; Secades, J.J.; et al. Double-blind placebo-controlled study with citicoline in APOE genotyped Alzheimer’s disease patients. Effects on cognitive performance, brain bioelectrical activity and cerebral perfusion. Methods Find. Exp. Clin. Pharmacol. 1999, 21, 633–644. [Google Scholar]
- Franco-Maside, A.; Caamano, J.; Gomez, M.J.; Cacabelos, R. Brain mapping activity and mental performance after chronic treatment with CDP-choline in Alzheimer’s disease. Methods Find. Exp. Clin. Pharmacol. 1994, 16, 597–607. [Google Scholar]
- Fioravanti, M.; Yanagi, M. Cytidinediphosphocholine (CDP-choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst. Rev. 2005. [Google Scholar] [CrossRef]
- Alvarez-Sabin, J.; Roman, G.C. The role of citicoline in neuroprotection and neurorepair in ischemic stroke. Brain Sci. 2013, 3, 1395–1414. [Google Scholar] [CrossRef] [Green Version]
- Davalos, A.; Alvarez-Sabin, J.; Castillo, J.; Diez-Tejedor, E.; Ferro, J.; Martinez-Vila, E.; Serena, J.; Segura, T.; Cruz, V.T.; Masjuan, J.; et al. Citicoline in the treatment of acute ischaemic stroke: An international, randomised, multicentre, placebo-controlled study (ICTUS trial). Lancet 2012, 380, 349–357. [Google Scholar] [CrossRef]
- Overgaard, K. The effects of citicoline on acute ischemic stroke: A review. J. Stroke Cerebrovasc. Dis. 2014, 23, 1764–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Sabin, J.; Ortega, G.; Jacas, C.; Santamarina, E.; Maisterra, O.; Ribo, M.; Molina, C.; Quintana, M.; Roman, G.C. Long-term treatment with citicoline may improve poststroke vascular cognitive impairment. Cerebrovasc. Dis. 2013, 35, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.C.; Bolzani, R.; Schiavi, C.; Baldi, A.; Porciatti, V. Cytidin-5′-diphosphocholine enhances the effect of part-time occlusion in amblyopia. Doc. Ophthalmol. 1996, 93, 247–263. [Google Scholar] [CrossRef]
- Campos, E.C.; Schiavi, C.; Benedetti, P.; Bolzani, R.; Porciatti, V. Effect of citicoline on visual acuity in amblyopia: Preliminary results. Graefes Arch. Clin. Exp. Ophthalmol. 1995, 233, 307–312. [Google Scholar] [CrossRef]
- Fresina, M.; Dickmann, A.; Salerni, A.; De Gregorio, F.; Campos, E.C. Effect of oral CDP-choline on visual function in young amblyopic patients. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 143–150. [Google Scholar] [CrossRef]
- Pawar, P.V.; Mumbare, S.S.; Patil, M.S.; Ramakrishnan, S. Effectiveness of the addition of citicoline to patching in the treatment of amblyopia around visual maturity: A randomized controlled trial. Indian J. Ophthalmol. 2014, 62, 124–129. [Google Scholar] [CrossRef]
- Porciatti, V.; Schiavi, C.; Benedetti, P.; Baldi, A.; Campos, E.C. Cytidine-5′-diphosphocholine improves visual acuity, contrast sensitivity and visually-evoked potentials of amblyopic subjects. Curr. Eye Res. 1998, 17, 141–148. [Google Scholar] [CrossRef]
- Parisi, V.; Coppola, G.; Ziccardi, L.; Gallinaro, G.; Falsini, B. Cytidine-5′-diphosphocholine (Citicoline): A pilot study in patients with non-arteritic ischaemic optic neuropathy. Eur. J. Neurol. 2008, 15, 465–474. [Google Scholar] [CrossRef]
- Parisi, V.; Barbano, L.; Di Renzo, A.; Coppola, G.; Ziccardi, L. Neuroenhancement and neuroprotection by oral solution citicoline in non-arteritic ischemic optic neuropathy as a model of neurodegeneration: A randomized pilot study. PLoS ONE 2019, 14, e0220435. [Google Scholar] [CrossRef]
- Secades, J.J. Citicoline: Pharmacological and clinical review, 2016 update. Rev. Neurol. 2016, 63, 1–73. [Google Scholar]
- Oshitari, T.; Fujimoto, N.; Adachi-Usami, E. Citicoline has a protective effect on damaged retinal ganglion cells in mouse culture retina. Neuroreport 2002, 13, 2109–2111. [Google Scholar] [CrossRef] [PubMed]
- Mir, C.; Clotet, J.; Aledo, R.; Durany, N.; Argemi, J.; Lozano, R.; Cervos-Navarro, J.; Casals, N. CDP-choline prevents glutamate-mediated cell death in cerebellar granule neurons. J. Mol. Neurosci. 2003, 20, 53–60. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, Y.S.; Noh, H.S.; Cheon, E.W.; Yang, Y.A.; Yoo, J.M.; Choi, W.S.; Cho, G.J. Neuroprotective effect of citicoline against KA-induced neurotoxicity in the rat retina. Exp. Eye Res. 2005, 81, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, A.; Varano, M.; Gaddini, L.; Mallozzi, C.; Villa, M.; Pricci, F.; Malchiodi-Albedi, F. Neuroprotective effects of citicoline in in vitro models of retinal neurodegeneration. Int. J. Mol. Sci. 2014, 15, 6286–6297. [Google Scholar] [CrossRef]
- Zerbini, G.; Bandello, F.; Lattanzio, R.; Gabellini, D.; Zucchiatti, I.; Spinello, A.; Capuano, V.; Preziosa, C.; Maestroni, S. In vivo evaluation of retinal and choroidal structure in a mouse model of long-lasting diabetes. Effect of topical treatment with citicoline. J. Ocul. Dis. Ther. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Rejdak, R.; Toczolowski, J.; Solski, J.; Duma, D.; Grieb, P. Citicoline treatment increases retinal dopamine content in rabbits. Ophthalmic Res. 2002, 34, 146–149. [Google Scholar] [CrossRef]
- Schuettauf, F.; Rejdak, R.; Thaler, S.; Bolz, S.; Lehaci, C.; Mankowska, A.; Zarnowski, T.; Junemann, A.; Zagorski, Z.; Zrenner, E.; et al. Citicoline and lithium rescue retinal ganglion cells following partial optic nerve crush in the rat. Exp. Eye Res. 2006, 83, 1128–1134. [Google Scholar] [CrossRef]
- Lanza, M.; Gironi Carnevale, U.A.; Mele, L.; Bifani Sconocchia, M.; Bartollino, S.; Costagliola, C. Morphological and functional evaluation of oral citicoline therapy in chronic open-angle glaucoma patients: A pilot study with a 2-year follow-up. Front. Pharmacol. 2019, 10, 1117. [Google Scholar] [CrossRef]
- Parisi, V.; Oddone, F.; Roberti, G.; Tanga, L.; Carnevale, C.; Ziccardi, L.; Manni, G. Enhancement of retinal function and of neural conduction along the visual pathway induced by treatment with citicoline eye drops in liposomal formulation in open angle glaucoma: A pilot electrofunctional study. Adv. Ther. 2019, 36, 987–996. [Google Scholar] [CrossRef]


| Authors | Study Design | Citicoline Concentration | Outcome Measures |
|---|---|---|---|
| Retinal cell cultures | |||
| Oshitari et al., 2002 [73] | Cultured mouse retina | 0.01–10 μM cultured for 9 days | TUNEL staining and assessment of the number of regenerating neurites on damaged RGCs |
| Matteucci et al., 2014 [76] | Cultured rat retinas | 10, 100 and 1000 μM for 96 h and 24 h before glutamate-induced excitotoxic insult and high glucose-promoted neuronal cell damage | Apoptotic analysis and immunostaining and morphometric analysis of glutamate- and hyperglycemia-induced RGC damage |
| Animal models | |||
| Rejdak et al., 2002 [78] | Case-control in albino rabbits | IP administration of 50 mg/kg twice day | Retinal catecholamine levels |
| Park et al., 2005 [75] | Case-control in adult Sprague-Dawley rats | IP administration of 500 mg/kg twice for 1, 3, and 7 days after KA injection | Retinal layer thickness and expression of ChAT and TH after KA-induced retinal damage |
| Schuettauf et al., 2006 [79] | Case-control in adult Brown Norway rats | IP administration of 1g/kg/daily and 300 mg/kg/daily | Density of RGCs and expression of the antiapoptotic protein Bcl-2 |
| Zerbini et al., 2015 [77] | Mouse model of type 1 diabetes | Topical application of 2% eye drops | Retinal layer thickness and choroidal thickness |
| Authors | Study Design | Study Population | Administration and Dosage | Treatment Schedule | Follow-up | Outcome Measures |
|---|---|---|---|---|---|---|
| Pecori Giraldi et al., 1989 [40] | Cohort | OAG (n = 30) | IM 1 g/day | 10 days | 3 months | Reduction in the scotomatous area (computerized central perimetry) and decrease in mean defect (automated perimetry) |
| Parisi et al., 1999 [39] | Double-blind placebo controlled | OAG (n = 40) −3 dB > MD < −6 dB | IM 1 g/day | 2 cycles of 60 days with 120-day washout period | 360 days | VEP and PERG parameters |
| Virno et al., 2000 [43] | Case-control | OAG (n = 23) | IM 1 g/day | 15 days treatment repeated every 6 months for 20 cycles | 10 years | Visual field worsening (increase in non-perception area >500 mm2) |
| Rejdak et al., 2003 [41] | Cohort | OAG (n = 21 eyes) | Oral 1 g/day | 14 days 2 days of washout (2 cycles) | 56 days | VEP parameters |
| Parisi V, 2005 [36] | Case-control | OAG (n = 30) −3 dB > MD < −6 dB | IM 1 g/day | 60-day cycles with 120 days of washout (14 cycles) | 8 years | VEP and PERG parameters |
| Parisi et al., 2008 [38] | Case-control | OAG (n = 60) −2 dB > MD < −14 dB | IM 1 g/day Oral 1600 mg/day | 60 days 120 days of washout (2 cycles) | 360 days | VEP and PERG parameters |
| Ottobelli et al., 2013 [35] | Retrospective cohort | Progressing OAG (n = 41) | Oral 500 mg/day | 120 days 60 days of washout (4 cycles) | 2 years | Rate of visual field progression |
| Roberti et al., 2014 [42] | Case-control | OAG (n = 34) −3 dB > MD < −12 dB | Intraocular (topical eye drops) 3 drops/day | 60 days | 90 days | VEP and PERG parameters |
| Parisi et al., 2015 [37] | Case-control | OAG (n = 56) MD > −10 dB | Intraocular (topical eye drops) 3 drops/day | 120 days 60 days of washout | 180 days | VEP and PERG parameters |
| Lanza et al., 2019 [80] | Case-control | OAG (n = 60) MD −6.51 dB | Oral 500 mg/day | 120 days 60 days of washout (4 cycles) | 2 years | SAP and OCT parameters |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandolfi, S.; Marchini, G.; Caporossi, A.; Scuderi, G.; Tomasso, L.; Brunoro, A. Cytidine 5?-Diphosphocholine (Citicoline): Evidence for a Neuroprotective Role in Glaucoma. Nutrients 2020, 12, 793. https://doi.org/10.3390/nu12030793
Gandolfi S, Marchini G, Caporossi A, Scuderi G, Tomasso L, Brunoro A. Cytidine 5?-Diphosphocholine (Citicoline): Evidence for a Neuroprotective Role in Glaucoma. Nutrients. 2020; 12(3):793. https://doi.org/10.3390/nu12030793
Chicago/Turabian StyleGandolfi, Stefano, Giorgio Marchini, Aldo Caporossi, Gianluca Scuderi, Livia Tomasso, and Andrea Brunoro. 2020. "Cytidine 5?-Diphosphocholine (Citicoline): Evidence for a Neuroprotective Role in Glaucoma" Nutrients 12, no. 3: 793. https://doi.org/10.3390/nu12030793
APA StyleGandolfi, S., Marchini, G., Caporossi, A., Scuderi, G., Tomasso, L., & Brunoro, A. (2020). Cytidine 5?-Diphosphocholine (Citicoline): Evidence for a Neuroprotective Role in Glaucoma. Nutrients, 12(3), 793. https://doi.org/10.3390/nu12030793





