1. Introduction
Obesity is a serious problem that affects human health leading to several diseases including, diabetes mellitus, hypertension, and cardiovascular diseases [
1]. The World Health Organization (WHO) has recognized that the high blood cholesterol is the main cause of cardiovascular diseases worldwide leading to death of about 4.4 million people each year. The main character of hyperlipidemia is the elevated levels of serum low density lipoprotein, very low density lipoprotein, and reduced level of high density lipoprotein [
2]. Consumption of human and rodents’ high fat diet induces obesity and hyperlipidemia associated with altered plasma and tissues cholesterol and triacylglycerol levels that promote the risk of coronary heart disease, fatty liver, and carcinogenesis [
3]. Liver plays vital roles in lipid metabolism and disturbance in lipid metabolism due to high access of fat to the liver, lower mobilization of fat from liver or defect in the structure and/or function of lipoproteins leads accumulation of fat in the liver, which subsequently induced hepatomegaly, changes in the shape and colors of the liver. It is well documented that HFD induced liver disturbances including hepatomegaly [
4,
5].
The development and progression of obesity, dyslipidemia and diabetes mellitus type 2 are regulated by some proteins and cytokines such as leptin, adiponectin and uncoupling protein 1. Leptin is an adipocyte derived hormone secreted from adipocyte and plays an important role in amelioration of complications and pathogenesis of obesity [
6,
7] as it acts on the hypothalamus to control appetite, food intake, energy metabolism, and sympathetic nervous system outflow [
7,
8]. Adiponectin is an adipocyte derived hormone, whose plasma concentrations are decreased in obese and type 2 diabetes mellitus subjects. It has anti-atherogenic and anti-inflammatory properties [
9]. Over nutrition leads to hypoadiponectinemia and increased TNF-α that enhanced insulin resistance [
10]. Adiponectin improves diabetic mice through enhancement of free fatty acid (FFA) oxidation in muscle, clearance of plasma FFA in HFD [
11] and decreases glucose release from hepatocyte [
12]. Uncoupling protein 1 (UCP1) is implicated in thermogenesis, energy expenditure, and reduction of oxidative stress that associated with the progress of obesity and diabetes mellitus type 2 (DM2) [
13]. In addition, UCP1 has a role in regulation of cold and diet-induced thermogenesis, metabolic and energy balance and reducing the mitochondrial production of reactive oxygen species (ROS) that involved in the pathogenesis and progression of obesity and/or DM2 [
14].
Treatment of obesity and dyslipidemia by synthetic chemical drugs causes several adverse side effects; therefore, it is of great interest to look for alternative safe natural agents. Medicinal plants contain various bioactive substances such as phytosterols, diterpenes, triterpenes, and polyphenolic compounds that enable them to prevent and treat many disorders [
2,
15].
Commiphora myrrha (CM) (family Burseraceae) is a small tropical tree that is widely distributed in East Africa, Arabia and India [
16]. Myrrh is a resinous exudate obtained from the trunk of CM trees [
17].
Commiphora myrrha contains many bioactive substances thus; it was used to treat a variety of illnesses such as obesity and lipid disorders [
18]. In addition, it has anti-hyperglycemic, antioxidant [
19], hepatoprotective [
20], analgesic, anti-inflammatory [
21], hypolipidemic [
22], and cholesterol lowering activities through inhibiting LDL oxidation [
23]. Studies concerning the anti-obesity activity of CM extract still scarce to the best of our knowledge. Therefore, this study was performed to evaluate the preventive and curative effects of CM resin alcoholic extract against HFD induced obesity and dyslipidemia with the respect to its impact on the expression of obesity development related cytokines, leptin, adiponectin and UCP1 in rats.
4. Discussion
Obesity and dyslipidemia are metabolic disorders that represent a big problem on human health that increase the risk of many diseases such as cardiovascular disease, diabetes mellitus and hypertension [
1]. However,
Commiphora myrrha has antioxidant, hypoglycemic, hypolipidemic and anti-diabetic activities [
37].
The results of our study revealed that feeding rats HFD increased food intake, liver and body weights and induced hyperglycemia, dyslipidemia, hyperketonemia, hypoleptinemia, hypoadiponectinemia and oxidative stress in hepatic tissue accompanied with alteration of liver structure and function and decreased protein expression of UCP1 in brown adipose tissue. This increase in body weight may attribute to the increase of food intake, which in turn enhanced excess energy and buildup of adiposity because HFD consumption favors more fat storage than fat oxidation in muscle [
38]. The other possible cause of HFD induced increased body weights, hyperglycemia and dyslipidemia in the current study is the reduction of leptin, adiponectin and UCP1 protein expression, which play important roles in the regulation of food intake, insulin sensitivity and energy metabolism. Leptin plays an important role in the control of appetite [
39] through its action on the hypothalamus to control appetite, food intake, energy metabolism and outflow of the sympathetic nervous system [
40]. Hence, impairment of its secretion and/or action results in weight gain by sending an inappropriate signal to the brain, which consequently reduced satiety response. The leptin secretion and circulating leptin level are manipulated by the kind of diet as reduced carbohydrate not fat intake in obese human subjects is associated with lower leptin concentration [
41]. In addition, feeding rats HFD for 4 to 14 weeks reduces leptin secretion leading to increased body weight gain [
42]. The possible reasons behind HFD reduced leptin serum levels may be HFD decreased insulin-mediated glucose metabolism in adipocytes, which associated with lower leptin expression and secretion from adipocytes [
43,
44] because HFD induced lipolysis increases the expression of peroxisome proliferator–activated receptor gamma [
45], which reduces the expression of leptin in adipose tissue [
46].
Adiponectin has anti-atherogenic and anti-inflammatory and insulin sensitizing properties and its plasma concentrations are decreased in obese individuals and type 2 diabetic mellitus (DM2) [
9,
47]. UCP1 is an important gene that controls the development of obesity and DM2 [
13]. As it plays a great role in the regulation of cold and diet induced thermogenesis, metabolic and energy balance and decreasing reactive oxygen species (ROS) production, which implicated in the pathogenesis of obesity and/or DM2 [
14]. Several studies stated that there was a relation between reduced expression of UCP1 in adipose tissue of obese subjects and the polymorphism of the 3826G allele of UCP1 gene [
48], which in turn associated with the obesity or other obesity disorders as DM2 [
49]. Therefore, HFD may contribute to weight gain by reducing leptin, adiponectin and UCP1 production.
The dyslipidemic effect of HFD in the current study may due to the consumption of HFD alters lipid profile [
38] through increasing fatty acid delivery to the liver that increases TG synthesis accompanied by an increase of VLDL synthesis [
50]. Moreover, HFD up regulates lipogenesis pathway [
51] leading to increases of the production of triglyceride and total cholesterol, which in turn enhanced insulin resistance and glucose intolerance [
52] and consequently hepatomegaly.
The HFD hyperketonemia and hepatic dysfunction may due to consumption of HFD increases serum level of ketone bodies and serum activities of ALT and AST [
4]. Obesity and overweight were correlated with a decrease in insulin sensitivity and development of diabetes mellitus type-2, which in turn increase ALT activity [
53]. The hyperglycemic and dyslipidemic effects of HFD in the current study may induce oxidative stress and alter the antioxidant enzyme system in obese rats, which represented by elevated level of lipid peroxidation biomarker, MDA, and reduced activity of GR. Glutathione reductase catalyzes the conversion of oxidized glutathione to reduced glutathione (GSH) in the hepatic tissues of obese rats. Reduced glutathione acts as antioxidant, involved in transport of amino acid, and it is a substrate for glutathione peroxidases and glutathione s-transferases, which responsible for organic peroxide detoxification and xenobiotics metabolism respectively [
54]. High fat diet induced oxidative stress in our study led to pathological changes in the hepatic tissues of HFD fed rats due to consumption of HFD induces diffuse and extensive microvesicular steatosis with great enlargement of hepatocyte and presence of intracytoplasmic acidophilic globular body [
4].
On the contrast, administration of rats fed HFD with CME ameliorated HFD increased food intake, body weight gain and livers weights in rats fed. This finding may be attributed to decreased food intake (
Figure 2). This decrease in food intake led to low caloric intake, low energy excess and decrease buildup of adiposity. These findings were parallel with those of [
21] who demonstrated that administration of obese rats with CM decreases body gain. These decreases in food intake and body weight gain may due to CME normalized serum leptin levels, which control food via its action on the satiety center in the brain and subsequently regulates body weight gain [
55].
Commiphora myrrha extract normalized serum leptin levels through different possible pathways such as increasing UCP1 expression in brown adipose tissue, which subsequently increased energy expenditure and reduced obesity development which reduced leptin synthesis and secretion by adipocytes [
43,
44]. In addition, CME increased insulin mediated glucose metabolism, which increased leptin synthesis and secretion by adipocytes [
43,
44].
Commiphora myrrha extract reversed the effect of HFD on UCP1 protein expression in brown adipose tissue may due to its content of retinol (vitamin A), which up regulates UCP1 gene expression in brown adipose tissue. [
56]. This explanation can be confirmed by the finding of [
57] who indicated that feeding obese rats’ vitamin A reduces body weight gain, adiposity index, and retroperitoneal white adipose tissue mass. In addition, guggulsterone (Myrrha sterols) acts as an anti-obesity agent as it decreases adipogenesis through preventing the differentiation of preadipocytes into mature adipocytes [
58] and decreases food intake and body weight gain in rats fed HFD due to it reduces the plasma ghrelin, glucose, triglyceride levels and increased plasma leptin, serotonin, dopamine levels [
59]. Moreover, it up-regulates the expression of a thermogenic marker, UCP1, in mature adipocytes. The hypocholestermic effect of CME may be related to the inhibition of cholesterol synthesis via antagonism of foresenoid X receptor and bile receptor [
60] as Guggulsterone is structurally similar to bile acids [
61] and through its effect on UCP1 expression. Thus, Guggulsterone acts as anti-obesity agent via reducing adipogenesis, increasing lipolysis in white adipose tissue and inducing trans-differentiation of white adipocytes into beige that decrease body weight. Furthermore, Ref. [
62] indicated that guggulsterone enhances mitochondrial density, biogenesis of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) and peroxisome proliferator-activated receptor-γ (PPARγ), and increased expression beige adipocyte phenotype markers, UCP1, t-box transcription factor 1 (TBX1), and β-3 adrenergic receptor (β-3AR). The anti-obesity effect of adiponectin may be attributed to the activation of AMPK increasing fatty acid oxidation and reduction of serum glucose [
12,
63]. From the above mentioned findings, it has become clear that the reduced protein expression of leptin, adiponectin and UCP1 is one the most of important possible causes that increased body weight; hyperglycemia and dyslipidemia in HFD fed rats that were restored by administration of HFD fed rats with CME. The hypoglycemic effect of CME in our study confirmed the findings of [
18,
19] that indicated the administration of myrrh extract decreases the fasting blood glucose in diabetic rats. This hypoglycemic effect of CME may be attributed to the presence of germacrene B and benzoquinone compounds in CME (
Table 3) as [
64] stated that administration of rats with Embelin, 1,4-benzoquinone, lowered blood glucose and serum insulin levels. In addition, CME contains anti-hyperglycemic compounds, furanoeudesma-1,3-diene and 2-O-acetyl-8,12-epoxygermacra-1 [
37] and has stimulatory effect on β cells division and insulin secretion [
18,
65]. Benzofuranebisindole hybrids have been shown to have in vitro antioxidant effect and in vivo anti-dyslipidemic activity as it decreases total cholesterol, triglycerides and phospholipids through increasing the plasma lecithin cholesterol acyltransferase activity, which plays a key role in lipoprotein metabolism increasing HDL-C in serum level [
66].
The demonstrated hypolipidemic effect of CME in our study confirmed the findings of the previous studies, which found that administration of rats fed high fructose diet with Commiphora Mukul decreases lipid profiles and the atherogenic index [
19] and Commiphora Molmol extract decreases lipid profile and atherogenic index in obese rats [
21] The hypocholesteremic effect might due to the presence of hexadecanoic acid, 9 and 12-octadecenoic acids in CME (
Table 3), which have been proven to have inhibit the activity of pancreatic lipase suppressing lipid digestion and thereby diminishing entry of lipids into the body [
67]. In addition, CME contains germacrene B, sesquiterpene (
Table 3), which have a hypolipidemic effect [
68].
The other possible reason behind the anti-obesity effect of CME in our study is its antioxidant activity as our results revealed that administration of rats fed HFD with CME alleviated HFD induced oxidative stress through decreasing lipid peroxidation and enhancing the antioxidant enzyme activity of hepatic tissues (
Table 6) and it has been reported that administration of high fructose diet fed rats with Commiphora Mukul decreases MDA levels while increases GR activity that preserving GSH contents in hepatic tissues [
19]. These results may be attributed to the presence of certain bioactive compounds in CME that have antioxidant effect such as 11, 14-eicosadienoic acid, 9, 12-octadecenoic acid methy ester, germacrene B and isochiapin B (
Table 3). These findings were in line with [
69] as he found that
Centaurea centaurium L. methanolic root extract has potent antioxidant properties due to the presence fatty acids: 11,14-eicosadienoic acid methyl ester, 9-octadecenoic acid and terpenes such as β-bisabolene, β and [
70] indicated that Juniperus phoenicea has powerful anti-diabetic, anti-obesity and antioxidant activities due to it contains Germacrene B and other antioxidants. Moreover, Ref. [
71] showed that elemene administration to atherosclerotic rabbit decreases obesity via reduction of the infiltration of macrophage, also reduction of the levels of TC, TG, and LDL-C and the inflammatory factors as TNF-Į and IL-6 levels in vitro. Ref. [
72] showed that the inhibitory effect of elemene on atherosclerosis mediated by reduction of lipid peroxidation, enhancement of anti-oxidant defense system, and suppression of inflammatory chemokine expression in atherosclerotic mice induced by HFD for 16 weeks. In addition, 1-heptatriacotanol is one of the most effective components in CM, as [
73] reported that Basella alba leaf extract, which contain1-heptatriacotanol has hypocholesterolemic effect through inhibition of HMG-CoA reductase enzyme. These antioxidant activities of CME protected hepatic tissues of HFD fed rats (
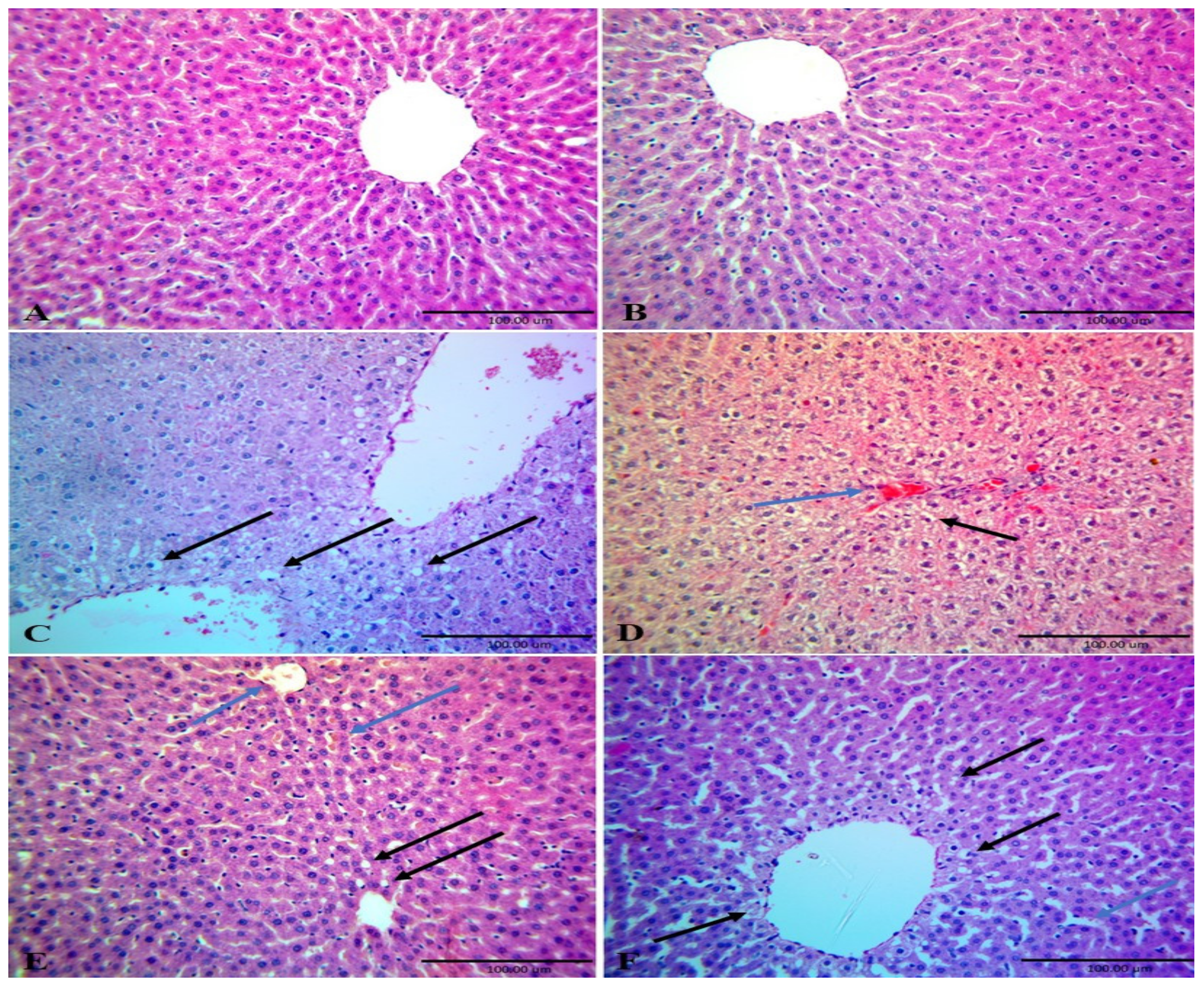
Figure 4). That antioxidant property of CME ameliorated the detrimental effects of HFD on the liver structure and function as it has been found that treatment of rats fed high fructose diet with CM improves liver tissues structure and induces regeneration of hepatocyte around central vein [
20]. The protective effects of CME on hepatocytes may be related to its phytochemical constituents, sesquiterpene, reynosin, bisabolene, elemene and curezerine (benzofuran) compounds in CME, which have antioxidant and hepatoprotective effects as [
20] indicated the presence of curezerine, delta-elemene, beta-elemene, 3-[(E)-2-phenyl-1-propenyl] cyc, bicycle [3.1.1] hept-2-ene-2-car, 8-isopropenyl-9-isopropyltetra, and 2,4-bis (3-methyl-1-pentynyl)-4 in CME. These compounds were demonstrated to have cytoprotective, antioxidant and anticancer effects [
74,
75]. The protective effects of CME on hepatic tissue improved liver function as our studies and [
18,
20] demonstrated that treatment of animals with CME reduced serum activities of AST and ALT. These results may due to the presence of reynosin compounds in CME (
Table 3) as reynosin has been shown to inhibit thioacetamide induced apoptosis and hepatocellular DNA damage through decreasing mRNA expression of proapoptotic Bax and increasing mRNA expression of antiapoptotic Bcl-2, Bcl-XL in primary rat hepatocytedecreases and reduced the activities of serum AST and ALT in thioacetamide intoxicated mice [
75]. Taken together from the aforementioned discussion it can be postulated that CME reduced HFD induced dyslipidemia and increased body weight gain through different pathways such as up-regulation of leptin, UCP1 and adiponectin expression and antioxidant activity.