Nutritional Challenges in Pregnant Women with Renal Diseases: Relevance to Fetal Outcomes
Abstract
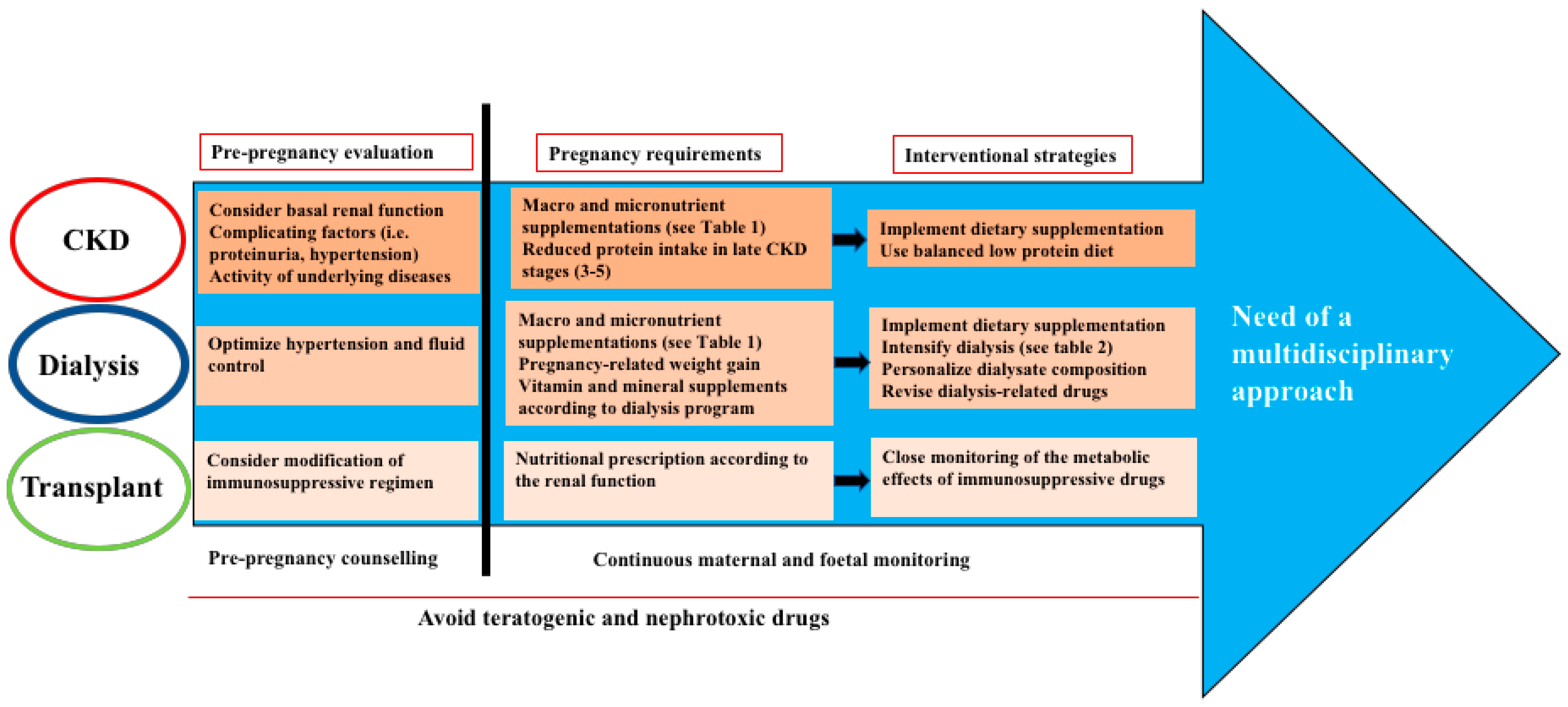
:1. Pregnancy in Women with Renal Disease: General Considerations
2. Pregnancy in Women with Renal Disease: Nutritional Issues
3. CKD Non-Dialysis
3.1. Pregnancy in CKD Non-Dialysis Women
3.2. Principles of Nutritional Management in Pregnant Women and Non-Dialysis Treated CKD
3.3. Clinical Experiences
4. Stage 5 CKD Women Treated with Maintenance Dialysis
4.1. Pregnancy in Women on Dialysis: A Still Challenging Condition
4.2. Principles of Nutritional Management in Pregnant Women on Dialysis
4.3. Clinical Experiences
5. Kidney Transplant
5.1. Pregnancy after Renal Transplantation
5.2. Nutritional Management in Pregnant Kidney Transplant Patients
6. Conclusions
Financial Disclosures
Author Contributions
Funding
Conflicts of Interest
References
- Williams, D.; Davison, J. Chronic kidney disease in pregnancy. BMJ 2008, 336, 211–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhunaizi, A.; Melamed, N.; Hladunewich, M.A. Pregnancy in advanced chronic kidney disease and end-stage renal disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.; Sharma, S.; Holmen, J.; Palit, S.; Nuccio, E.; Chonchol, M. Kidney disease and maternal and fetal outcomes in pregnancy. Am. J. Kidney Dis. 2015, 66, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, A.; Mohammadi, F.; Jesudason, S. Managing pregnancy in chronic kidney disease: Improving outcomes for mother and baby. Int. J. Womens Health 2016, 8, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacchetta, J.; Harambat, J.; Dubourg, L.; Guy, B.; Liutkus, A.; Canterino, I.; Kassaı, B.; Putet, G.; Cochat, P. Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney Int. 2009, 76, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Piccoli, G.B.; Cabiddu, G.; Attini, R.; Vigotti, F.N.; Maxia, S.; Lepori, N.; Tuveri, M.; Massidda, M.; Marchi, C.; Mura, S.; et al. Risk of Adverse Pregnancy Outcomes in Women with CKD. J. Am. Soc. Nephrol. 2015, 26, 2011–2022. [Google Scholar] [CrossRef] [Green Version]
- Imbasciati, E.; Gregorini, G.; Cabiddu, G.; Gammaro, L.; Ambroso, G.; Del Giudice, A.; Ravani, P. Pregnancy in CKD stages 3 to 5: Fetal and maternal outcomes. Am. J. Kidney Dis. 2007, 49, 753–762. [Google Scholar] [CrossRef]
- Wiles, K.S.; Nelson-Piercy, C.; Bramham, K. Reproductive health and pregnancy in women with chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 165–184. [Google Scholar] [CrossRef]
- Limardo, M.; Imbasciati, E.; Ravani, P.; Surian, M.; Torres, D.; Gregorini, G.; Magistroni, R.; Casellato, D.; Gammaro, L.; Pozzi, C. Rene e Gravidanza Collaborative Group of the Italian Society of Nephrology. Pregnancy and progression of IgA nephropathy: Results of an Italian multicenter study. Am. J. Kidney Dis. 2010, 56, 506–512. [Google Scholar] [CrossRef] [Green Version]
- Snoek, R.; van der Graaf, R.; Meinderts, J.R.; van Reekum, F.; Bloemenkamp, K.W.M.; Knoers, N.V.A.M.; van Eerde, A.M.; Lely, A.T. Pregnancy in Advanced Kidney Disease: Clinical Practice Considerations on a Challenging Combination. Nephron 2020, 1–5. [Google Scholar] [CrossRef]
- Cabiddu, G.; Castellino, S.; Gernone, G.; Santoro, D.; Moroni, G.; Giannattasio, M.; Gregorini, G.; Giacchino, F.; Attini, R.; Loi, V.; et al. A best practice position statement on pregnancy in chronic kidney disease: The Italian Study Group on Kidney and Pregnancy. J. Nephrol. 2016, 29, 277–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Recommendations on Antenatal Care for a Positive Pregnancy Experience; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Imdad, A.; Bhutta, Z.A. Maternal nutrition and birth outcomes: Effect of balanced protein-energy supplementation. Paediatr. Perinat. Epidemiol. 2012, 26, 178–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood-Bradley, R.J.; Barrand, S.; Giot, A.; Armitage, J.A. Understanding the role of maternal diet on kidney development; an opportunity to improve cardiovascular and renal health for future generations. Nutrients 2015, 7, 1881–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cupisti, A.; Brunori, G.; Di Iorio, B.R.; D’Alessandro, C.; Pasticci, F.; Cosola, C.; Bellizzi, V.; Bolasco, P.; Capitanini, A.; Fantuzzi, A.L.; et al. Nutritional treatment of advanced CKD: Twenty consensus statements. J. Nephrol. 2018, 31, 457–473. [Google Scholar] [CrossRef] [Green Version]
- Stover, J. Nutritional management of pregnancy in chronic kidney disease. Adv. Chronic Kidney Dis. 2007, 14, 212–214. [Google Scholar] [CrossRef]
- Moroni, G.; Doria, A.; Giglio, E.; Tani, C.; Zen, M.; Strigini, F.; Zaina, B.; Tincani, A.; de Liso, F.; Matinato, C.; et al. Fetal outcome and recommendations of pregnancies in lupus nephritis in the 21st century. A prospective multicenter study. J. Autoimmun. 2016, 74, 6–12. [Google Scholar] [CrossRef]
- Bell, R.; Glinianaia, S.V.; Tennant, P.W.; Bilous, R.W.; Rankin, J. Peri-conception hyperglycaemia and nephropathy are associated with risk of congenital anomaly in women with pre-existing diabetes: A population-based cohort study. Diabetologia 2012, 55, 936–947. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Wang, D.; Zand, L.; Harris, P.C.; White, W.M.; Garovic, V.D.; Kermott, C.A. Pregnancy outcomes in autosomal dominant polycystic kidney disease: A case-control study. J. Matern. Fetal Neonatal Med. 2016, 29, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Pradeep, Y. Pregnancy in women with chronic kidney disease. Clin. Queries Nephrol. 2012, 3, 205–214. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Piccoli, G.B.; Calella, P.; Brunori, G.; Pasticci, F.; Egidi, M.F.; Capizzi, I.; Bellizzi, V.; Cupisti, A. “Dietaly”: Practical issues for the nutritional management of CKD patients in Italy. BMC Nephrol. 2016, 17, 102. [Google Scholar] [CrossRef]
- James, G.; Jackson, H. European Guidelines for the Nutritional Care of Adult Renal Patients. EDTNA-ERCA J. 2003, 29, 23–43. [Google Scholar] [CrossRef]
- Hanson, M.A.; Bardsley, A.; De-Regil, L.M.; Moore, S.E.; Oken, E.; Poston, L.; Ma, R.C.; McAuliffe, F.M.; Maleta, K.; Purandare, C.N.; et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First”. Int. J. Gynaecol. Obstet. 2015, 131, S213. [Google Scholar] [CrossRef] [Green Version]
- A Concise, Practical Resource for Comprehensive Nutrition Care in CKD. In Pocket Guide to Nutrition Assessment of the Patient with Chronic Kidney Disease, 4th ed.; National Kidney Foundation: New York, NY, USA, 2009.
- Reynolds, R.M.; Allan, K.M.; Raja, E.A.; Bhattacharya, S.; McNeill, G.; Hannaford, P.C.; Sarwar, N.; Lee, A.J.; Bhattacharya, S.; Norman, J.E. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: Follow-up of 1,323,275 person years. BMJ 2013, 347, f4539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppe, C.C.; Evans, R.G.; Bertram, J.F.; Moritz, K.M. Effects of dietary protein restriction on nephron number in the mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1768–R1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symonds, M.E.; Stephenson, T.; Gardner, D.S.; Budge, H. Long-term effects of nutritional programming of the embryo and fetus: Mechanisms and critical windows. Reprod. Fertil. Dev. 2007, 19, 53–63. [Google Scholar] [CrossRef]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S.; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008, 371, 340–357. [Google Scholar] [CrossRef] [Green Version]
- Barsotti, G.; Guiducci, A.; Ciardella, F.; Giovannetti, S. Effects on renal function of a low-nitrogen diet supplementedwith essential amino acids and ketoanalogues and of hemodialysis and free protein supply in patients with chronic renal failure. Nephron 1981, 27, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Ferraresi, M.; Deagostini, M.C.; Vigotti, F.N.; Consiglio, V.; Scognamiglio, S.; Moro, I.; Clari, R.; Fassio, F.; Biolcati, M.; et al. Vegetarian low-protein diets supplemented with keto analogues: A niche for the few or an option for many. Nephrol. Dial. Transpl. 2013, 28, 2295–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-López, M.A.; Piccoli, G.B.; Leone, F.; Orozco-Guillén, A.; Perichart-Perera, O. Nutrition care for chronic kidney disease during pregnancy: An updated review. Eur. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Attini, R.; Vasario, E.; Gaglioti, P.; Piccoli, E.; Consiglio, V.; Deagostini, C.; Oberto, M.; Todros, T. Vegetarian supplemented low-protein diets. A safe option for pregnant CKD patients: Report of 12 pregnancies in 11 patients. Nephrol. Dial. Transpl. 2011, 26, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Piccoli, G.B.; Leone, F.; Attini, R.; Parisi, S.; Fassio, F.; Deagostini, M.C.; Ferraresi, M.; Clari, R.; Ghiotto, S.; Biolcati, M.; et al. Association of low-protein supplemented diets with fetal growth in pregnant women with CKD. Clin. J. Am. Soc. Nephrol. 2014, 9, 864–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attini, R.; Leone, F.; Parisi, S.; Fassio, F.; Capizzi, I.; Loi, V.; Colla, L.; Rossetti, M.; Gerbino, M.; Maxia, S.; et al. Vegan-vegetarian low-protein supplemented diets in pregnant CKD patients: Fifteen years of experience. BMC Nephrol. 2016, 17, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attini, R.; Leone, F.; Montersino, B.; Fassio, F.; Minelli, F.; Colla, L.; Rossetti, M.; Rollino, C.; Alemanno, M.G.; Barreca, A.; et al. Pregnancy, Proteinuria, Plant-Based Supplemented Diets and Focal Segmental Glomerulosclerosis: A Report on Three Cases and Critical Appraisal of the Literature. Nutrients 2017, 9, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nava, J.; Moran, S.; Figueroa, V.; Salinas, A.; Lopez, M.; Urbina, R.; Gutierrez, A.; Lujan, J.L.; Orozco, A.; Montufar, R.; et al. Successful pregnancy in a CKD patient on a low-protein, supplemented diet: An opportunity to reflect on CKD and pregnancy in Mexico, an emerging country. J. Nephrol. 2017, 30, 877–882. [Google Scholar] [CrossRef]
- Gao, X.; Wu, J.; Dong, Z.; Hua, C.; Hu, H.; Mei, C. A low-protein diet supplemented with ketoacids plays a more protective role against oxidative stress of rat kidney tissue with 5/6 nephrectomy than a low-protein diet alone. Br. J. Nutr. 2010, 103, 608–616. [Google Scholar] [CrossRef] [Green Version]
- Piccoli, G.B.; Cabiddu, G.; Daidone, G.; Guzzo, G.; Maxia, S.; Ciniglio, I.; Postorino, V.; Loi, V.; Ghiotto, S.; Nichelatti, M.; et al. The children of dialysis: Live-born babies from on-dialysis mothers in Italy--an epidemiological perspective comparing dialysis, kidney transplantation and the overall population. Nephrol. Dial. Transplant. 2014, 29, 1578–1586. [Google Scholar] [CrossRef] [Green Version]
- Basok, E.K.; Atsu, N.; Rifaioglu, M.M.; Kantarci, G.; Yildirim, A.; Tokuc, R. Assessment of female sexual function and quality of life in predialysis, peritoneal dialysis, hemodialysis, and renal transplant patients. Int. Urol. Nephrol. 2009, 41, 473–481. [Google Scholar] [CrossRef]
- Öyekçin, D.G.; Gülpek, D.; Sahin, E.M.; Mete, L. Depression, anxiety, body image, sexual functioning, and dyadic adjustment associated with dialysis type in chronic renal failure. Int. J. Psychiatry Med. 2012, 43, 227–241. [Google Scholar] [CrossRef]
- Okundaye, I.; Abrinko, P.; Hou, S. Registry of pregnancy in dialysis patients. Am. J. Kidney Dis. 1998, 31, 766–773. [Google Scholar] [CrossRef]
- Hou, S. Pregnancy in women treated with dialysis: Lessons from a large series over 20 years. Am. J. Kidney Dis. 2010, 56, 5–6. [Google Scholar] [CrossRef]
- Shahir, A.K.; Briggs, N.; Katsoulis, J.; Levidiotis, V. An observational outcomes study from 1966–2008, examining pregnancy and neonatal outcomes from dialysed women using data from the ANZDATA Registry. Nephrology 2013, 18, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Conijn, A.; Consiglio, V.; Vasario, E.; Attini, R.; Deagostini, M.C.; Bontempo, S.; Todros, T. Pregnancy in dialysis patients: Is the evidence strong enough to lead us to change our counseling policy? Clin. J. Am. Soc. Nephrol. 2010, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Alp Ikizler, T.; Flakoll, P.J.; Parker, R.A.; Hakim, R.M. Amino acid and albumin losses during hemodialysis. Kidney Int. 1994, 46, 830–837. [Google Scholar] [CrossRef] [Green Version]
- Hou, S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am. J. Kidney Dis. 1999, 33, 235–252. [Google Scholar] [CrossRef]
- Wiggins, K.L. Nutrition care of adult pregnant ESRD patients. In Nutrition Care of Renal Patients, 3rd ed.; Burrowes, J., Ed.; The American Dietetic Association: Chicago, IL, USA, 2002. [Google Scholar]
- Shemin, D. Dialysis in pregnant women with chronic kidney disease. Semin. Dial. 2003, 16, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Molloy, A.M.; Kirke, P.N.; Brody, L.C.; Scott, J.M.; Mills, J.L. Effects of folate and vitamin B12 deficiencies during pregnancy on fetal, infant, and child development. Food Nutr. Bull. 2008, 29, S101–S115. [Google Scholar] [CrossRef] [PubMed]
- Bastos Maia, S.; Rolland Souza, A.S.; Caminha, C.; Lins da Silva, S.; Callou Cruz, R.D.S.B.L.; Carvalho dos Santos, C.; Batista Filho, M. Vitamin A and Pregnancy: A Narrative Review. Nutrients 2019, 11, 681. [Google Scholar] [CrossRef] [Green Version]
- Esposito, P.; Rampino, T.; Gregorini, M.; Tinelli, C.; De Silvestri, A.; Malberti, F.; Coppo, R.; Dal Canton, A.; IAMM Group. Management of mineral metabolism in hemodialysis patients: Discrepancy between interventions and perceived causes of failure. J. Nephrol. 2014, 27, 689–697. [Google Scholar] [CrossRef]
- Karras, S.N.; Wagner, C.L.; Castracane, V.D. Understanding vitamin D metabolism in pregnancy: From physiology to pathophysiology and clinical outcomes. Metab. Clin. Exp. 2018, 86, 112–123. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Vitamin D: Screening and Supplementation during Pregnancy; American College of Obstetricians and Gynecologists: Washington, DC, USA, 2011. [Google Scholar]
- Reddy, S.S.; Holley, J.L. Management of the pregnant chronic dialysis patient. Adv. Chronic Kidney Dis. 2007, 14, 146–155. [Google Scholar] [CrossRef]
- Vidal, M.; Ursu, M.; Martínez, A.; Sánchez, R.; Pereira, D. Nutritional control of pregnant women on chronic hemodialysis. J. Ren. Nutr. 1998, 8, 150–156. [Google Scholar] [CrossRef]
- Neogi, S.B.; Devasenapathy, N.; Singh, R.; Bhushan, H.; Shah, D.; Divakar, H.; Zodpey, S.; Malik, S.; Nanda, S.; Mittal, P.; et al. Safety and effectiveness of intravenous iron sucrose versus standard oral iron therapy in pregnant women with moderate-to-severe anaemia in India: A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Glob. Health 2019, 7, e1706–e1716. [Google Scholar] [CrossRef] [Green Version]
- Jagielski, J.B. Optimizing nutritional care for pregnant patients on hemodialysis. J. Ren. Nutr. 2015, 25, e19–e21. [Google Scholar] [CrossRef]
- Campos-Collado, A.X.; . Reyes-López, M.A.; Orozco-Guillén, A.; Muñoz-Manrique, C.; Perichart-Perera, O. Medical Nutrition Therapy for Chronic Kidney Disease in Pregnancy: A Case Report. J. Acad. Nutr. Diet. 2016, 116, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hladunewich, M.; Schatell, D. Intensive dialysis and pregnancy. Hemodial. Int. 2016, 20, 339–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccoli, G.B.; Minelli, F.; Versino, E.; Cabiddu, G.; Attini, R.; Vigotti, F.N.; Rolfo, A.; Giuffrida, D.; Colombi, N.; Pani, A.; et al. Pregnancy in dialysis patients in the new millennium: A systematic review and meta-regression analysis correlating dialysis schedules and pregnancy outcomes. Nephrol. Dial. Transpl. 2016, 31, 1915–1934. [Google Scholar] [CrossRef] [PubMed]
- Hladunewich, M.A.; Hou, S.; Odutayo, A.; Cornelis, T.; Pierratos, A.; Goldstein, M.; Tennankore, K.; Keunen, J.; Hui, D.; Chan, C.T. Intensive hemodialysis associates with improved pregnancy outcomes: A Canadian and United States cohort comparison. J. Am. Soc. Nephrol. 2014, 25, 1103–1109. [Google Scholar] [CrossRef] [Green Version]
- Asamiya, Y.; Otsubo, S.; Matsuda, Y.; Kimata, N.; Kikuchi, K.A.N.; Miwa, N.; Uchida, K.; Mineshima, M.; Mitani, M.; Ohta, H.; et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009, 75, 1217–1222. [Google Scholar] [CrossRef] [Green Version]
- Haase, M.; Morgera, S.; Bamberg, C.; Halle, H.; Martini, S.; Hocher, B.; Diekmann, F.; Dragun, D.; Peters, H.; Neumayer, H.H.; et al. A systematic approach to managing pregnant dialysis patients—The importance of an intensified haemodialfiltration protocol. Nephrol. Dial. Transpl. 2005, 20, 2537–2542. [Google Scholar] [CrossRef] [Green Version]
- Gangji, A.S.; Windrim, R.; Gandhi, S.; Silverman, J.A.; Chan, C.T. Successful pregnancy with nocturnal hemodialysis. Am. J. Kidney Dis. 2004, 44, 912–916. [Google Scholar] [CrossRef]
- Wiles, K.; Chappell, L.; Clark, K.; Elman, L.; Hall, M.; Lightstone, L.; Mohamed, G.; Mukherjee, D.; Nelson-Piercy, C.; Webster, P.; et al. Clinical practice guideline on pregnancy and renal disease. BMC Nephrol. 2019, 20, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morfin, J.A.; Fluck, R.J.; Weinhandl, E.D.; Kansal, S.; McCullough, P.A.; Komenda, P. Intensive Hemodialysis and Treatment Complications and Tolerability. Am. J. Kidney Dis. 2016, 68, S43–S50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangren, J.; Nadel, M.; Hladunewich, M.A. Pregnancy and End-Stage Renal Disease. Blood Purif. 2018, 45, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Quiroga, B.; Abad, S.; Aragoncillo, I.; Arroyo, D.; Panizo, N.; López-Gómez, J.M. Alumin leakage in online hemodiafiltration, more convective transport, more losses? Ther. Apher. Dial. 2015, 19, 267–271. [Google Scholar] [CrossRef]
- Hussain, S.; Savin, V.; Piering, W.; Tomasi, J.; Blumenthal, S. Phosphorus-enriched hemodialysis during pregnancy: Two case reports. Hemodial. Int. 2005, 9, 147–152. [Google Scholar] [CrossRef]
- Thomas, A.K.; McVie, R.; Levine, S.N. Disorders of maternal calcium metabolism implicated by abnormal calcium metabolism in the neonate. Am. J. Perinatol. 1999, 16, 515–520. [Google Scholar] [CrossRef]
- McKay, D.B.; Josephson, M.A.; Armenti, V.T.; August, P.; Coscia, L.A.; Davis, C.L.; Davison, J.M.; Easterling, T.; Friedman, J.E.; Hou, S.; et al. Reproduction and transplantation: Report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am. J. Transpl. 2005, 5, 1592–1599. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, F.; Budde, K.; Gerland, M.; Wiechers, C.; Heyne, N.; Nadalin, S.; Brucker, S. and Bachmann, C. Pregnancy following kidney transplantation—Impact on mother and graft function and focus on childrens’ longitudinal development. BMC Pregnancy Childbirth 2019, 19, 376. [Google Scholar] [CrossRef]
- Miniero, R.; Tardivo, I.; Curtoni, E.S.; Segoloni, G.P.; La Rocca, E.; Nino, A.; Todeschini, P.; Tregnaghi, C.; Rosati, A.; Zanelli, P.; et al. Pregnancy after renal transplantation in Italian patients: Focus on fetal outcome. J. Nephrol. 2002, 15, 626–632. [Google Scholar]
- Pregnancy Outcomes in Renal Transplant Recipients. Available online: https://clinicaltrials.gov/ct2/show/NCT03073434 (accessed on 10 March 2020).
- Cabiddu, G.; Spotti, D.; Gernone, G.; Santoro, D.; Moroni, G.; Gregorini, G.; Giacchino, F.; Attini, R.; Limardo, M.; Gammaro, L.; et al. A best-practice position statement on pregnancy after kidney transplantation: Focusing on the unsolved questions. The Kidney and Pregnancy Study Group of the Italian Society of Nephrology. J. Nephrol. 2018, 31, 665–681. [Google Scholar] [CrossRef] [Green Version]
- McGrory, C.H.; Ondeck-Williams, M.; Hilburt, N.; Constantinescu, S.; Silva, P.; Daller, J.A.; Coscia, L.A.; Armenti, V.T. Nutrition, pregnancy, and transplantation. Nutr. Clin. Pract. 2007, 22, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Park-Wyllie, L.; Mazzotta, P.; Pastuszak, A.; Moretti, M.E.; Beique, L.; Hunnisett, L.; Friesen, M.H.; Jacobson, S.; Kasapinovic, S.; Chang, D.; et al. Birth defects after maternal exposure to corticosteroids: Prospective cohort study and meta-analysis of epidemiological studies. Teratology 2000, 62, 385–392. [Google Scholar] [CrossRef]
- Chakkera, H.A.; Kudva, Y.; Kaplan, B. Calcineurin Inhibitors: Pharmacologic Mechanisms Impacting Both Insulin Resistance and Insulin Secretion Leading to Glucose Dysregulation and Diabetes Mellitus. Clin. Pharmacol. Ther. 2017, 101, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Zaza, G.; Tomei, P.; Ria, P.; Granata, S.; Boschiero, L.; Lupo, A. Systemic and nonrenal adverse effects occurring in renal transplant patients treated with mTOR inhibitors. Clin. Dev. Immunol. 2013, 2013, 403280. [Google Scholar] [CrossRef]
- Deshpande, N.A.; Coscia, L.A.; Gomez-Lobo, V.; Moritz, M.J.; Armenti, V.T. Pregnancy after solid organ transplantation: A guide for obstetric management. Rev. Obstet. Gynecol. 2013, 6, 116–125. [Google Scholar]
- Battaglia, Y.; Cojocaru, E.; Fiorini, F.; Granata, A.; Esposito, P.; Russo, L.; Bortoluzzi, A.; Storari, A.; Russo, D. Vitamin D in kidney transplant recipients. Clin. Nephrol. 2020, 93, 57–64. [Google Scholar] [CrossRef]
- Gregorini, M.; Sileno, G.; Pattonieri, E.F.; Corradetti, V.; Abelli, M.; Ticozzelli, E.; Scudeller, L.; Grignano, M.A.; Esposito, P.; Bogliolo, L.; et al. Understanding Bone Damage After Kidney Transplantation: A Retrospective Monocentric Cross Sectional Analysis. Transpl. Proc. 2017, 49, 650–657. [Google Scholar] [CrossRef]
| Non-Dialysis (CKD Stages 3–5) | HD | PD | |||
|---|---|---|---|---|---|
| Macronutrients | |||||
| Calories (Kcal/kg/day) * | |||||
| Trimester | First | 35 | 30–35 | 25–30 | |
| Second-Third | 30–35 (+300 Kcal) | 30–35 (+300 Kcal) | 25–30 (+300 Kcal) | ||
| Proteins (g/kg/day) * | 0.6–0.8 (+10 g) if uremic syndrome is not controlled, start dialysis | 1.2 (+10 g) | 1.2 (+10 gr) | ||
| Micronutrients | |||||
| Folic acid (mg/day) | 6 | 2–5 | |||
| 25-OH vitamin D (IU/day) | 1000–2000 | 1000–2000 | |||
| Zinc (mg/day) | 15 | 15 | |||
| Iron (mg/day) | 20–30 | 20–30 | |||
| Others § | |||||
| Electrolytes | |||||
| Calcium (mg/day) | <2000 | 1500–2000 | |||
| Phosphate (mg/day ) | CKD stages 4–5: 800–1000 | 1200 | |||
| Potassium, mEq/L/day (gr) | According to the serum levels | <75 (3 gr) |
| Approaches | Recommendations | Notes | Ref | |
|---|---|---|---|---|
| Pre-pregnancy counselling | Consider clinical stability, comorbidities, potential teratogen medications and social conditions | [11] | ||
| Diet modifications | Nutritional assessment | To perform early Consider nutritional habits, economic conditions Define individual nutritional needs | Consider Medical Nutrition Therapy approach | [58] |
| Use of supplements | According to general and disease-specific recommendations (see Table 1) | [12,31] | ||
| Dialysis management | Dialysis dose | Intensify dialysis: HD: at least >20 h/week HD PD: Increase exchanges (not defined)—consider switch to HD | Maintain predialysis BUN < 50 mg/dL Associated with increased nutrient removal | [62,65] |
| Fluid management | To schedule according to the expected weigh gain | [11] | ||
| Dialysate composition | Possibility to individualize potassium, calcium and phosphate concentrations | High calcium content often required | [61,69] | |
| Dialysis-related drug therapy modification | Phosphate-binders | Often discontinued, according to phosphate serum levels | If necessary use calcium-based phosphate-binders | [67] |
| Vitamin D and iron supplementation | Both oral and iv supplements are considered safe | Frequent monitoring of mineral metabolism and anemia | [43] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, P.; Garibotto, G.; Picciotto, D.; Costigliolo, F.; Viazzi, F.; Conti, N.E. Nutritional Challenges in Pregnant Women with Renal Diseases: Relevance to Fetal Outcomes. Nutrients 2020, 12, 873. https://doi.org/10.3390/nu12030873
Esposito P, Garibotto G, Picciotto D, Costigliolo F, Viazzi F, Conti NE. Nutritional Challenges in Pregnant Women with Renal Diseases: Relevance to Fetal Outcomes. Nutrients. 2020; 12(3):873. https://doi.org/10.3390/nu12030873
Chicago/Turabian StyleEsposito, Pasquale, Giacomo Garibotto, Daniela Picciotto, Francesca Costigliolo, Francesca Viazzi, and Novella Evelina Conti. 2020. "Nutritional Challenges in Pregnant Women with Renal Diseases: Relevance to Fetal Outcomes" Nutrients 12, no. 3: 873. https://doi.org/10.3390/nu12030873
APA StyleEsposito, P., Garibotto, G., Picciotto, D., Costigliolo, F., Viazzi, F., & Conti, N. E. (2020). Nutritional Challenges in Pregnant Women with Renal Diseases: Relevance to Fetal Outcomes. Nutrients, 12(3), 873. https://doi.org/10.3390/nu12030873






