Meal Patterns and Changes in Cardiometabolic Risk Factors in Children: A Longitudinal Analysis
Abstract
:1. Introduction
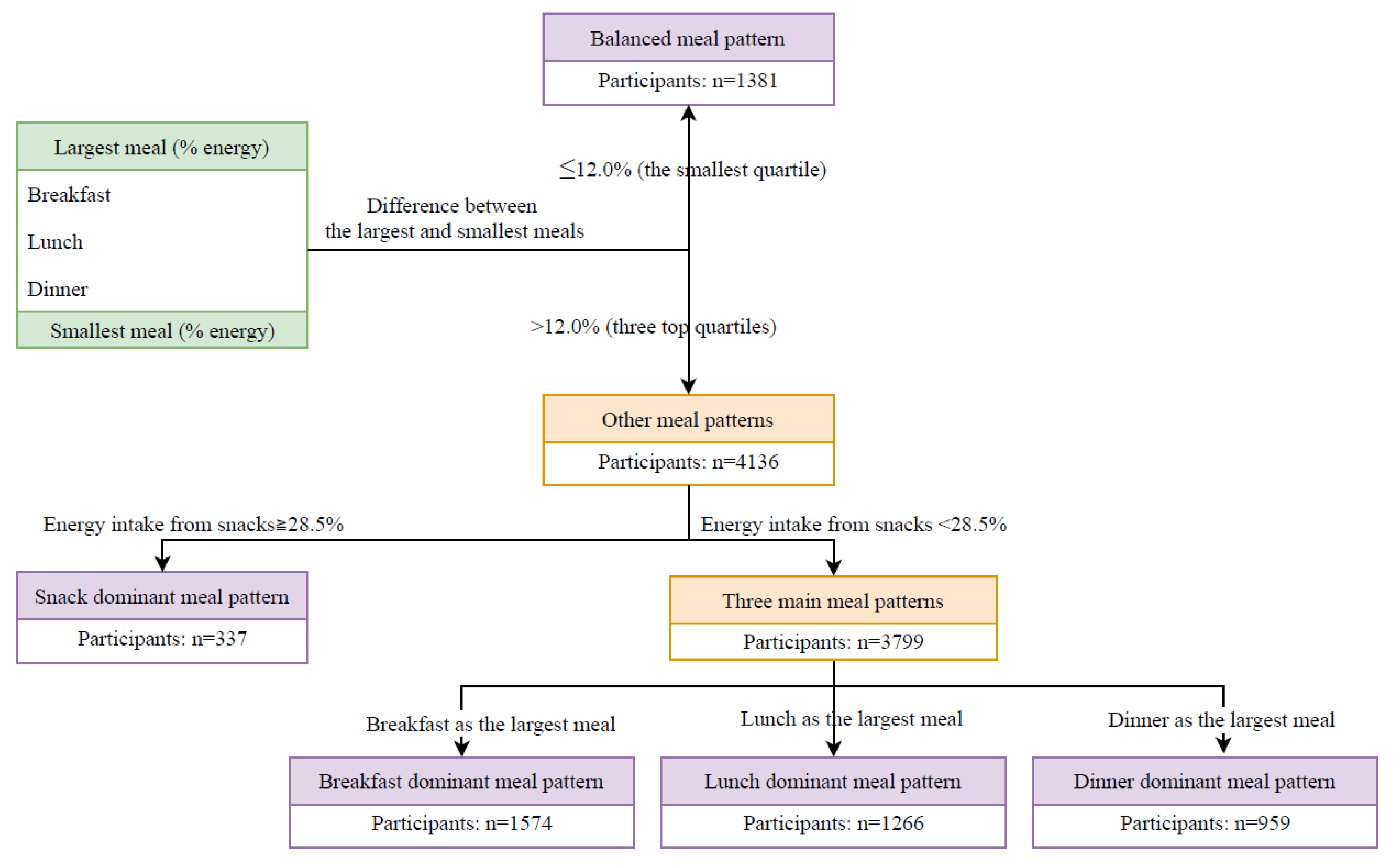
2. Materials and Methods
2.1. Participant Selection
2.2. Dietary Assessment
2.3. Confounders
2.4. Physical Examinations and Blood Tests
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
3.2. Energy and Macronutrients from Meals
3.3. Meal Patterns and Changes in CMR Factors
3.4. Energy Intake from Different Meals and Changes in CMRS
3.5. Macronutrients Intake at Different Meals and Changes in CMRS
3.6. Change in Macronutrients Intake at Different Meals and Changes in CMRS
3.7. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adair, L.S.; Gordon-Larsen, P.; Du, S.F.; Zhang, B.; Popkin, B.M. The emergence of cardiometabolic disease risk in Chinese children and adults: Consequences of changes in diet, physical activity and obesity. Obes. Rev. 2014, 15, 49–59. [Google Scholar] [CrossRef]
- Haffner, S.M. Relationship of metabolic risk factors and development of cardiovascular disease and diabetes. Obesity 2006, 14, 121–127. [Google Scholar] [CrossRef]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laitinen, T.T.; Pahkala, K.; Magnussen, C.G.; Viikari, J.S.; Oikonen, M.; Taittonen, L.; Mikkila, V.; Jokinen, E.; Hutri-Kahonen, N.; Laitinen, T.; et al. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: The cardiovascular risk in young finns study. Circulation 2012, 125, 1971–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, W.; Srinivasan, S.R.; Wattigney, W.A.; Berenson, G.S. Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood. The bogalusa heart study. Arch. Intern. Med. 1994, 154, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Berenson, G.S. Childhood risk factors predict adult risk associated with subclinical cardiovascular disease. The bogalusa heart study. Am. J. Cardiol. 2002, 90, 3l–7l. [Google Scholar] [CrossRef]
- Reilly, J.J.; Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int. J. Obes. 2011, 35, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Park, M.H.; Falconer, C.; Viner, R.M.; Kinra, S. The impact of childhood obesity on morbidity and mortality in adulthood: A systematic review. Obes. Rev. 2012, 13, 985–1000. [Google Scholar] [CrossRef]
- Ezzati, M.; Riboli, E. Behavioral and dietary risk factors for noncommunicable diseases. N. Engl. J. Med. 2013, 369, 954–964. [Google Scholar] [CrossRef] [Green Version]
- Lachat, C.; Otchere, S.; Roberfroid, D.; Abdulai, A.; Seret, F.M.; Milesevic, J.; Xuereb, G.; Candeias, V.; Kolsteren, P. Diet and physical activity for the prevention of noncommunicable diseases in low- and middle-income countries: A systematic policy review. PLoS Med. 2013, 10, e1001465. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.B.; Liu, Y.; Willett, W.C. Preventing chronic diseases by promoting healthy diet and lifestyle: Public policy implications for China. Obes. Rev. 2011, 12, 552–559. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K. Meal timing and frequency: Implications for cardiovascular disease prevention: A scientific statement from the american heart association. Circulation 2017, 135, 96–121. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Tinsley, G.; Bianco, A.; Moro, T. The influence of meal frequency and timing on health in humans: The role of fasting. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, M.P.; Allison, D.B.; Fontana, L.; Harvie, M.; Longo, V.D.; Malaisse, W.J.; Mosley, M.; Notterpek, L.; Ravussin, E.; Scheer, F.A.; et al. Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA 2014, 111, 16647–16653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Steffen, L.M.; Van Horn, L.; Ludwig, D.S.; Pereira, M.A. Breakfast frequency and development of metabolic risk. Diabetes Care 2013, 36, 3100–3106. [Google Scholar] [CrossRef] [Green Version]
- Hallstrom, L.; Labayen, I.; Ruiz, J.R.; Patterson, E.; Vereecken, C.A.; Breidenassel, C.; Gottrand, F.; Huybrechts, I.; Manios, Y.; Mistura, L.; et al. Breakfast consumption and CVD risk factors in European adolescents: The HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) study. Public Health Nutr. 2013, 16, 1296–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.J.; Gall, S.L.; McNaughton, S.A.; Blizzard, L.; Dwyer, T.; Venn, A.J. Skipping breakfast: Longitudinal associations with cardiometabolic risk factors in the Childhood Determinants of Adult Health Study. Am. J. Clin. Nutr. 2010, 92, 1316–1325. [Google Scholar] [CrossRef]
- Titan, S.M.; Bingham, S.; Welch, A.; Luben, R.; Oakes, S.; Day, N.; Khaw, K.T. Frequency of eating and concentrations of serum cholesterol in the Norfolk population of the European prospective investigation into cancer (EPIC-Norfolk): Cross sectional study. BMJ 2001, 323, 1286–1288. [Google Scholar] [CrossRef] [Green Version]
- Holmback, I.; Ericson, U.; Gullberg, B.; Wirfalt, E. A high eating frequency is associated with an overall healthy lifestyle in middle-aged men and women and reduced likelihood of general and central obesity in men. Br. J. Nutr. 2010, 104, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Mekary, R.A.; Giovannucci, E.; Willett, W.C.; van Dam, R.M.; Hu, F.B. Eating patterns and type 2 diabetes risk in men: Breakfast omission, eating frequency, and snacking. Am. J. Clin. Nutr. 2012, 95, 1182–1189. [Google Scholar] [CrossRef] [Green Version]
- Leech, R.M.; Timperio, A.; Livingstone, K.M.; Worsley, A.; McNaughton, S.A. Temporal eating patterns: Associations with nutrient intakes, diet quality, and measures of adiposity. Am. J. Clin. Nutr. 2017, 106, 1121–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahleova, H.; Lloren, J.I.; Mashchak, A.; Hill, M.; Fraser, G.E. Meal Frequency and timing are associated with changes in body mass index in Adventist Health Study 2. J. Nutr. 2017, 147, 1722–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapelot, D. The role of snacking in energy balance: A biobehavioral approach. J. Nutr. 2011, 141, 158–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanaley, J.A.; Heden, T.D.; Liu, Y.; Fairchild, T.J. Alteration of postprandial glucose and insulin concentrations with meal frequency and composition. Br. J. Nutr. 2014, 112, 1484–1493. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hu, X.; Zhang, Q.; Liu, A.; Fang, H.; Hao, L.; Duan, Y.; Xu, H.; Shang, X.; Ma, J.; et al. The nutrition-based comprehensive intervention study on childhood obesity in China (NISCOC): A randomised cluster controlled trial. BMC Public Health 2010, 10, 229. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, G.; Pan, X. China Food Composition; Peking Medical University: Beijing, China, 2009. [Google Scholar]
- Liu, A.L.; Ma, G.S.; Zhang, Q.; Ma, W.J. Reliability and validity of a 7-day physical activity questionnaire for elementary students. Zhonghua Liu Xing Bing Xue Za Zhi 2003, 24, 901–904. [Google Scholar]
- Deurenberg, P.; van der Kooy, K.; Leenen, R.; Weststrate, J.A.; Seidell, J.C. Sex and age specific prediction formulas for estimating body composition from bioelectrical impedance: A cross-validation study. Int. J. Obes. 1991, 15, 17–25. [Google Scholar]
- Eisenmann, J.C. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc. Diabetol. 2008, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Almoosawi, S.; Vingeliene, S.; Karagounis, L.G.; Pot, G.K. Chrono-nutrition: A review of current evidence from observational studies on global trends in time-of-day of energy intake and its association with obesity. Proc. Nutr. Soc. 2016, 75, 487–500. [Google Scholar] [CrossRef] [Green Version]
- Leidy, H.J.; Clifton, P.M.; Astrup, A.; Wycherley, T.P.; Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.D.; Woods, S.C.; Mattes, R.D. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 2015, 101, 1320–1329. [Google Scholar] [CrossRef] [Green Version]
- Almoosawi, S.; Prynne, C.J.; Hardy, R.; Stephen, A.M. Time-of-day and nutrient composition of eating occasions: Prospective association with the metabolic syndrome in the 1946 British birth cohort. Int. J. Obes. 2013, 37, 725–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paoli, A.; Bosco, G.; Camporesi, E.M.; Mangar, D. Ketosis, ketogenic diet and food intake control: A complex relationship. Front. Psychol. 2015, 6, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leidy, H.J.; Armstrong, C.L.; Tang, M.; Mattes, R.D.; Campbell, W.W. The influence of higher protein intake and greater eating frequency on appetite control in overweight and obese men. Obesity 2010, 18, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 1281–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huffman, S.L.; Harika, R.K.; Eilander, A.; Osendarp, S.J. Essential fats: How do they affect growth and development of infants and young children in developing countries? A literature review. Matern. Child Nutr. 2011, 7, 44–65. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]

| Meal Pattern | p-Value * | |||||
|---|---|---|---|---|---|---|
| Balanced | Breakfast Dominant | Lunch Dominant | Dinner Dominant | Snack Dominant | ||
| Age (years) | 9.54 ± 1.18 † | 9.54 ± 1.19 | 9.56 ± 1.18 | 9.59 ± 1.19 | 9.38 ± 1.17 | 0.66 |
| BMI (kg/m2) | 16.98 ± 3.09 | 17.15 ± 3.12 | 17.44 ± 3.24 | 17.34 ± 3.40 | 16.90 ± 2.94 | 0.0413 |
| WC (cm) | 57.88 ± 8.39 | 58.43 ± 8.88 | 59.23 ± 9.11 | 58.87 ± 9.14 | 56.92 ± 8.29 | 0.21 |
| PBF (%) | 23.65 ± 4.90 | 24.00 ± 4.70 | 24.24 ± 4.94 | 23.82 ± 4.92 | 23.87 ± 4.49 | 0.25 |
| SBP (mm Hg) | 100.04 ± 10.80 | 100.43 ± 10.79 | 100.89 ± 10.49 | 101.25 ± 11.59 | 101.26 ± 10.80 | 0.0026 |
| DBP (mm Hg) | 64.14 ± 8.95 | 64.01 ± 8.88 | 64.10 ± 8.97 | 64.56 ± 9.91 | 64.51 ± 8.40 | 0.22 |
| TC (mmol/L) | 4.11 ± 0.78 | 4.00 ± 0.77 | 4.12 ± 0.78 | 4.18 ± 0.83 | 3.92 ± 0.80 | 0.62 |
| HDL-C (mmol/L) | 1.50 ± 0.31 | 1.45 ± 0.30 | 1.46 ± 0.30 | 1.48 ± 0.31 | 1.45 ± 0.30 | 0.0917 |
| LDL-C (mmol/L) | 2.13 ± 0.60 | 2.04 ± 0.65 | 2.18 ± 0.62 | 2.21 ± 0.66 | 2.06 ± 0.64 | 0.0100 |
| TG (mmol/L) | 0.80 ± 0.45 | 0.80 ± 0.42 | 0.84 ± 0.43 | 0.84 ± 0.51 | 0.85 ± 0.46 | 0.0045 |
| Fasting glucose (mmol/L) | 4.55 ± 0.54 | 4.50 ± 0.58 | 4.52 ± 0.55 | 4.56 ± 0.54 | 4.36 ± 0.61 | 0.0196 |
| Log insulin | 1.64 ± 0.62 | 1.64 ± 0.58 | 1.66 ± 0.64 | 1.74 ± 0.60 | 1.54 ± 0.56 | 0.18 |
| Log HOMA-IR | −2.86 ± 0.66 | −2.87 ± 0.62 | −2.84 ± 0.68 | −2.75 ± 0.63 | −2.99 ± 0.62 | 0.32 |
| CMRS | −0.33 ± 2.37 | −0.26 ± 2.37 | −0.04 ± 2.38 | −0.22 ± 2.45 | −0.34 ± 2.48 | 0.22 |
| Physical activity (MET/week) | 597.96 ± 537.13 | 665.70 ± 577.08 | 624.26 ± 577.29 | 626.43 ± 605.05 | 613.57 ± 389.78 | 0.76 |
| Energy (kcal/day) | 1387.47 ± 584.22 | 1111.51 ± 535.18 | 1260.94 ± 570.12 | 1360.68 ± 598.81 | 1361.39 ± 644.53 | 0.11 |
| Protein intake (g/100 Kcal/day) | 4.31 ± 1.04 | 4.30 ± 1.06 | 4.39 ± 1.17 | 4.54 ± 1.22 | 3.90 ± 1.04 | 0.62 |
| Fat intake (g/100 Kcal/day) | 2.93 ± 1.11 | 2.93 ± 1.07 | 2.86 ± 1.19 | 2.99 ± 1.25 | 3.36 ± 1.08 | 0.0002 |
| Carbohydrate intake (g/100 Kcal/day) | 14.25 ± 2.80 | 14.28 ± 2.76 | 14.30 ± 3.13 | 13.88 ± 3.16 | 13.70 ± 2.74 | 0.0003 |
| Fibre intake (g/100 Kcal/day) | 0.52 ± 0.30 | 0.51 ± 0.30 | 0.55 ± 0.37 | 0.54 ± 0.38 | 0.48 ± 0.24 | 0.70 |
| Vitamin C intake (mg/100 Kcal/day) | 3.21 ± 2.51 | 3.16 ± 2.55 | 3.24 ± 2.63 | 3.43 ± 2.83 | 3.36 ± 2.51 | 0.0317 |
| Vitamin E intake (mg/100 Kcal/day) | 0.26 ± 0.17 | 0.28 ± 0.19 | 0.25 ± 0.16 | 0.26 ± 0.17 | 0.36 ± 0.35 | 0.0034 |
| Carotene intake (ug/100 Kcal/day) | 76.54 ± 74.69 | 73.63 ± 81.28 | 75.26 ± 86.98 | 81.69 ± 95.16 | 75.02 ± 71.97 | 0.31 |
| Magnesium intake (mg/100 Kcal/day) | 14.78 ± 3.43 | 14.96 ± 3.54 | 15.15 ± 3.92 | 15.09 ± 4.16 | 14.47 ± 3.68 | 0.42 |
| Potassium intake (mg/100 Kcal/day) | 99.25 ± 29.71 | 100.61 ± 30.67 | 98.93 ± 32.49 | 101.62 ± 35.67 | 103.58 ± 31.28 | 0.0453 |
| Phosphorus intake (mg/100 Kcal/day) | 60.66 ± 12.77 | 62.48 ± 13.14 | 61.06 ± 13.46 | 62.58 ± 14.55 | 57.38 ± 13.74 | 0.32 |
| Calcium intake (mg/100 Kcal/day) | 30.29 ± 13.97 | 32.76 ± 16.83 | 27.43 ± 13.46 | 27.95 ± 13.82 | 34.97 ± 18.91 | 0.0203 |
| Iron intake (mg/100 Kcal/day) | 1.22 ± 0.68 | 1.12 ± 0.55 | 1.29 ± 0.89 | 1.21 ± 0.63 | 1.05 ± 0.50 | 0.50 |
| Sex | 0.83 | |||||
| Boys | 668 (48.4) ‡ | 791 (50.3) | 628 (49.6) | 481 (50.2) | 152 (45.1) | |
| Girls | 713 (51.6) | 783 (49.7) | 638 (50.4) | 478 (49.8) | 185 (54.9) | |
| Grade | 0.60 | |||||
| Two | 390 (28.2) | 442 (28.1) | 363 (28.7) | 260 (27.1) | 111 (32.9) | |
| Three | 373 (27.0) | 475 (30.2) | 301 (23.8) | 253 (26.4) | 101 (30.0) | |
| Four | 365 (26.4) | 422 (26.8) | 377 (29.8) | 260 (27.1) | 90 (26.7) | |
| Five | 253 (18.3) | 235 (14.9) | 225 (17.8) | 186 (19.4) | 35 (10.4) | |
| Puberty | 0.07 | |||||
| Yes | 1275 (92.3) | 1470 (93.4) | 1165 (92.0) | 862 (89.9) | 312 (92.6) | |
| No | 106 (7.7) | 104 (6.6) | 101 (8.0) | 97 (10.1) | 25 (7.4) | |
| Meal Pattern | p-Value * | |||||
|---|---|---|---|---|---|---|
| Balanced | Breakfast Dominant | Lunch Dominant | Dinner Dominant | Snack Dominant | ||
| Change in BMI | ||||||
| Participants | 1367 | 1553 | 1249 | 945 | 334 | |
| β (95% CI) † | 0.032 (−0.0114, 0.076) | 0.039 (−0.006, 0.084) | 0.003 (−0.045, 0.052) | 0.010 (−0.061, 0.081) | 0.37 | |
| Change in WC | ||||||
| Participants | 1360 | 1549 | 1245 | 945 | 334 | |
| β (95% CI) | −0.012 (−0.046, 0.022) | 0.032 (−0.003, 0.067) | −0.006 (−0.044, 0.032) | 0.012 (−0.043, 0.068) | 0.13 | |
| Change in PBF | ||||||
| Participants | 1337 | 1510 | 1209 | 915 | 326 | |
| β (95% CI) | 0.042 (−0.011, 0.095) | 0.012 (−0.043, 0.067) | −0.035 (−0.094, 0.024) | −0.069 (−0.156, 0.018) | 0.0320 | |
| Change in SBP | ||||||
| Participants | 1361 | 1551 | 1246 | 942 | 333 | |
| β (95% CI) | 0.054 (−0.015, 0.123) | 0.046 (−0.025, 0.117) | 0.057 (−0.019, 0.133) | −0.060 (−0.173, 0.052) | 0.17 | |
| Change in DBP | ||||||
| Participants | 1363 | 1552 | 1248 | 943 | 334 | |
| β (95% CI) | 0.023 (−0.047, 0.094) | 0.007 (−0.066, 0.079) | −0.013 (−0.092, 0.065) | −0.057 (−0.172, 0.058) | 0.68 | |
| Change in MAP | ||||||
| Participants | 1361 | 1550 | 1246 | 943 | 334 | |
| β (95% CI) | 0.038 (−0.032, 0.108) | 0.026 (−0.046, 0.098) | 0.016 (−0.061, 0.094) | −0.053 (−0.167, 0.061) | 0.56 | |
| Change in TC | ||||||
| Participants | 1283 | 1460 | 1175 | 892 | 316 | |
| β (95% CI) | −0.062 (−0.115, −0.008) | 0.001 (−0.054, 0.056) | −0.025 (−0.084, 0.035) | 0.043 (−0.044, 0.130) | 0.0513 | |
| Change in HDL-C | ||||||
| Participants | 1284 | 1459 | 1175 | 891 | 314 | |
| β (95% CI) | 0.027 (−0.049, 0.103) | −0.066 (−0.144, 0.012) | −0.027 (−0.1107, 0.057) | 0.270 (0.146, 0.393) | <0.0001 | |
| Change in LDL-C | ||||||
| Participants | 1284 | 1461 | 1176 | 891 | 316 | |
| β (95% CI) | −0.051 (−0.108, 0.006) | −0.063 (−0.122, −0.004) | −0.037 (−0.100, 0.026) | 0.004 (−0.089, 0.096) | 0.21 | |
| Change in TG | ||||||
| Participants | 1282 | 1461 | 1176 | 894 | 317 | |
| β (95% CI) | −0.029 (−0.098, 0.039) | 0.001 (−0.070, 0.071) | 0.017 (−0.059, 0.093) | −0.193 (−0.304, −0.082) | 0.0075 | |
| Change in fasting glucose | ||||||
| Participants | 1284 | 1460 | 1176 | 892 | 317 | |
| β (95% CI) | 0.030 (−0.028, 0.088) | −0.047 (−0.107, 0.013) | 0.015 (−0.050, 0.079) | 0.040 (−0.054, 0.135) | 0.11 | |
| Change in insulin | ||||||
| Participants | 1132 | 1278 | 1035 | 795 | 273 | |
| β (95% CI) | −0.048 (−0.156, 0.059) | −0.055 (−0.166, 0.055) | 0.119 (−0.0001, 0.237) | −0.125 (−0.300, 0.051) | 0.0181 | |
| Change in HOMA-IR | ||||||
| Participants | 1132 | 1277 | 1034 | 795 | 273 | |
| β (95% CI) | −0.036 (−0.142, 0.069) | −0.069 (−0.177, 0.039) | 0.114 (−0.002, 0.230) | −0.104 (−0.275, 0.068) | 0.0199 | |
| Change in CMRS ‡ | ||||||
| Participants | 1179 | 1331 | 1066 | 798 | 300 | |
| β (95% CI) | 0.059 (−0.107, 0.225) | 0.113 (−0.058, 0.284) | 0.079 (−0.105, 0.264) | −0.324 (−0.590, −0.058) | 0.031 | |
| Consumption Level | p-Trend† | |||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Energy from breakfast | ||||||
| Range (%) | <21.06 | 21.06–27.39 | 27.40–33.40 | 33.41–41.67 | >41.67 | |
| Participants | 940 | 930 | 933 | 925 | 946 | |
| β (95% CI), Model 1 ‡ | 0.019 (−0.179, 0.216) | 0.036 (−0.161, 0.234) | 0.193 (−0.006, 0.391) | 0.042 (−0.158, 0.242) | 0.32 | |
| β (95% CI), Model 2 § | 0.041 (−0.147, 0.228) | 0.026 (−0.162, 0.213) | 0.202 (0.013, 0.391) | 0.049 (−0.144, 0.241) | 0.24 | |
| β (95% CI), Model 3 ¶ | 0.044 (−0.143, 0.232) | 0.023 (−0.164, 0.210) | 0.189 (0.0002, 0.377) | 0.043 (−0.149, 0.236) | 0.31 | |
| Energy from lunch | ||||||
| Range (%) | <20.81 | 20.81–27.07 | 27.08–32.59 | 32.60–39.09 | >39.09 | |
| Participants | 954 | 942 | 920 | 932 | 926 | |
| β (95% CI), Model 1 | 0.037 (−0.161, 0.235) | 0.080 (−0.122, 0.283) | 0.135 (−0.068, 0.338) | 0.248 (0.044, 0.452) | 0.14 | |
| β (95% CI), Model 2 | 0.016 (−0.172, 0.203) | 0.100 (−0.091, 0.292) | 0.138 (−0.054, 0.330) | 0.194 (0.0002, 0.387) | 0.24 | |
| β (95% CI), Model 3 | 0.035 (−0.152, 0.223) | 0.091 (−0.1001, 0.283) | 0.143 (−0.049, 0.335) | 0.173 (−0.021, 0.367) | 0.38 | |
| Energy from dinner | ||||||
| Range (%) | <18.08 | 18.09–24.31 | 24.32–29.88 | 29.89–36.64 | >36.64 | |
| Participants | 957 | 943 | 913 | 934 | 927 | |
| β (95% CI), Model 1 | 0.185 (−0.012, 0.381) | 0.167 (−0.032, 0.367) | −0.027 (−0.225, 0.172) | 0.015 (−0.184, 0.213) | 0.10 | |
| β (95% CI), Model 2 | 0.128 (−0.058, 0.314) | 0.126 (−0.063, 0.315) | −0.049 (−0.237, 0.139) | 0.010 (−0.179, 0.199) | 0.23 | |
| β (95% CI), Model 3 | 0.136 (−0.051, 0.322) | 0.145 (−0.045, 0.335) | −0.029 (−0.218, 0.159) | 0.016 (−0.173, 0.205) | 0.22 | |
| Energy from snacks | ||||||
| Range (%) | 0 | 0–2.47 | 2.48–8.32 | 8.33–19.03 | >19.03 | |
| Participants | 1399 | 457 | 923 | 944 | 951 | |
| β (95% CI), Model 1 | −0.069 (−0.303, 0.164) | −0.037 (−0.219, 0.145) | −0.158 (−0.340, 0.024) | −0.101 (−0.284, 0.082) | 0.51 | |
| β (95% CI), Model 2 | −0.003 (−0.224, 0.2184) | −0.0140 (−0.1872, 0.1593) | −0.1305 (−0.3058, 0.0448) | −0.0827 (−0.2700, 0.1046) | 0.61 | |
| β (95% CI), Model 3 | 0.018 (−0.204, 0.239) | −0.010 (−0.183, 0.164) | −0.102 (−0.277, 0.074) | −0.064 (−0.251, 0.124) | 0.77 | |
| Consumption Level | p-Trend† | |||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Carbohydrate at breakfast | ||||||
| Range (% energy) | <47.54 | 47.54–56.39 | 56.40–63.19 | 63.20–72.05 | >72.05 | |
| Participants | 948 | 924 | 920 | 946 | 936 | |
| β (95% CI) ‡ | −0.032 (−0.228, 0.163) | −0.128 (−0.337, 0.080) | −0.194 (−0.419, 0.030) | −0.054 (−0.310, 0.202) | 0.31 | |
| Carbohydrate at lunch | ||||||
| Range (% energy) | <41.24 | 41.24–52.63 | 52.63–62.15 | 62.16–72.16 | >72.16 | |
| Participants | 923 | 920 | 922 | 938 | 971 | |
| β (95% CI) | 0.217 (0.018, 0.416) | 0.315 (0.098, 0.531) | 0.603 (0.368, 0.837) | 0.777 (0.509, 1.046) | <0.0001 | |
| Carbohydrate at dinner | ||||||
| Range (% energy) | <36.61 | 36.61–49.79 | 49.80–60.70 | 60.71–72.70 | >72.70 | |
| Participants | 927 | 927 | 939 | 944 | 937 | |
| β (95% CI) | 0.345 (0.145, 0.545) | 0.601 (0.383, 0.818) | 0.662 (0.428, 0.907) | 0.663 (0.387, 0.938) | <0.0001 | |
| Carbohydrate from snacks | ||||||
| Range (% energy) | 0–41.12 | 41.13–63.92 | 63.93–82.98 | >82.98 | ||
| Participants | 1393 | 469 | 922 | 961 | 929 | |
| β (95% CI) | 0.048 (−0.174, 0.269) | −0.108 (−0.287, 0.072) | −0.117 (−0.294, 0.060) | 0.077 (−0.097, 0.251) | 0.18 | |
| Protein at breakfast | ||||||
| Range (% energy) | <11.83 | 11.83–13.77 | 13.78–15.76 | 15.77–18.61 | >18.61 | |
| Participants | 954 | 919 | 947 | 929 | 925 | |
| β (95% CI) | 0.010 (−0.177, 0.196) | 0.026 (−0.169, 0.220) | 0.048 (−0.161, 0.257) | −0.165 (−0.417, 0.086) | 0.30 | |
| Protein at lunch | ||||||
| Range (% energy) | <12.78 | 12.78–15.84 | 15.85–19.14 | 19.15–23.92 | >23.92 | |
| Participants | 959 | 960 | 931 | 921 | 903 | |
| β (95% CI) | 0.025 (−0.162, 0.213) | 0.143 (−0.054, 0.339) | −0.263 (−0.474, −0.052) | −0.4632 (−0.710, −0.217) | <0.0001 | |
| Protein at dinner | ||||||
| Range (% energy) | <13.01 | 13.01–16.86 | 16.87–20.75 | 20.76–26.47 | >26.47 | |
| Participants | 933 | 934 | 933 | 946 | 928 | |
| β (95% CI) | 0.080 (−0.114, 0.273) | −0.048 (−0.248, 0.153) | 0.102 (−0.111, 0.314) | −0.360 (−0.602, −0.117) | <0.0001 | |
| Protein from snacks | ||||||
| Range (% energy) | 0 | 0–3.95 | 3.96–8.14 | 8.15–12.40 | >12.40 | |
| Participants | 1412 | 457 | 921 | 940 | 944 | |
| β (95% CI) | −0.041 (−0.263, 0.180) | −0.043 (−0.219, 0.134) | −0.144 (−0.320, 0.033) | 0.064 (−0.113, 0.241) | 0.27 | |
| Fat at breakfast | ||||||
| Range (% energy) | <14.23 | 14.23–21.35 | 21.35–27.59 | 27.60–35.20 | >35.20 | |
| Participants | 935 | 940 | 925 | 922 | 952 | |
| β (95% CI) | −0.106 (−0.296, 0.085) | −0.019 (−0.221, 0.183) | −0.030 (−0.243, 0.183) | 0.086 (−0.158, 0.329) | 0.39 | |
| Fat at lunch | ||||||
| Range (% energy) | <11.13 | 11.13–18.98 | 18.99–26.27 | 26.28–35.87 | >35.87 | |
| Participants | 976 | 925 | 923 | 916 | 934 | |
| β (95% CI) | −0.260 (−0.453, −0.067) | −0.296 (−0.504, −0.089) | −0.507 (−0.726, −0.289) | −0.441 (−0.685, −0.197) | 0.0003 | |
| Fat at dinner | ||||||
| Range (% energy) | <9.75 | 9.75–17.95 | 17.96–26.72 | 26.73–37.30 | >37.30 | |
| Participants | 947 | 940 | 930 | 922 | 935 | |
| β (95% CI) | 0.169 (−0.027, 0.365) | 0.110 (−0.101, 0.321) | −0.017 (−0.244, 0.210) | −0.146 (−0.400, 0.109) | 0.10 | |
| Fat from snacks | ||||||
| Range (% energy) | 0–3.26 | 3.27–16.78 | 16.79–33.34 | >33.34 | ||
| Participants | 1426 | 443 | 926 | 939 | 940 | |
| β (95% CI) | 0.040 (−0.184, 0.264) | 0.006 (−0.168, 0.180) | −0.029 (−0.206, 0.149) | −0.068 (−0.244, 0.107) | 0.89 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, X.; Li, Y.; Xu, H.; Zhang, Q.; Liu, A.; Du, S.; Ma, G. Meal Patterns and Changes in Cardiometabolic Risk Factors in Children: A Longitudinal Analysis. Nutrients 2020, 12, 799. https://doi.org/10.3390/nu12030799
Shang X, Li Y, Xu H, Zhang Q, Liu A, Du S, Ma G. Meal Patterns and Changes in Cardiometabolic Risk Factors in Children: A Longitudinal Analysis. Nutrients. 2020; 12(3):799. https://doi.org/10.3390/nu12030799
Chicago/Turabian StyleShang, Xianwen, Yanping Li, Haiquan Xu, Qian Zhang, Ailing Liu, Songming Du, and Guansheng Ma. 2020. "Meal Patterns and Changes in Cardiometabolic Risk Factors in Children: A Longitudinal Analysis" Nutrients 12, no. 3: 799. https://doi.org/10.3390/nu12030799





