Diet and Skin Aging—From the Perspective of Food Nutrition
Abstract
:1. Introduction
2. Changes and Molecular Mechanisms in Skin Aging
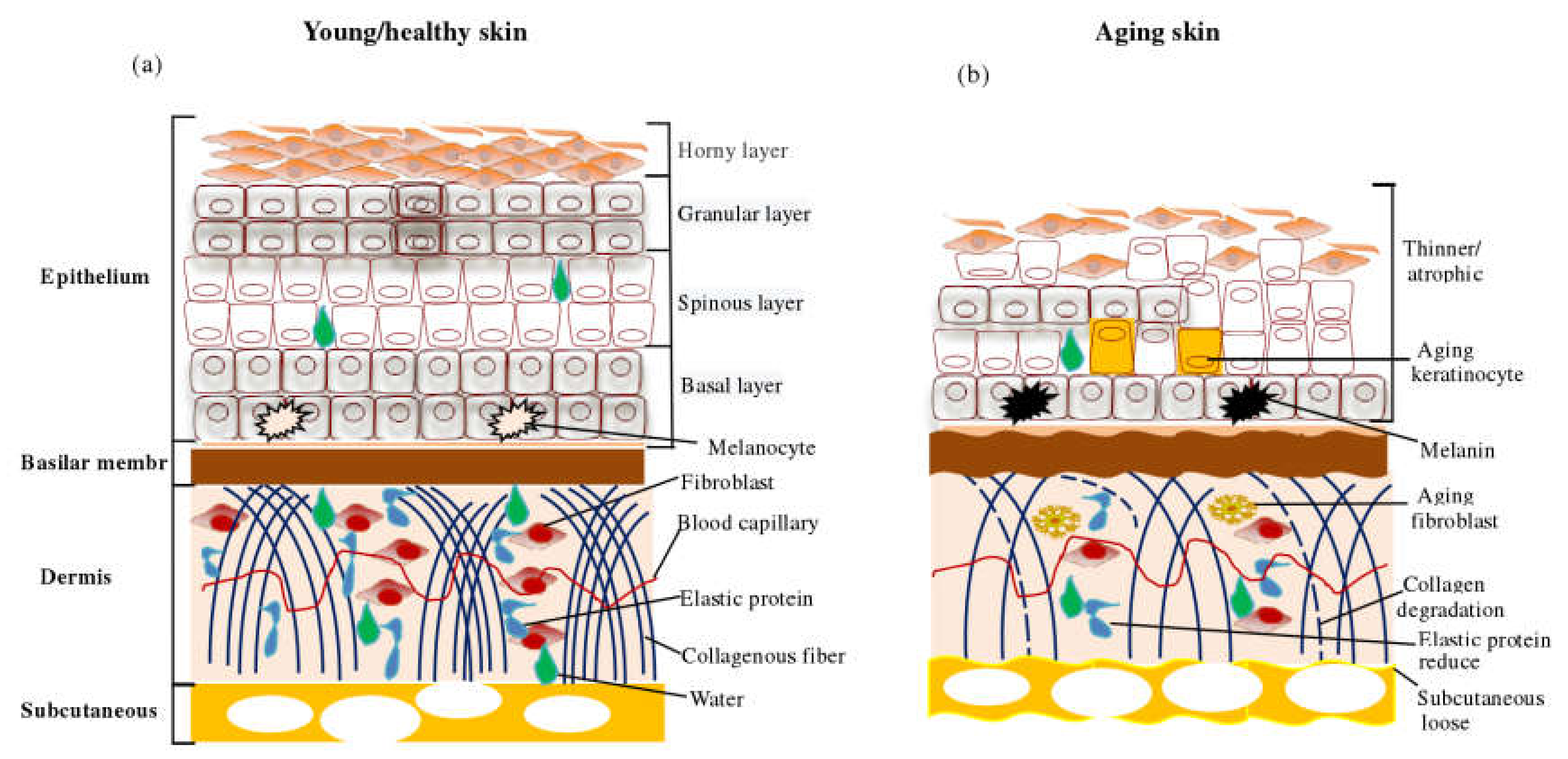
2.1. Apparent Changes in Skin Aging
2.2. Molecular Mechanism of Skin Aging
- a.
- Oxidative stress. Oxidative stress plays an important role in skin aging and skin damage processes, and its main feature is increased intracellular ROS. The skin’s oxidative metabolism and UV exposure lead to the production of ROS. The accumulation of ROS causes DNA damage, induces skin inflammatory response, reduces antioxidant enzymes, activates nuclear factor kappa B (NF-kB) and activator protein1 (AP–1) to inhibit collagen production, and increases matrix metalloproteinases to decompose collagen and binding proteins in the dermis, which eventually leads to skin aging [30,31,32,33].
- b.
- DNA damage and gene mutation. Earlier studies reviewed the mechanisms of UV-induced DNA damage and classified them into direct damage and indirect damage. Direct damage occurs when DNA absorbs the UV-B photon, leading to rearrangement in the nucleotide sequence, resulting in DNA strand deletion or mutation. During indirect damage, DNA molecules absorb UV-A and promote electron and energy transfer to oxygen molecules to form free radicals’ singlet oxygen ions, causing DNA damage [34,35]. DNA damage can be repaired by photolytic enzymes, while UV-induced skin DNA damage can be prevented by applying sunscreen [36,37].
- c.
- Shortening of the telomere. Telomeres are a small piece of DNA-protein complex at the end tips of eukaryotic linear chromosomes, which are important components in maintaining the chromosomal integrity and controlling the cell cycle. A telomere is shortened with cell division and is associated closely with cell division and senescence [38,39]. Telomerase is an enzyme responsible for telomere elongation, and its synthesis is essential for telomere maintenance and long-term survival of the organism. Epithelial stem cells with short telomeres have a poor proliferative capacity, which can be corrected by introducing telomerase. The reactive oxygen generated by UV radiation induces telomere mutation, cell death, or senescence. Nevertheless, some studies opine that the relationship between telomeres and aging may be speculation and, therefore, this relationship needs to be demonstrated [38,39,40,41,42].
- d.
- The role of microRNA. miRNAs are a type of conserved non-coding RNA. Chronic UV-B alters the expression of mir–34 family proteins in the skin. MiR–34 in human dermal fibroblast (HDFs) cells regulates cell function and expression of MMP–1, α1 type1 collagen (COL1A1), and elastin. miRNA 378b inhibits mRNA expression of COL1A1 by interfering with Sirtuin 6 (SIRT6) in HDFs, miRNA 217 regulates the senescence of human skin fibroblast by directly targeting DNA methyltransferase 1, and miR–23a–3p controls cellular senescence by targeting enzymes to control hyaluronic acid synthesis. These studies thus show that microRNAs regulate the skin aging process [43,44,45,46,47].
- e.
- Accumulation of advanced glycation end products (AGEs). AGEs are the products of excess sugar and protein binding, usually derived from body synthesis and food intake. The nonenzymatic glycosylation aging theory has been widely recognized by many scholars. As the final product of nonenzymatic glycosylation reaction, AGEs accumulate in photo-aging skin, affect protein function in the dermis, and promote skin aging [48,49,50].
- f.
- Aging due to inflammation. Continuous UV radiation exposure induces oxidative stress in epidermal cells, causing cell damage, fat oxidation, and finally leads to cell inflammation. When the degree of inflammation exceeds the ability of macrophages to clear up, macrophages also begin secreting pro-inflammatory factors and ROS to accelerate dermal inflammation and injury [51,52]. With the continuous advancement in dermatology in the past two decades, methods such as stem cell transplantation, hormone therapy, telomerase modification, and use of antioxidants and retinoic acid have been promoted to address skin aging. However, some of these treatment methods have certain disadvantages and serious side effects. For example, hormone therapy increases the risk of breast cancer, retinoic acid may cause osteoporosis, and telomerase modification increases the risk of skin cancer. Therefore, improving skin condition through diet management is being increasingly accepted by people.
3. Diet Management and Skin Aging
3.1. Nutrition Level
3.2. Eating Habits
| Nutrients/Diet | Relationship with the Skin | References |
|---|---|---|
| Water | Maintain skin internal balance and tissue function (e.g., aging and inflammation) | [56,57] |
| Proteins | Constitution and repair of skin tissues (involved in protein synthesis and metabolism), mediation of skin physiological functions and supply of energy. | [74] |
| Trace Elements | ||
| Copper | Involved in extracellular matrix, synthesis and stabilization of skin proteins, and angiogenesis. | [63] |
| Zinc | Participates in the proliferation and differentiation of epidermal keratinocytes. | [60,61] |
| Iron | Closely related to the activity of antioxidant enzymes in skin cells. | [64,65] |
| Selenium | 1. Essential for the development and function of skin keratinocytes.2. Related to skin antioxidant enzyme activity. | [67] |
| Vitamins | ||
| VA | Commonly used anti-aging ingredients prevent skin aging by regulating the expression of genes and matrix metalloproteinases. | [97,98] |
| VB | Associated with skin inflammation and pigmentation. | [99] |
| VC | Involved in skin collagen synthesis and elimination of intracellular reactive oxygen species. | [100] |
| VD | Reduces skin DNA damage, inflammation, and photocarcinogenesis. | [101] |
| VE | Prevent skin aging by inhibiting lipid peroxidation. | [102,103] |
| Diet | ||
| Fat | High fat is associated with skin inflammation, essential fatty acids are involved in skin lipid synthesis and metabolism. | [92,104] |
| Tobacco | Change skin cuticle thickness, accelerate skin pigmentation and skin necrosis. | [76,77,78,79] |
| Alcohol | Promote the proliferation of keratinocytes, change the skin permeability, destroy the barrier function of the skin, affect the skin lipid composition. | [81,82] |
| Sugar and baked goods | Associated with skin thickness, AGEs, autophagy, and inflammation. | [93,94,95,96] |
4. Foodborne Antioxidants and Skin Aging
4.1. Collagen Peptide
4.2. Polyphenols
4.3. Polysaccharides
4.4. Vitamins
4.5. Fatty Acids
4.6. Other Anti-Aging Nutrients
| References/Country/Study Type | Antioxidants/Source | Participants/Age | Induction Factors | Group/Dose/Time | Main Result | Main Conclusion |
|---|---|---|---|---|---|---|
| Effects of Oral Collagen Peptides on Skin Aging | ||||||
| [133]/China/animal | High-, medium- and low-antioxidant peptides (HCP, MCP and LCP)/silver carp skin | KM mice/5 week (25 ± 2 g) | 3UV-A + 1UV-B | Tg: HCP, MCP, LCP (0,200 mg/kg.bw.d)/0/1/2 weeks | 1. ACPs significantly alleviated skin composition and antioxidant index abnormalities induced by UVs. 2. HCP has the best protection effect on skin photoaging, and the difference between MCP and LCP is not obvious. | ACPs have the potential to resist photoaging of the skin. |
| [134]/China/animal | Gelatin (SG)and gelatin hydrolysate (SGH)/ salmon skin | ICR male mice/ 20 to 22 g | UV-B | Tg: SG (100, 500 mg/kg.bw.d), SGH (100, 500 mg/kg.bw.d); Cg: Vc 100 mg/kg.bw.d/5 week | 1. Antioxidant activity of SG and SGH is related to dose, molecular weight and amino acid composition. 2. SG and SGH alleviate oxidative damage by enhancing antioxidant enzyme activity and thymus index | SGH has the potential to be used as an antioxidant in health products and cosmetics. |
| [135]/Korea/animal | Collagen peptide (CP)/ tilapia scale | SKH–1 hairless mice/ 6 weeks old | UV-B | Tg:CP (0,500, 1000 mg/kg.bw.d) Cg: N–acetyl glucosamine (1000 mg/kg.bw.d)/ 9 weeks | 1. Oral CP increased skin hydration, reduced wrinkle formation, changed the expression of HAS–1,–2, and maintained the stability of HA. 2. CP regulate the expression of skin moisturizing factor filagglutinin and total chain protein | CP can be used as a nutrient to relieve UV-B-induced skin wrinkles, dehydration and water loss. |
| [136]/Brazil/cell | Collagen Hydrolysate (CH)/cow | HDFs | Cg:CH (0.5, 1.0, 2.5 and 5.0 mg/mL)/48 h | 1. CH regulates cell metabolism without cytotoxicity. 2. CH maintains intracellular protein stability by inhibiting the activity of MMP 1 and 2. | This CH has protective effects on skin cells and has the potential to become a food supplement. | |
| [137]/Korea/clinical | Low-molecular-weight Collagen peptide (LMWCP)/ catfish’s skin | Women/40–60 years old | Age | Tg:LMWCP; 1000 mg/d. Cg: placebo; (0/6/12) weeks | Oral LMWCP protects photoaged skin by improving skin wrinkles, hydration and elasticity | LMWCP can be used as a functional food ingredient to relieve skin photoaging. |
| [138]/China/animal | Collagen hydrolysates (CHs)/Nile tilapia skin | ICRmice/38 ± 4 g, 9-month-old | Age | Tg:CHs (0%, 2.5%, 5%, 10%); Ng: weaned mice; Cg: (WC, 10% whey protein hydrolysates)/180 days | 1. CHs significantly improves skin visual appearance, tissue structure and matrix homeostasis. 2. CHs alleviates oxidative stress by increasing skin antioxidant activity | CHs can be used as a functional nutritional food against skin aging, but its molecular mechanism is not clear. |
| [139]/China/animal | Elastin peptides (EH)/bovine arteries | Female mice/ (20 ± 2 g) | UV | Nc:vehicle-treated mice; Mg:vehicle-treated + UV. EH group:UV + EH (100 mg/kg.bw.d)/8 weeks | EH can significantly reduce UV-induced epidermal hyperplasia and fibroblast apoptosis, and increase skin hydroxyproline and water content | EH has the potential to prevent and regulate skin photoaging |
| [140]/China/animal | Collagen peptides (CPs)/silver carp skin | Mice/(8, 13-month-old (28 ± 2, 45 ± 5 g) | Age | Cg: young mice (normal saline); Tg: old mice (CPs: 400 mg/kg.bw.d); Mg: Old mice (normal saline)/2 months | 1. CPs promotes skin collagen synthesis by regulating cytokines in skin and serum. 2. Intake of CPs inhibited platelet release. | CPs has the potential to be an anti-aging, anti- cancer and anti- cardiovascular health product |
| [141]/Canada/cell | Collagen peptides (CPs)/Chicken meat | HDFs cells/ human skin | DCF-DA | Tg: Two peptides, hydrolyzed by two enzymes (0, 2.5 mg/mL)/24 h | Two chicken collagen peptides have significant effects on inflammatory changes, oxidative stress, type I collagen synthesis, and cell proliferation in skin HDFs | CPs hydrolyzed by different enzymes have different protective and regulatory effects on skin fibroblasts |
| [142]/Canada/cell | Collagen peptides (CPs)/porcine/ bovine/tilapia/hen skin | HDFs/ human skin | UV-A | Tg: Four kinds of collagen peptides (0, 0.5, 1, 2, 4 mg/mL)/24 h | 1. Bovine CH inhibits the MMP–1 production. 2. Tilapia CH promotes cell viability and type I collagen generation, while inhibiting ROS and MMP–3 generation. 3. Hen CH promotes collagen production and reduces ROS, MMP–1 and 9 generation and the expression of apoptotic genes. | Hen CH protects HDFs from UV-A-induced damage better than pigs, cattle and tilapia. |
| [143]/China/animal | High, low molecular weight collagen hydrolysates (HMCH/LMCH)/Silver Carp | Mice/5weeks (25 ± 2 g) | UV-A + UV-B | Tg1:UV + LMCH (HMCH)(50, 100, 200 mg/kg.bw.d)/6 weeks; Tg2:UV+LMCH (HMCH) (200 mg/kg.bw.d)/ 2 weeks | 1. Both HMCH and LMCH increase skin components and antioxidant enzyme activity in skin and serum. 2. LMCH is more effective than HMCH. 3. Skin hydroxyproline, HA, and moisture content depend on peptide dose. | LMCH extracted from silver carp skin can be used as a dietary supplement to prevent skin aging. |
| [144]/Japan /clinical | High, low purity collagen hydrolysate (H-CP/L- CP)/fish gelatin | Female/(35–55 years old) | Age | H-CP group: 5 g/d; L-CP group: 5 g/d; Cp: placebo; 0/4/8 weeks. | H-CP is more significant than L-CP in improving facial skin moisture, elasticity, wrinkles, and roughness. | L-CP and H-CP are both effective dietary supplements to improve skin conditions. |
| [145] /Thailand /cell/animal | Collagen hydrolysate (HC)/Lates calcarifer skin | HDFs/human; Wistar rats (214 ± 26 g) | Mice Tg: (0,2000,5000 mg/kg.bw.d)/15d; Cell Tg: (50, 100, 150 and 200 µg/mL)/24 h. | 1. Animal and cell experiments prove that HC is non-toxic. 2. HC can promote the growth of fibroblasts and the synthesis of cellular collagen, but not as effective as HC combined with VC. | Single HC or HC combined with VC can be used as nutritional health products for skin care. | |
| [146]/China /cell | Gelatin hydrolysates (CGH)/Cod skin | HDF cells/ Mouse skin | UV-B | Tg: CGH (0, 0.001, 0.01, 0.1,1, 10) mg/mL /24 h. | 1. CGH inhibits the expression of MMP–1 in fibroblasts induced by UV-B. 2. Purified MMP–1 inhibitory peptides have significant inhibitory effects on MMP–1, p-ER and p-p38. | CGH can be used as a functional supplement for skin care. |
| [147]/China /cell/animal | High, medium, low antioxidant peptide (HCP/ MCP/LCP)/Silver carp skin; Serum collagen peptides (SCP)/ rat serum | SD rat (8 week); ESF cells/skin | UV-A | Rats Tg (HCP, MCP and LCP)/(2.4 g/kg.bw.d)/2 h; Cell Tg: (SHCP, SMCP and SLCP)/(0, 50, 200 µM/mL)/24 h. | 1. SCP is the active component of serum metabolites, which shows repair effect by removing ROS. 2. SCP promotes collagen synthesis and inhibits its degradation by activating TGF-β/Smad3 pathway and inhibiting the expression of AP–1 and MMP–1,3,SHCP is the best one. | CP promotes photoaging skin repair by activating the TGF- TGF/Smad pathway and inhibiting collagen reduction. |
| [148]/China /animal | Alcalase, Collagenase Collagen peptide (ACP/CCP)/ bovine bone | Mice/(8, 13-month-old (28 ± 2, 45 ± 5 g) | Age | Cg: young mice (normal saline); Tg: old mice/ACP (200, 400, and 800 mg/kg.bw.d), CCP (400 mg/kg.bw.d)/8 weeks | Oral CPs improve skin relaxation, increase collagen content and antioxidant enzyme activity, repair collagen fibers, and normalize the ratio of skin collagen. ACP is better than CCP. | CP can alleviate the chronological aging of the skin and has the potential to become an anti-aging functional food. |
| [149]/Korea /animal/cell | Collagen peptide NS (CPNS)/fish scale | HDF cells /human, Mice/8 weeks old (25–30 g) | UV-B | Cell Tg: CPNS (0, 50, 100, 250, 500 µg/mL)/24 h; Mice Tg: CPNS (300, 500 mg/kg.bw.d)/12weeks | 1. CPNS treatment reduced the production of MMP–1 and increased the synthesis of type 1 procollagen in HFD cells. 2. Oral CPNS significantly reduced skin wrinkle formation, epidermal water loss, epidermal thickness, and increased hydration. | CPNS are a potential food supplement to prevent skin aging. |
| [150]/China /animal | Gelatin/Amur sturgeon swim bladder | Female SD rat/6 months old | Age | Cg (8% whey protein); Tg (8%, 4%, 2%)/12 months. | 1. Oral administration of 3.85 g/kg.bw.d gelatin significantly improved skin histological structure and collagen ratio. 2. Skin antioxidant activity increased. | The gelatin improves the foundation for the development of anti-aging foods. |
| [110]/China /animal | Protein hydrolysate (WPH)/Walnut | SD rats/ 180–200 g | UV-A + UV-B | Cg:( distilled water); Tg: UV-R + WPH (0, 0.32, 0.98, 2.88 g/L)/18 weeks | 1. WPH significantly enhances skin elasticity and promotes the biosynthesis of Col I, Hyp, and HA. 2. WPH inhibits MMP–1 activity and repairs skin damage. 3. WPH repair effect becomes dose dependent, high dose is best. | WPH has potential as a functional food ingredient against photoaging. |
| Effects of Oral Polyphenol on Skin Aging | ||||||
| [151,152] /China/cell /animal | Polyphenol extract (HPE)/hawthorn | HDFs and HaCaT/human; mice/5–6 weeks old | UV-B | Cell Tg: HPE (0, 5, 10 µg/mL)/24 h; Mice Tg: HPE (0, 100, 300 mg/kg.bw.day)/12 weeks | 1. HPE treatment can promote cell proliferation, increase intracellular collagen and reduce MMP–1 production. 2. Oral HPE reduces UV-B-induced skin damage by eliminating ROS, reducing DNA damage and inhibiting p53 expression. | HPE can be used as an anti-aging food or cosmetic ingredient. |
| [153]/Spain /clinical | Products rich in polyphenol (NutroxsunTM)/rosemary and citrus | Adult female | UV-B + UV-A | Long-term: NutroxsunTM (250 mg/day)/ 2 weeks; Short-term: NutroxsunTM (100, 250 mg/day)/24, 48 h | 1. Dietary NutroxsunTM reduces UV- induced skin changes, wrinkles and elasticity improvements. 2. The improvement effect between two doses is not obvious. | Long-term oral NutroxsunTM can be used as a nutritional supplement to improve skin conditions. |
| [154]/Korea /cell | Polyphenolic- rich extract (SSE and SSW)/Spatholobus Suberectus stem | HaCaT/Human skin | UV-B | Tg1: SSE (0, 3, 10, 30, 300 µg/mL); Tg2: SSW (0, 3, 10, 30, 300 µg/mL)/24 h | 1. SSE and SSW inhibited ROS production and cell damage. 2. SSE repairs skin by upregulating the expression of enzymes and proteins in cells, blocking UV-B-induced MAPKs phosphorylation and its downstream transcription factor. | SSE can be used as a natural biomaterial to inhibit UV-B-induced photoaging. |
| [155]/China /animal | Rambutan peel phenolics (RPP)/Nephelium lappaceum; Leu-Ser-Gly-Tyr-Gly-Pro (LSGYGP)/synthetic | Male BALB/c nude mice/20–22 g | UV-B | Single group: RPP (100 mg/kg.bw. d), SGYGP (100 mg/kg.bw.d); Composite group: (50 RPP+ 50 LSGYGP) mg/kg.bw.d, (100 RPP + 100 LSGYGP)mg/kg.bw.d/10 weeks | 1. RPP and LSGYGP improve skin biochemical indicators, tissue structure and collagen levels. 2. RPP enhances the regulation of oxidative stress and inflammatory factor levels. 3. LSGYGP significantly affects skin collagen and HA content. | Oral RPP and LSGYGP can alleviate UV-B- induced skin aging. |
| [156]/Korea /animal | Polyphenols/Flavonoid hesperidin exerts | Male hairless mice/6- week-old | UV-B | Cg: water; Tg: UV-B + hesperidin (0, 100 mg/kg.bw.d)/12 weeks | 1. Oral hesperidin inhibited UV-B-induced skin thickening and wrinkle formation. 2. Hesperidin inhibited UV-B-induced expression of MMP–9 and cytokines, and protected collagen fiber loss. | Oral hesperidin regulates MMP–9 expression by inhibiting MAPK- dependent signaling pathways to relieve skin photo-aging. |
| [157]/Korea /cell | Polyphenols/3,5,6,7,8,3,4-heptam-ethoxy flavone (HMF)/C. unshiu peels | HDFn cells/human dermal | UV-B | HMF (0, 50, 100, 200 µg/mL)/24 h | 1. HMF protects UV-induced HDFn cell damage by inhibiting MMP–1 expression through phosphorylated MAPK signals. 2. HMF regulates the expression of Smad3 and Smad7 proteins in a dose-dependent manner. | HMF has the potential to be an anti-aging cosmetic or food supplement. |
| [158]/Korea /cell | Polyphenols/ Tectorigenin/ Belamcanda chinensis L | HaCaT cells/human | UV-B | Tg: Tectorigenin (0, 0.1, 1,10 µM); Cg: VC (200 µM)/24 h | 1. Tectorigenin lowers ROS levels by increasing intracellular antioxidant enzymes. 2. Tectorigenin reduces mmp–1 and inhibits collagen degradation. 3. Tectorigenin inhibits apoptosis by regulating the levels of caspase–3 and bcl–2 related proteins. | Tectorigenin alleviates skin damage by inhibiting UV-B-induced cellular oxidation, apoptosis and collagen degradation. |
| Effects of Oral Polysaccharides on Skin Aging | ||||||
| [116]/China /animal | Polysaccharides (TP)/T. fuciformis | SD rats/6~7 weeks old (180–220 g) | UV-A + UV-B | Cg: no irradiation; Tg group: UV + TP (0, 100, 200, 300 mg/kg.bw.d)/12 weeks | Oral TP can alleviate UV-induced skin structural changes, repair collagen damage, maintain the I/III collagen ratio and enhance skin antioxidant enzyme activity. | TP has the potential to become a skin-protective functional food additive. |
| [117]/Korea /cell | Polysaccharide (HFPS)/Hizikia fusiforme | HDF cells | UV-B | Cg: no irradiation; Tp: UV + HFPS (0, 25, 50, 100 µg/mL)/24 h | 1. HFPS significantly reduces cell ROS and increases the pure activity rate. 2.HFPS inhibits UV-induced skin damage by regulating NF-κB, ap–1 and MAPKs signaling pathways. | HFPS has a strong anti-ultraviolet effect and is a potential pharmaceutical, food, and cosmetic ingredient. |
| [118]/China /cell | Polysaccharide (LBP)/Lycium barbarum | HaCaT cells | UV-B | Tg1: LBP (0, 50, 100, 300, 600, 1500, 3000 µg/mL) 24 h; Tp2: UV-B + LBP (0, 300 µg/mL)/24 h | LBP mainly eliminates ROS and reduces DNA damage. In part, the Nrf2/ARE pathway is activated to inhibit the p38 MAP pathway, thereby inhibiting the activation of caspase–3 and the expression of mmp–9 to protect the aging cells. | LBP may be used as a protective agent or food additive against skin oxidative damage. |
| [119]/China /cell | Polysaccharide (GL-PS)/Ganoderma lucidum | Fibroblast/men foreskin | UV-B | Tg: UV-B + GL-PS (0, 10, 20, 40 µg/mL) 24 h; Tg: no UV-B and GL-PS/24 h | After GL-PS treatment, cell activity increased, senescent cells decreased, CICP protein expression increased, MMP–1 protein expression decreased, and cell ROS level decreased. | GL-PS protects UV-B- induced cell photoaging by eliminating intracellular ROS, which will provide strategies for subsequent studies. |
| [120]/China /animal | Polysaccharide (SFP)/Sargassum fusiforme | Female KM mice/7 weeks old (20–25 g) | UV-B | Cg: UV-B + sodium hyaluronate (400 mg/kg. bw/d); SFP Tg: UV-B + SFP (0, 200, 400, 600 mg/kg.bw/d)/9 weeks | 1. SFP regulates mouse chest, spleen index and skin water content. 2. SFP increases skin antioxidant enzyme activity, reduces ROS, and reduces oxidative damage.3.SFP inhibits MMP–1 and 9 levels in the skin. | SFP can be a potential functional food additive for skin protection. |
| [121]/China /cell/animal | Purified, crude polysaccharide (TLH–3,TLH)/ Tricholoma lobayense | HELF cells/human; Mice/8 weeks (23 ± 2 g) | t-BHP/D-galactose | Cell Tg: TLH–3 (0, 50, 100, 200, 400 µg/mL), Pc: Vc (50 ug/mL)/24 h; Mice Tg: TLH–3 and TLH (200 mg/kg. bw/d),Pc: Vc (100 mg/kg. bw/d)/5 weeks | 1. TLH–3 relieves cell senescence by regulating the expression of bcl–2, bax, caspase–3 proteins, inhibiting senescence-related enzyme levels. 2. TLH–3 reduced skin pathological lesions by reducing IL–6, LPF, AGEs, and enhanced MAO activity. | TLH–3 is an active polysaccharide that protects cells and mice from oxidative stress aging. |
| Effects of Oral Vitamins on Skin Aging | ||||||
| [159]/China /cell | Vitamin Coenzyme Q10 (CoQ10) | ESF and HaCaT cells/ Human | UV-A, UV-B | Cg: ESF, HaCat (CoQ10 (0, 0.5, 1, 2 µM))/24 h; Tg: ESF, HaCat (UV-A or UV-B + CoQ10 (0, 1, 5, 10 µM))/24 h | 1. CoQ10 treatment promoted ESF cell proliferation, type IV collagen and elastin gene expression. 2. CoQ10 treatment inhibited UV-induced IL–1a production in HaCaT cells. | CoQ10 has anti-aging effect on chronological aging and photo-aging and can be used in food and cosmetics. |
| [122]/Japan /clinical | VC, VE, and Astaxanthin (AX) | Female/(mean age 37.26 years) | - | Tg1:AX (6 mg) + VC (1000 mg) + VE (10 mg)/d; Tg2:VC (1000 mg) + VE (10 mg)/d/20 weeks | Tg 1 significantly improved skin moisture content, skin elasticity and wrinkles; Tg 2 did not improve the skin significantly. | Oral formulations containing astaxanthin and vitamin C and E have skin-improving effects. |
| [160]/Iran /animal | Silymarin, Vitamin C | Balb/C mice/6 weeks old (30 ± 2 g) | UV-B | Cg: Silymarin (100),VC (40 mg/kg.bw/d)/; Tg: UV-B + Silymarin (0, 100 mg/kg.bw/d), UV-B + VC: (0, 40 mg/kg.bw/d)/4 weeks. | Oral VC enhances skin antioxidant enzyme activity, reduces skin wrinkle formation and thickness increase in mice induced by UV-B. | Salicylic acid and vitamin C can be used as food or cosmetic ingredients to resist skin photo-aging. |
| [161]/Korea /cell | Niacinamide (NIA) | HaCaT/ human | PM2.5 | Cg: NIA (0, 12.5, 25, 50, 100, or 200 µM); Tg: NIA (0, 12.5, 25, 50, 100, or 200 µM) + PM2.5 (50 µM)/24 h | NIA treatment can inhibit the oxidation of lipid, protein, DNA and other molecules induced by PM 2.5, as well as inhibit apoptosis and ROS production. | NIA protects cells from PM 2.5-induced oxidative stress and cell damage. |
| Effects of Oral Fatty Acids on Skin Aging | ||||||
| [125]/Brazil /animal | 0live oil | Swiss mice/8–12 weeks age | Rotational stress | Stress group: stress + olive (1.5 g/kg.bw. d), Cg: olive (1.5 g/kg. bw/d)/29 d | Olive inhibited skin ROS, lipid peroxidation, protein carbonylation, phenolamine synthesis, MMP–8 expression and promotes collagen deposition in mice through NF-κB and NRF2 pathways. | Oral administration of olive oil can reduce mice skin aging induced by stress. |
| [126]/Korea /animal | 7-MEGATM500/> 50% of palmitoleic acid containing fish oil, omega–7 | H–1 mice/5-week old (18–20 g) | UV-B | Cg: 30% EtOH; Tp: 7-MEGATM500 (50, 100, 200 mg/kg.bw/d)/4 weeks | 1.7-MEGATM 500 improves skin histological indicators and significantly down-regulated the expression levels of MMP–3 and c-jun genes and proteins in the skin. | 7-MEGATM500 can alleviate UV-B induced skin photoaging in mice |
| [129]/Korea /cell | Fermented Fish Oil (FFO) | HaCaT/ human | PM2.5 | Cg: PM2.5; Tp:PM2.5 + FFO (0, 20 µg/mL)/24 h | FFO can inhibit PM 2.5-induced intracellular ROS, Ca 2+ levels and MMPs–1,2,9 production, and block the MAPK/AP–1 pathway. | FFO can alleviate PM 2.5 induced skin aging. |
| [162]/Japan /animal | Coconut oil | Female mice/(6 weeks old) | DNFB | Cg: Coconut or soybean oil (4%)/2 months; Tg: Coconut or soybean oil (4%)/after 2 months + DNFB | Oral coconut oil improves BDFB-induced skin inflammation in mice. Mechanistically related to elevated mead acid in serum inhibiting directional migration of neutrophils. | Dietary coconut oil improved skin contact allergies in mice by producing midic acid. |
5. Conclusions and Prospects
- People’s current understanding of diet to improve skin aging is still insufficient. While it is difficult for us to accurately define what is a healthy diet, and to quantify the relationship between diet and skin aging that convince the public, it is difficult for them to change their original lifestyle and diet, even if people have such knowledge.
- The issues of accurately quantifying the skin improvement effect of each nutrient intake, and the negative effects of smoking, drinking, grilling, etc., on skin aging still need to be addressed.
- The functional anti-aging ingredients in food mainly relieve skin aging in three ways. First, anti-aging ingredients (such as protein peptides and essential fatty acids) enter the skin as a precursor after digestion and absorption and participate in the synthesis and metabolism of skin components. Second, anti-aging ingredients relieve skin oxidative damage by removing cellular ROS and enhancing antioxidant enzyme activity. Third, the anti-aging component acts as an enzymatic factor, and regulates the expression of enzymes such as MMPs and AP–1, inhibiting the degradation of skin components and maintaining the integrity of the skin structure.
- The limitations of foodborne antioxidants such as unstable storage, low skin bioavailability, and poor solubility, can be improved by chemical modification, collagen drug delivery, and a combination of supplements.
- Only oral supplementation is not enough to improve the skin. The combination of oral and external skin penetration should be the safest and the most effective way to improve skin aging.
- Diet causes skin aging or improves skin aging and is difficult to simply apply to clinical research. While on the one hand, there is an ethical controversy, on the other hand, the experimental period is too long to control the diet of volunteers for a single, long duration, and the uniformity of clinical experimental conditions is not guaranteed, resulting in vague experimental results and insufficient credibility.
- Improvement in skin aging through diet should not be rushed, because skin aging caused by diet and improvement of aging performance by diet are long-term processes. There is also the problem of metabolic processing of food and nutrients until they reach the skin. They have to travel a long way, and there is still a lot to study in this process.
Author Contributions
Funding
Conflicts of Interest
References
- Blanpain, C.; Fuchs, E. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 2006, 22, 339–373. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Murphree, R.W. Impairments in skin integrity. Nurs. Clin. 2017, 52, 405–417. [Google Scholar] [CrossRef]
- Veltri, A.; Lang, C.; Lien, W.H. Concise review: Wnt signaling pathways in skin development and epidermal stem cells. Stem Cells 2018, 36, 22–35. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.C.; Li, L.; Fuchs, E. Emerging interactions between skin stem cells and their niches. Nat. Med. 2014, 20, 847. [Google Scholar] [CrossRef] [Green Version]
- Arseni, L.; Lombardi, A.; Orioli, D. From structure to phenotype: Impact of collagen alterations on human health. Int. J. Mol. Sci. 2018, 19, 1407. [Google Scholar] [CrossRef] [Green Version]
- Orioli, D.; Dellambra, E. Epigenetic regulation of skin cells in natural aging and premature aging diseases. Cells 2018, 7, 268. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Park, S.Y.; Moon, S.; Lee, J.; Kim, S. Autophagy in human skin fibroblasts: Impact of Age. Int. J. Mol. Sci. 2018, 19, 2254. [Google Scholar] [CrossRef] [Green Version]
- Liberato, T.; Pessotti, D.S.; Fukushima, I.; Kitano, E.S.; Serrano, S.M.; Zelanis, A. Signatures of protein expression revealed by secretome analyses of cancer associated fibroblasts and melanoma cell lines. J. Proteom. 2018, 174, 1–8. [Google Scholar] [CrossRef]
- DesJardins-Park, H.E.; Foster, D.S.; Longaker, M.T. Fibroblasts and wound healing: An update. Regen. Med. 2018, 13, 491. [Google Scholar] [CrossRef] [Green Version]
- Pincha, N.; Hajam, E.Y.; Badarinath, K.; Batta, S.P.R.; Masudi, T.; Dey, R.; Andreasen, P.A.; Kawakami, T.; Samuel, R.; George, R.; et al. PAI1 mediates fibroblast–mast cell interactions in skin fibrosis. J. Clin. Investig. 2018, 128, 1807–1819. [Google Scholar] [CrossRef]
- Guerrero-Juarez, C.F.; Plikus, M.V. Emerging nonmetabolic functions of skin fat. Nat. Rev. Endocrinol. 2018, 14, 163. [Google Scholar] [CrossRef]
- Driskell, R.; Lichtenberger, B.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.; Hérault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Yanyan, F.; Xiongming, P. Natural aging and photoaging of skin. Int. J. Dermatovener. 2004, 30, 354–356. [Google Scholar]
- Landau, M. Exogenous factors in skin aging. Curr. Probl. Dermatol. 2007, 35, 1–13. [Google Scholar]
- Fuchs, E. Epithelial skin biology: Three decades of developmental biology, a hundred questions answered and a thousand new ones to address. Curr. Top. Dev. Biol. 2016, 116, 357–374. [Google Scholar]
- Tigges, J.; Krutmann, J.; Fritsche, E.; Haendeler, J.; Schaal, H.; Fischer, J.W.; Kalfalah, F.; Reinke, H.; Reifenberger, G.; Stühler, K.; et al. The hallmarks of fibroblast ageing. Mech. Ageing Dev. 2014, 138, 26–44. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Voorhees, J.J.; Fisher, G.J. Cysteine-rich protein 61 (CCN1) mediates replicative senescence-associated aberrant collagen homeostasis in human skin fibroblasts. J. Cell. Biochem. 2012, 113, 3011–3018. [Google Scholar] [CrossRef]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [Green Version]
- Bernerd, F.; Vioux, C.; Asselineau, D. Evaluation of the protective effect of sunscreens on in vitro reconstructed human skin exposed to UV-B or UV-A irradiation. Photochem. Photobiol. 2000, 71, 314–320. [Google Scholar] [CrossRef]
- Bernerd, F.; Asselineau, D. An organotypic model of skin to study photodamage and photoprotection in vitro. J. Am. Acad. Dermatol. 2008, 58, 155–159. [Google Scholar] [CrossRef]
- Bernerd, F.; Marionnet, C.; Duval, C. Solar ultraviolet radiation induces biological alterations in human skin in vitro: Relevance of a well-balanced UV-A/UV-B protection. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 15. [Google Scholar] [CrossRef]
- Fagot, D.; Asselineau, D.; Bernerd, F. Matrix Metalloproteinase-1 Production Observed After Solar-Simulated Radiation Exposure is Assumed by Dermal Fibroblasts but Involves a Paracrine Activation Through Epidermal Keratinocytes. Photochem. Photobiol. 2004, 79, 499–506. [Google Scholar] [CrossRef]
- D’Errico, M.; Lemma, T.; Calcagnile, A.; De Santis, L.P.; Dogliotti, E. Cell type and DNA damage specific response of human skin cells to environmental agents. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2007, 614, 37–47. [Google Scholar] [CrossRef]
- Duval, C.; Cohen, C.; Chagnoleau, C.; Flouret, V.; Bourreau, E.; Bernerd, F. Key regulatory role of dermal fibroblasts in pigmentation as demonstrated using a reconstructed skin model: Impact of photo-aging. PLoS ONE 2014, 9, e114182. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Makrantonaki, E. Clinical aspects and molecular diagnostics of skin aging. Clin. Dermatol. 2011, 29, 3–14. [Google Scholar] [CrossRef]
- Natarajan, V.T.; Ganju, P.; Ramkumar, A.; Grover, R.; Gokhale, R.S. Multifaceted pathways protect human skin from UV radiation. Nat. Chem. Boil. 2014, 10, 542. [Google Scholar] [CrossRef]
- Gonzaga, E.R. Role of UV light in photodamage, skin aging, and skin cancer. Am. J. Clin. Dermatol. 2009, 10, 19–24. [Google Scholar] [CrossRef]
- Ravanat, J.L.; Douki, T.; Cadet, J. Direct and indirect effects of UV radiation on DNA and its components. J. Photochem. Photobiol. B Biol. 2001, 63, 88–102. [Google Scholar] [CrossRef]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet radiation-induced skin aging: The role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wang, L.; Zhong, D. Dynamics and Mechanisms of Ultraviolet-Damaged DNA Repair by Photolyases; Jenny Stanford Publishing: New York, NY, USA, 2017; Volume 3, pp. 109–144. [Google Scholar]
- Narbutt, J.; Philipsen, P.; Harrison, G.; Morgan, K.; Lawrence, K.; Baczynska, K.; Grys, K.; Rogowski-Tylman, M.; Olejniczak-Staruch, I.; Tewari, A.; et al. Sunscreen applied at ≥ 2 mg cm−2 during a sunny holiday prevents erythema, a biomarker of ultraviolet radiation-induced DNA damage and suppression of acquired immunity. Br. J. Dermatol. 2018, 180, 604–614. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, E.H. Structure and function of telomeres. Nature 1991, 350, 569. [Google Scholar] [CrossRef]
- Shay, J.W. Telomeres and aging. Curr. Opin. Cell Biol. 2018, 52, 1–7. [Google Scholar] [CrossRef]
- Siegl-Cachedenier, I.; Flores, I.; Klatt, P.; Blasco, M.A. Telomerase reverses epidermal hair follicle stem cell defects and loss of long-term survival associated with critically short telomeres. J. Cell Biol. 2007, 179, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Buckingham, E.M.; Klingelhutz, A.J. The role of telomeres in the ageing of human skin. Exp. Dermatol. 2011, 20, 297–302. [Google Scholar] [CrossRef]
- Simons, M.J. Questioning causal involvement of telomeres in aging. Ageing Res. Rev. 2015, 24, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Blackstone, B.N.; Wilgus, T.; Roy, S.; Wulff, B.; Powell, H.M. Skin Biomechanics and miRNA expression Following.Chronic UV-B Irradiation. Adv. Wound Care 2020, 9, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Du, R.; Xiao, X.; Deng, Z.L.; Jian, D.; Xie, H.F.; Li, J. Microrna-217 modulates human skin fibroblast senescence by directly targeting DNA methyltransferase 1. Oncotarget 2017, 8, 33475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röck, K.; Tigges, J.; Sass, S.; Schütze, A.; Florea, A.-M.; Fender, A.C.; Theis, F.J.; Krutmann, J.; Boege, F.; Fritsche, E.; et al. miR-23a-3p Causes Cellular Senescence by Targeting Hyaluronan Synthase 2: Possible Implication for Skin Aging. J. Investig. Dermatol. 2015, 135, 369–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, D.; An, S.; Choi, B.G.; Kim, K.; Choi, Y.M.; Ahn, K.J.; An, I.-S.; Cha, H.J. MicroRNA-378b regulates α-1-type 1 collagen expression via sirtuin 6 interference. Mol. Med. Rep. 2017, 16, 8520–8524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Yan, X.; Jiang, M.; Xiang, L. The comparison of microRNA profile of the dermis between the young and elderly. J. Dermatol. Sci. 2016, 82, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, E.; Kawada, A.; Ono, K.; Fujimoto, E.; Wachi, H.; Harumiya, S.; Nagai, R.; Tajima, S. Nε-(carboxy- methyl) lysine modification of elastin alters its biological properties: Implications for the accumulation of abnormal elastic fibers in actinic elastosis. J. Investig. Dermatol. 2012, 132, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrar, M.D. Advanced glycation end products in skin ageing and photoageing: What are the implications for epidermal function. Exp. Dermatol. 2016, 25, 947–948. [Google Scholar] [CrossRef] [Green Version]
- Radjei, S.; Gareil, M.; Moreau, M.; Leblanc, E.; Schnebert, S.; Friguet, B.; Nizard, C.; Petropoulos, I. The glyoxalase enzymes are differentially localized in epidermis and regulated during ageing and photoageing. Exp. Dermatol. 2016, 25, 492–494. [Google Scholar] [CrossRef] [Green Version]
- Handoko, H.Y.; Rodero, M.P.; Boyle, G.M.; Ferguson, B.; Engwerda, C.R.; Hill, G.; Muller, H.K.; Khosrotehrani, K.; Walker, G.J. UV-B-Induced Melanocyte Proliferation in Neonatal Mice Driven by CCR2-Independent Recruitment of Ly6clowMHCIIhi Macrophages. J. Investig. Dermatol. 2013, 133, 1803–1812. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Lyga, J. Inflammaging in skin and other tissues-the roles of complement system and macrophage. Inflamm. Allergy Drug Targets Former. Curr. Drug Targets Inflamm. Allergy 2014, 13, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, hydration, and health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef]
- Jéquier, E.; Constant, F. Water as an essential nutrient: The physiological basis of hydration. Eur. J. Clin. Nutr. 2010, 64, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnaud, M.J.; Noakes, T.D. Should humans be encouraged to drink water to excess? Eur. J. Clin. Nutr. 2011, 65, 875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma, M.L.; Monteiro, C.; Tavares, L.; Julia, M.; Rodrigues, L.M. Relationship between the dietary intake of water and skin hydration. Biomed. Biopharm. Res. 2012, 9, 173–181. [Google Scholar] [CrossRef]
- Palma, L.; Marques, L.T.; Bujan, J.; Rodrigues, L.M. Dietary water affects human skin hydration and biomechanics. Clin. Cosmet. Investig. Dermatol. 2015, 8, 413. [Google Scholar]
- Weidong, Y.; Jiesheng, L.; Xichun, P. Chapter1, pp. 1–10. Trace Elements and Health; Huazhong University of Science and Technology Press: Wuhan, China, 2007. [Google Scholar]
- Chen, W.; Zhou, X.; Zhu, W. Trace Elements Homeostatic Imbalance in Psoriasis: A Meta-Analysis. Biol. Trace Elem. Res. 2019, 191, 313–322. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kawamura, T.; Shimada, S. Zinc and skin biology. Arch. Biochem. Biophys. 2016, 611, 113–119. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kinoshita, M.; Shimada, S.; Kawamura, T. Zinc and skin disorders. Nutrients 2018, 10, 199. [Google Scholar] [CrossRef] [Green Version]
- Bauer, B.U.; Rapp, C.; Mülling, C.K.; Meissner, J.; Vogel, C.; Humann-Ziehank, E. Influence of dietary zinc on the claw and interdigital skin of sheep. J. Trace Elem. Med. Biol. 2018, 50, 368–376. [Google Scholar] [CrossRef]
- Borkow, G. Using copper to improve the well-being of the skin. Curr. Chem. Boil. 2014, 8, 89–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reelfs, O.; MEggleston, I.; Pourzand, C. Skin protection against UV-A-induced iron damage by multiantioxidants and iron chelating drugs/prodrugs. Curr. Drug Metab. 2010, 11, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Pelle, E.; Jian, J.; Zhang, Q.; Muizzuddin, N.; Yang, Q.; Dai, J.; Maes, D.; Pernodet, N.; Yarosh, D.B.; Frenkel, K.; et al. Menopause increases the iron storage protein ferritin in skin. J. Cosmet. Sci. 2013, 64, 175–179. [Google Scholar] [PubMed]
- Zhu, X.; Jiang, M.; Song, E.; Jiang, X.; Song, Y. Selenium deficiency sensitizes the skin for UV-B-induced oxidative damage and inflammation which involved the activation of p38 MAPK signaling. Food Chem. Toxicol. 2015, 75, 139–145. [Google Scholar] [CrossRef]
- Sengupta, A.; Lichti, U.F.; Carlson, B.A.; Ryscavage, A.O.; Gladyshev, V.N.; Yuspa, S.H.; Hatfield, D.L. Selenoproteins are essential for proper keratinocyte function and skin development. PLoS ONE 2010, 5, e12249. [Google Scholar] [CrossRef] [Green Version]
- Alqanatish, J.T.; Alqahtani, F.; Alsewairi, W.M.; Al-Kenaizan, S. Childhood scurvy: An unusual cause of refusal to walk in a child. Pediatr. Rheumatol. 2015, 13, 23. [Google Scholar] [CrossRef] [Green Version]
- Ellinger, S.; Stehle, P. Efficacy of vitamin supplementation in situations with wound healing disorders: Results from clinical intervention studies. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 588–595. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- Pasini, E.; Corsetti, G.; Aquilani, R.; Romano, C.; Picca, A.; Calvani, R.; Dioguardi, F. Protein- amino acid metabolism disarrangements: The hidden enemy of chronic age-related conditions. Nutrients 2018, 10, 391. [Google Scholar] [CrossRef] [Green Version]
- Bellizzi, V.; Calella, P.; Carrero, J.J.; Fouque, D. Very low-protein diet to postpone renal failure: Pathophysiology and clinical applications in chronic kidney disease. Chronic Dis. Transl. Med. 2018, 4, 45–50. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Chung, M.; Fu, Z.; Insogna, K.L.; Karlsen, M.C.; LeBoff, M.S.; Shapses, S.A.; Sackey, J.; Shi, J.; Wallace, T.C.; et al. Animal versus plant protein and adult bone health: A systematic review and meta-analysis from the National Osteoporosis Foundation. PLoS ONE 2018, 13, e0192459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strasser, B.; Volaklis, K.; Fuchs, D.; Burtscher, M. Role of dietary protein and muscular fitness on longevity and aging. Aging Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanjani, N.A.; Vafa, M. Protein restriction, epigenetic diet, intermittent fasting as new approaches for preventing age-associated diseases. Int. J. Prev. Med. 2018, 9, 58. [Google Scholar] [PubMed]
- Haresaku, S.; Hanioka, T.; Tsutsui, A.; Watanabe, T. Association of lip pigmentation with smoking and gingival melanin pigmentation. Oral Dis. 2007, 13, 71–76. [Google Scholar] [CrossRef]
- Sandby-Moller, J.; Poulsen, T.; Wulf, H.C. Epidermal thickness at different body sites: Relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm. Venereol. 2003, 83, 410–413. [Google Scholar] [CrossRef]
- Fatani, A.Z.; Alshamrani, H.M.; Alshehri, K.A.; Almaghrabi, A.Y.; Alzahrani, Y.A.; Abduljabbar, M.H. Awareness on the association between skin aging and smoking: Impact on smoking quitting. Imam J. Appl. Sci. 2020, 5, 33. [Google Scholar]
- Dupati, A.; Helfrich, Y.R. Effect of cigarette smoking on skin aging. Expert Rev. Dermatol. 2014, 4, 371–378. [Google Scholar] [CrossRef]
- Theocharidis, V.; Katsaros, I.; Sgouromallis, E.; Serifis, N.; Boikou, V.; Tasigiorgos, S.; Kokosis, G.; Economopoulos, K. Current evidence on the role of smoking in plastic surgery elective procedures: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 624–636. [Google Scholar] [CrossRef]
- Farkas, A.; Kemény, L.; Széll, M.; Dobozy, A. Ethanol and acetone stimulate the proliferation of Ha Ca T keratinocytes: The possible role of alcohol in exacerbating psoriasis. Arch. Dermatol. Res. 2003, 295, 56–62. [Google Scholar] [CrossRef]
- Park, H.; Kim, K. Association of alcohol consumption with lipid profile in hypertensive men. Alcohol Alcohol. 2012, 47, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Goodman, G.D.; Kaufman, J.; Day, D.; Weiss, R.; Kawata, A.K.; Garcia, J.K.; Santangelo, S.; Gallagher, C.J. Impact of Smoking and Alcohol Use on Facial Aging in Women: Results of a Large Multinational, Multiracial, Cross-sectional Survey. J. Clin. Aesthet. Dermatol. 2019, 12, 28–39. [Google Scholar] [PubMed]
- Kuprys, P.V.; Tsukamoto, H.; Gao, B.; Jia, L.; McGowan, J.; Coopersmith, C.M.; Moreno, M.C.; Hulsebus, H.; Meena, A.S.; Souza-Smith, F.M.; et al. Summary of the 2018 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol 2019, 77, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandini, S.; Masala, G.; Palli, D.; Cavicchi, B.; Saieva, C.; Ermini, I.; Baldini, F.; Gnagnarella, P.; Caini, S. Alcohol, alcoholic beverages, and melanoma risk: A systematic literature review and dose–response meta-analysis. Eur. J. Nutr. 2018, 57, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Meksiarun, P.; Maeda, Y.; Hiroi, T.; Andriana, B.B.; Sato, H. Analysis of the effects of dietary fat on body and skin lipids of hamsters by Raman spectroscopy. Analyst 2015, 140, 4238–4244. [Google Scholar] [CrossRef]
- Rosa, D.F.; Sarandy, M.M.; Novaes, R.D.; Freitas, M.B.; do Carmo Gouveia Pelúzio, M.; Gonçalves, R.V. High-fat diet and alcohol intake promotes inflammation and impairs skin wound healing in Wistar rats. Mediat. Inflamm. 2018, 2018, 4658583. [Google Scholar] [CrossRef]
- Rosa, D.F.; Sarandy, M.M.; Novaes, R.D.; da Matta, S.L.P.; Goncalves, R.V. Effect of a high-fat diet and alcohol on cutaneous repair: A systematic review of murine experimental models. PLoS ONE 2017, 12, e0176240. [Google Scholar] [CrossRef] [Green Version]
- Vaid, M.; Singh, T.; Prasad, R.; Katiyar, S.K. Intake of high-fat diet stimulates the risk of ultraviolet radiation-induced skin tumors and malignant progression of papillomas to carcinoma in skh-1 hairless mice. Toxicol. Appl. Pharmacol. 2014, 274, 147–155. [Google Scholar] [CrossRef]
- Herbert, D.; Franz, S.; Popkova, Y.; Anderegg, U.; Schiller, J.; Schwede, K.; Lorz, A.; Simon, J.C.; Saalbach, A. High-Fat Diet Exacerbates Early Psoriatic Skin Inflammation Independent of Obesity: Saturated Fatty Acids as Key Players. J. Investig. Dermatol. 2018, 138, 1999–2009. [Google Scholar] [CrossRef] [Green Version]
- Higashi, Y.; Yamakuchi, M.; Fukushige, T.; Ibusuki, A.; Hashiguchi, T.; Kanekura, T. High-fat diet exacerbates imiquimod-induced psoriasis-like dermatitis in mice. Exp. Dermatol. 2018, 27, 178–184. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Rao, E.; Sun, Y.; Grossmann, M.E.; Morris, R.J.; Cleary, M.P.; Li, B. Epidermal fatty acid binding protein promotes skin inflammation induced by high-fat diet. Immunity 2015, 42, 953–964. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.P.; Katta, R. Sugar sag: Glycation and the role of diet in aging skin. Skin Ther. Lett. 2015, 20, 1–5. [Google Scholar]
- Draelos, Z.D. Aging skin: The role of diet: Facts and controversies. Clin. Dermatol. 2013, 31, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Danby, F.W. Nutrition and aging skin: Sugar and glycation. Clin. Dermatol. 2010, 28, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Shuang, E.; Yamamoto, K.; Tsuduki, T. Carbohydrate-restricted diet promotes skin senescence in senescence-accelerated prone mice. Biogerontology 2019, 20, 71–82. [Google Scholar] [CrossRef]
- Fuchs, E.; Green, H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell 1981, 25, 617–625. [Google Scholar] [CrossRef]
- Fisher, G.J.; Talwar, H.S.; Lin, J.; Voorhees, J.J. Molecular mechanisms of photoaging in human skin in vivo and their prevention by all-trans retinoic acid. Photochem. Photobiol. 1999, 69, 154–157. [Google Scholar] [CrossRef]
- Brescoll, J.; Daveluy, S. A review of vitamin B12 in dermatology. Am. J. Clin. Dermatol. 2015, 16, 27–33. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [Green Version]
- Gordon-Thomson, C.; Tongkao-on, W.; Song, E.J.; Carter, S.E.; Dixon, K.M.; Mason, R.S. Protection from ultraviolet damage and photocarcinogenesis by vitamin D compounds. In Sunlight, Vitamin D and Skin Cancer; Springer: New York, NY, USA, 2014; Volume 17, pp. 303–328. [Google Scholar]
- Wu, Y.; Zheng, X.; Xu, X.-G.; Li, Y.-H.; Wang, B.; Gao, X.; Chen, H.; Yatskayer, M.; Oresajo, C. Protective effects of a topical antioxidant complex containing vitamins C and E and ferulic acid against ultraviolet irradiation-induced photodamage in Chinese women. J. Drugs Dermatol. JDD 2013, 12, 464–468. [Google Scholar]
- Schempp, C.M.; Meinke, M.C.; Lademann, J.; Ferrari, Y.; Brecht, T.; Gehring, W. Topical antioxidants protect the skin from chemical-induced irritation in the repetitive washing test: A placebo-controlled, double-blind study. Contact Dermat. 2012, 67, 234–237. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef] [Green Version]
- Callaghan, T.M.; Wilhelm, K.P. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part I: Cellular and molecular perspectives of skin ageing. Int. J. Cosmet. Sci. 2008, 30, 313–322. [Google Scholar] [CrossRef]
- Ratnam, D.V.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Kumar, M.R. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control. Release 2006, 113, 189–207. [Google Scholar] [CrossRef]
- Kandola, K.; Bowman, A.; Birch-Machin, M.A. Oxidative stress-a key emerging impact factor in health, ageing, lifestyle and aesthetics. Int. J. Cosmet. Sci. 2015, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Suzuki, N. Isolation of collagen from fish waste material-skin, bone and fins. Food Chem. 2000, 68, 277–281. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Qian, J.; Liang, Q.; Wang, Z.; Xu, J.; He, S.; Ma, H. Bioavailability and Bioavailable Forms of Collagen after Oral Administration to Rats. J. Agric. Food Chem. 2015, 63, 3752–3756. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, D.; Zhao, Z.; Wu, J.; Zhao, M. Regulation by walnut protein hydrolysate on the components and structural degradation of photoaged skin in SD rats. Food Funct. 2019, 10, 6792–6802. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Bertoglio, J.C.; Polimeni, A.; Scapagnini, G. Cytoprotective polyphenols against chronological skin aging and cutaneous photodamage. Curr. Pharm. Des. 2018, 24, 99–105. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Lin, Y.K.; Lin, C.F.; Wang, P.W.; Chen, E.L.; Fang, J.Y. Elucidating the skin delivery of aglycone and glycoside flavonoids: How the structures affect cutaneous absorption. Nutrients 2017, 9, 1304. [Google Scholar] [CrossRef] [Green Version]
- Korkina, L.; De Luca, C.; Pastore, S. Plant polyphenols and human skin: Friends or foes. Ann. N. Y. Acad. Sci. 2012, 1259, 77–86. [Google Scholar] [CrossRef]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Yuan, Z.; He, X. Research progress on pharmacological effects of astragalus polysaccharides. Chin. J. Biochem. Med. 2012, 5, 692–694. [Google Scholar]
- Wen, L.; Gao, Q.; Ma, C.-W.; Ge, Y.; You, L.; Liu, R.H.; Fu, X.; Liu, N. Effect of polysaccharides from Tremella fuciformis on UV-induced photoaging. J. Funct. Foods 2016, 20, 400–410. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.J. Protective effect of sulfated polysaccharides from celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-Induced skin damage by regulating NF-κB, AP-1, and MAPKs signaling pathways in vitro in human dermal fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, Z.; Peng, L.; Jiang, N.; Liu, Q.; Zhang, E.; Liang, B.; Li, R.; Zhu, H. Lycium barbarum polysaccharide protects human keratinocytes against UV-B-induced photo-damage. Free Radic. Res. 2017, 51, 200–210. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhou, F.; Lei, L.; Chen, J.; Lu, J.; Zhou, J.; Cao, K.; Gao, L.; Xia, F.; Ding, S.; et al. Ganoderma lucidum polysaccharides protect fibroblasts against UV-B-induced photoaging. Mol. Med. Rep. 2016, 15, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Ji, D.; You, L.; Zhou, L.; Zhao, Z.; Brennan, C. Structural properties and protective effect of Sargassum fusiforme polysaccharides against ultraviolet B radiation in hairless Kun Ming mice. J. Funct. Foods 2018, 43, 8–16. [Google Scholar] [CrossRef]
- Pan, W.-J.; Ding, Q.-Y.; Wang, Y.; Wang, D.-D.; Lu, Y.; Yang, W.-W.; Cai, Z.-N.; Cheng, X.-D.; Zhang, W.-N.; Chen, Y. A bioactive polysaccharide TLH-3 isolated from Tricholoma lobayense protects against oxidative stress-induced premature senescence in cells and mice. J. Funct. Foods 2018, 42, 159–170. [Google Scholar] [CrossRef]
- Suganuma, K.; Shiobara, M.; Sato, Y.; Nakanuma, C.; Maekawa, T.; Ohtsuki, M.; Yazawa, K.; Imokawa, G. Anti-aging and functional improvement effects for the skin by functional foods intakes: Clinical effects on skin by oral ingestion of preparations containing Astaxanthin and Vitamins C and E. Jichi Med. Univ. J. 2012, 35, 25–33. [Google Scholar]
- Pappas, A.; Fantasia, J.; Chen, T. Age and ethnic variations in sebaceous lipids. Derm. Endocrinol. 2013, 5, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Horrobin, D.F. Essential fatty acids in clinical dermatology. J. Am. Acad. Dermatol. 1989, 20, 1045–1053. [Google Scholar] [CrossRef]
- Romana-Souza, B.; Monte-Alto-Costa, A. Olive oil reduces chronic psychological stress-induced skin aging in mice through the NF-κB and NRF2 pathways. J. Funct. Foods 2019, 54, 310–319. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, J.; Jung, S.; Sung, K.H.; Son, Y.K.; Bae, J.M.; Kim, B.H. Alleviation of ultraviolet B-induced photoaging by 7-MEGATM 500 in hairless mouse skin. Toxicol. Res. 2019, 35, 353. [Google Scholar] [CrossRef] [PubMed]
- Song, I.B.; Gu, H.; Han, H.J.; Lee, N.Y.; Cha, J.Y.; Son, Y.K.; Kwon, J. Effects of 7-MEGA? 500 on oxidative stress, inflammation, and skin regeneration in H2O2-treated skin cells. Toxicol. Res. 2018, 34, 103–110. [Google Scholar] [CrossRef]
- Balkrishna, A.; Nain, P.; Chauhan, A.; Sharma, N.; Gupta, A.; Ranjan, R.; Varshney, A. Super Critical Fluid Extracted Fatty Acids from Withania somnifera Seeds Repair Psoriasis-Like Skin Lesions and Attenuate Pro-Inflammatory Cytokines (TNF-α and IL-6) Release. Biomolecules 2020, 10, 185. [Google Scholar] [CrossRef] [Green Version]
- Hyun, Y.J.; Piao, M.J.; Kang, K.A.; Zhen, A.X.; Fernando, P.D.S.M.; Kang, H.K.; Ahn, Y.S.; Hyun, J.W. Effect of Fermented Fish Oil on Fine Particulate Matter-Induced Skin Aging. Mar. Drugs 2019, 17, 61. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wu, J. Modulating effect of fatty acids and sterols on skin aging. J. Funct. Foods 2019, 57, 135–140. [Google Scholar] [CrossRef]
- Tsuji, R.; Komano, Y.; Ohshio, K.; Ishii, N.; Kanauchi, O. Long-term administration of pDC stimulative lactic acid bacteria, Lactococcus lactis strain Plasma, prevents immune-senescence and decelerates individual senescence. Exp. Gerontol. 2018, 111, 10–16. [Google Scholar] [CrossRef]
- Shin, D.; Lee, Y.; Huang, Y.H.; Lim, H.W.; Jang, K.; Kim, D.D.; Lim, C.J. Probiotic fermentation augments the skin anti-photoaging properties of Agastache rugosa through up-regulating antioxidant components in UV-B-irradiated HaCaT keratinocytes. BMC Complement. Altern. Med. 2018, 18, 196. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.; Cheng, X.; Meng, M.; Luo, Y.; Li, B. The anti-photoaging effect of antioxidant collagen peptides from silver carp (Hypophthalmichthys molitrix) skin is preferable to tea polyphenols and casein peptides. Food Funct. 2017, 8, 1698–1707. [Google Scholar] [CrossRef]
- Chen, T.; Hou, H.; Lu, J.; Zhang, K.; Li, B. Protective effect of gelatin and gelatin hydrolysate from salmon skin on UV irradiation- induced photoaging of mice skin. J. Ocean Univ. China 2016, 15, 711–718. [Google Scholar] [CrossRef]
- Kang, M.C.; Yumnam, S.; Kim, S.Y. Oral intake of collagen peptide attenuates ultraviolet B irradiation- induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int. J. Mol. Sci. 2018, 19, 3551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zague, V.; do Amaral, J.B.; Rezende Teixeira, P.; de Oliveira Niero, E.L.; Lauand, C.; Machado-Santelli, G.M. Collagen peptides modulate the metabolism of extracellular matrix by human dermal fibroblasts derived from sun-protected and sun-exposed body sites. Cell Biol. Int. 2018, 42, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.U.; Chung, H.C.; Choi, J.; Sakai, Y.; Lee, B.Y. Oral intake of low-molecular-weight collagen peptide improves hydration, elasticity, and wrinkling in human skin: A randomized, double-blind, placebo- controlled study. Nutrients 2018, 10, 826. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Jiang, Y.; Wang, X.; Zhou, J.; Cui, H.; Xu, W.; He, Y.; Ma, H.; Gao, R. Effect of oral administration of collagen hydrolysates from Nile tilapia on the chronologically aged skin. J. Funct. Foods 2018, 44, 112–117. [Google Scholar] [CrossRef]
- Liu, Y.; Su, G.; Zhou, F.; Zhang, J.; Zheng, L.; Zhao, M. Protective effect of bovine elastin peptides against photoaging in mice and identification of novel antiphotoaging peptides. J. Agric. Food Chem. 2018, 66, 10760–10768. [Google Scholar] [CrossRef]
- Song, H.; Zhang, L.; Luo, Y.; Zhang, S.; Li, B. Effects of collagen peptides intake on skin ageing and platelet release in chronologically aged mice revealed by cytokine array analysis. J. Cell. Mol. Med. 2018, 22, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Offengenden, M.; Chakrabarti, S.; Wu, J. Chicken collagen hydrolysates differentially mediate anti- inflammatory activity and type I collagen synthesis on human dermal fibroblasts. Food Sci. Hum. Wellness 2018, 7, 138–147. [Google Scholar] [CrossRef]
- Wang, X.; Hong, H.; Wu, J. Hen collagen hydrolysate alleviates UV-A-induced damage in human dermal fibroblasts. J. Funct. Foods 2019, 63, 103574. [Google Scholar] [CrossRef]
- Song, H.; Meng, M.; Cheng, X.; Li, B.; Wang, C. The effect of collagen hydrolysates from silver carp (Hypophthalmichthys molitrix) skin on UV-induced photoaging in mice: Molecular weight affects skin repair. Food Funct. 2017, 8, 1538–1546. [Google Scholar] [CrossRef]
- Inoue, N.; Sugihara, F.; Wang, X. Ingestion of bioactive collagen hydrolysates enhance facial skin moisture and elasticity and reduce facial ageing signs in a randomised double-blind placebo-controlled clinical study. J. Sci. Food Agric. 2016, 96, 4077–4081. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Karnjanapratum, S.; Visessanguan, W. Hydrolysed collagen from Lates calcarifer skin: Its acute toxicity and impact on cell proliferation and collagen production of fibroblasts. Int. J. Food Sci. Technol. 2018, 53, 1871–1879. [Google Scholar] [CrossRef]
- Lu, J.; Hou, H.; Fan, Y.; Yang, T.; Li, B. Identification of MMP-1 inhibitory peptides from cod skin gelatin hydrolysates and the inhibition mechanism by MAPK signaling pathway. J. Funct. Foods 2017, 33, 251–260. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Song, H.; He, J.; Li, G.; Zheng, Y.; Li, B. Collagen peptides promote photoaging skin cell repair by activating the TGF-β/Smad pathway and depressing collagen degradation. Food Funct. 2019, 10, 6121–6134. [Google Scholar] [CrossRef]
- Song, H.; Zhang, S.; Zhang, L.; Li, B. Effect of orally administered collagen peptides from bovine bone on skin aging in chronologically aged mice. Nutrients 2017, 9, 1209. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-J.; Jang, H.-L.; Ahn, D.-K.; Kim, H.-J.; Jeon, H.Y.; Seo, D.B.; Lee, J.-H.; Choi, J.K.; Kang, S.-S. Orally administered collagen peptide protects against UV-B-induced skin aging through the absorption of dipeptide forms, Gly-Pro and Pro-Hyp. Biosci. Biotechnol. Biochem. 2019, 83, 1146–1156. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Bai, F.; Fang, Y.; Wang, J.; Gao, R. The anti-skin-aging effect of oral administration of gelatin from the swim bladder of Amur sturgeon (Acipenser schrenckii). Food Funct. 2019, 10, 3890–3897. [Google Scholar] [CrossRef]
- Liu, S.; You, L.; Zhao, Y.; Chang, X. Hawthorn polyphenol extract inhibits UV-B-induced skin photoaging by regulating MMP expression and type I procollagen production in mice. J. Agric. Food Chem. 2018, 66, 8537–8546. [Google Scholar] [CrossRef]
- Liu, S.; Sui, Q.; Zou, J.; Zhao, Y.; Chang, X. Protective effects of hawthorn (Crataegus pinnatifida) polyphenol extract against UV-B-induced skin damage by modulating the p53 mitochondrial pathway in vitro and in vivo. J. Food Biochem. 2019, 43, e12708. [Google Scholar] [CrossRef]
- Nobile, V.; Michelotti, A.; Cestone, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Pérez-Sánchez, A.; Micol, V. Skin photoprotective and antiageing effects of a combination of rosemary (Rosmarinus officinalis) and grapefruit (Citrus paradisi) polyphenols. Food Nutr. Res. 2016, 60, 31871. [Google Scholar] [CrossRef]
- Kwon, K.R.; Alam, M.B.; Park, J.H.; Kim, T.H.; Lee, S.H. Attenuation of UV-B-induced photo-aging by polyphenolic-rich Spatholobus Suberectus stem extract via modulation of MAPK/AP-1/MMPs signaling in human keratinocytes. Nutrients 2019, 11, 1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Liu, B.; Zhuang, Y. Effects of rambutan (Nephelium lappaceum) peel phenolics and Leu-Ser-Gly-Tyr-Gly-Pro on hairless mice skin photoaging induced by ultraviolet irradiation. Food Chem. Toxicol. 2019, 129, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.I.; Bae, S.; Ahn, K.J. Flavonoid Silibinin Increases Hair-Inductive Property Via Akt and Wnt/β-Catenin Signaling Activation in 3-Dimensional-Spheroid Cultured Human Dermal Papilla Cells. J. Microbiol. Biotechnol. 2019, 29, 321–329. [Google Scholar] [CrossRef]
- Kim, H.I.; Jeong, Y.U.; Kim, J.H.; Park, Y.J. 3, 5, 6, 7, 8, 3′, 4′-Heptamethoxyflavone, a citrus flavonoid, inhibits collagenase activity and induces type I procollagen synthesis in HDFn cells. Int. J. Mol. Sci. 2018, 19, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, D.; Choi, J.G.; Huh, E.; Oh, M.S. Tectorigenin, a Flavonoid-Based Compound of Leopard Lily Rhizome, Attenuates UV-B-Induced Apoptosis and Collagen Degradation by Inhibiting Oxidative Stress in Human Keratinocytes. Nutrients 2018, 10, 1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Dang, L.; Guo, F.; Wang, X.; Zhao, W.; Zhao, R. Coenzyme Q10 enhances dermal elastin expression, inhibits IL-1α production and melanin synthesis in vitro. Int. J. Cosmet. Sci. 2012, 34, 273–279. [Google Scholar] [CrossRef]
- Ahmadi Ashtiani, H.R.; Rastegar, H.; Salarian, A.A.; Rahmani, F.; Rezazadeh, S.; Sedghi Zadeh, H. Study the Effect of Silymarin and Vitamin C in Skin Aging Induced by UV-B Rays on the Mice Skin Redox System. J. Med. Plants 2019, 3, 130–144. [Google Scholar]
- Zhen, A.X.; Piao, M.J.; Kang, K.A.; Fernando, P.D.S.M.; Kang, H.K.; Koh, Y.S.; Yi, J.M.; Hyun, J.W. Niacinamide Protects Skin Cells from Oxidative Stress Induced by Particulate Matter. Biomol. Ther. 2019, 27, 562–569. [Google Scholar] [CrossRef]
- Tiwari, P.; Nagatake, T.; Hirata, S.-I.; Sawane, K.; Saika, A.; Shibata, Y.; Morimoto, S.; Honda, T.; Adachi, J.; Abe, Y.; et al. Dietary coconut oil ameliorates skin contact hypersensitivity through mead acid production in mice. Allergy 2019, 74, 1522–1532. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging—From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. https://doi.org/10.3390/nu12030870
Cao C, Xiao Z, Wu Y, Ge C. Diet and Skin Aging—From the Perspective of Food Nutrition. Nutrients. 2020; 12(3):870. https://doi.org/10.3390/nu12030870
Chicago/Turabian StyleCao, Changwei, Zhichao Xiao, Yinglong Wu, and Changrong Ge. 2020. "Diet and Skin Aging—From the Perspective of Food Nutrition" Nutrients 12, no. 3: 870. https://doi.org/10.3390/nu12030870
APA StyleCao, C., Xiao, Z., Wu, Y., & Ge, C. (2020). Diet and Skin Aging—From the Perspective of Food Nutrition. Nutrients, 12(3), 870. https://doi.org/10.3390/nu12030870





