Abstract
Exclusive enteral nutrition (EEN) has been shown to be more effective than corticosteroids in achieving mucosal healing in children with Crohn´s disease (CD) without the adverse effects of these drugs. The aims of this study were to determine the efficacy of EEN in terms of inducing clinical remission in children newly diagnosed with CD, to describe the predictive factors of response to EEN and the need for treatment with biological agents during the first 12 months of the disease. We conducted an observational retrospective multicentre study that included paediatric patients newly diagnosed with CD between 2014–2016 who underwent EEN. Two hundred and twenty-two patients (140 males) from 35 paediatric centres were included, with a mean age at diagnosis of 11.6 ± 2.5 years. The median EEN duration was 8 weeks (IQR 6.6–8.5), and 184 of the patients (83%) achieved clinical remission (weighted paediatric Crohn’s Disease activity index [wPCDAI] < 12.5). Faecal calprotectin (FC) levels (μg/g) decreased significantly after EEN (830 [IQR 500–1800] to 256 [IQR 120–585] p < 0.0001). Patients with wPCDAI ≤ 57.5, FC < 500 μg/g, CRP >15 mg/L and ileal involvement tended to respond better to EEN. EEN administered for 6–8 weeks is effective for inducing clinical remission. Due to the high response rate in our series, EEN should be used as the first-line therapy in luminal paediatric Crohn’s disease regardless of the location of disease and disease activity.
1. Background
Exclusive enteral nutrition (EEN) is the use of a complete liquid formula as the sole source of food for 6 to 8 weeks. It is still the therapeutic modality of choice for treating the first flare-up of paediatric luminal Crohn’s disease (CD) [1]. Although the efficacy of EEN in inducing remission has been known since the 1980s, there have been few studies published on the subject in Spain [2]. In 2014, the Inflammatory Bowel Disease (IBD) working group of the Spanish Society of Gastroenterology, Hepatology, and Nutrition (SEGHNP) published the PRESENT (PREScription of Enteral Nutrition in pediaTric Crohn’s disease in Spain) survey (70-item questionnaire) [3]. The aim of the PRESENT study was to investigate the frequency and characteristics of the use of EEN, including barriers and enablers, in pediatric gastroenterology units in Spain. The PRESENT study revealed that many paediatric gastroenterologists limited their indication of EEN as first-line therapy at the onset of the disease to specific cases. In this way, 43% of the physicians only indicated EEN for inflammatory forms (B1), 37.3% indicated EEN only when there was ileal (L1) or ileocolonic (L3) involvement, 41.1% only indicated EEN for mild-moderate disease, 62.7% only indicated EEN if the patient and their family were cooperative, and only 25.5% offered EEN as the only therapeutic option (they allowed them to choose between ENN and steroids). From this study, it appears that a group of newly diagnosed Crohn’s disease children, according to their phenotypic characteristics and severity of the disease (measured by Paediatric Crohn’s Disease Activity Index, PCDAI), would not benefit from receiving EEN as first-line therapy, as they were not considered, a priori, a candidate by their treating physician.
Our working hypothesis is that the response to EEN in patients with Crohn’s disease does not depend on the severity of the flare-up measured by weighted Pediatric Crohn’s Disease Activity Index (wPCDAI) [4], on the patient’s age, or on the location of the disease (according to Paris classification [5]). There are other factors, probably still unknown, that limit the success of this therapeutic modality. The aims of the present study were to determine the rate of remission after induction to remission therapy with EEN in newly diagnosed Crohn’s disease children, the potential predictors of response to EEN and the need for treatment with biological agents during the first 12 months of the disease.
2. Material and Methods
We conducted a retrospective cohort study that included paediatric patients diagnosed with CD between 1 January 2014 and 31 December 2016, based on their clinical, laboratory, endoscopic, radiological, and histological criteria, according to ESPGHAN Revised Porto Criteria for the Diagnosis of Inflammatory Bowel Disease in Children and Adolescents [6], and who were treated with EEN for their first flare-up and were followed up for 12 months after diagnosis. The participating centres were invited through the distribution list of the Spanish Society of Gastroenterology, Hepatology, and Nutrition (Sociedad Española de Gastroenterología, Hepatología y Nutrición, SEGHNP) in January 2017. A Case Report Form (CRF) was designed and distributed by email to the participating centers. The deadline for sending the CRFs to the study coordinator was 28 February 2018. All patients received one of the commercially prepared formulas (lactose and gluten free), normal or hypercaloric polymeric flavored formulas (ready for drink) or Modulen IBD® or Resource IBD® (the same formula but with a different name, Nestlé Health Science, Vevey, Switzerland), both prepared the same way by mixing 1700 ml water with 400 g of product to produce 2000 ml of formula (1 kcal/ml). No other food was allowed during the EEN period. The prescribed volumes were based on the caloric needs of each patient according to age, sex, and nutritional status. Feeds were gradually increased to target volumes in 3–5 days and patients were only allowed to drink water during treatment. EEN was given for a 6- to 8-week period. After the EEN period, food was introduced according to local protocol.
The data collected were age, gender, type of delivery, breastfeeding, previous appendectomy, rural or urban (more than 10.000 population) environment, family history of IBD, season of the year in which it was diagnosed, time from the onset of symptoms to diagnosis, and concomitant pharmacological treatment. Disease phenotype was determined according to the Paris classification [5].
All the patients were assessed at the start and after completing the EEN period, and the variables analyzed were weight, height, body mass index (BMI), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), albumin, complete blood count (CBC), faecal calprotectin (FC), and wPCDAI [4]. The wPCDAI was derived by reweighting the PCDAI mathematically, and composed of three domains—clinical history symptoms with a 1 week recall (abdominal pain, patient functioning, general well-being, and stools per day), physical examination (weight, perirectal disease, and extraintestinal manifestations), and laboratory parameters (ESR and albumin) with individual items mathematically weighted to produce an overall score that classifies patients into four disease activity categories: none, < 12.5; mild, 12.5 to 40; moderate, >40 to 57.5; and severe, >57.5. Weight and height were measured with the patient barefoot and in underwear. Weight, height, and BMI z scores were calculated using data from Spanish growth charts [7]. Samples for the determination of FC were collected at home by the patient the day before and were delivered, refrigerated, to the laboratory for immediate analysis. Blood samples were collected at each participating hospital and processed on site. There was no centralized analysis of blood or faecal samples. We considered remission a wPCDAI <12.5 points after completing the EEN period and defined a response to EEN as a >17.5-point change in the initial wPCDAI value without achieving remission. Biological remission was defined as wPCDAI <12.5, ESR <20 mm/h, CRP <5 mg/L, and FC <300 μg/g. We excluded patients with ulcerative colitis (UC), those with inflammatory bowel disease unclassified (IBD-U), those undergoing concomitant treatment with steroids or anti-tumour necrosis factor (anti-TNF) during induction with EEN, and those who were treated with EEN in successive flare-ups. Written informed consent was obtained from parents and also from patients older than 12 years old before collecting the data on the CRF.
2.1. Statistical Analysis
Variables with a normal distribution were expressed as mean ± standard deviation, and those without a normal distribution as median and interquartile range (IQR). We employed the Kolmogorov–Smirnov test to evaluate the normality of the distribution. We employed the Wilcoxon signed-rank test for paired samples (wPCDAI, CRP, FC, ESR, albumin, and haemoglobin values before and after EEN) and the chi-square test for comparing the proportions (A1b of Paris, wPCDAI ≤ 57.5, Ileal involvement, CRP >15 mg/L and FC <500 μg/g). To compare the variables with a normal distribution, we employed the t-Student (age at diagnosis in responders and non-responders) and the Mann–Whitney U test in those without normal distribution (time to diagnosis, months; wPCDAI; CRP, mg/L and FC, μg/g values; and time to anti-TNF between responders and non-responders; EEN duration between those who responded and those who went into remission after EEN). We estimated the hazard ratio and its confidence interval using the Cox proportional hazards model. We considered a p < 0.05 as statistically significant. We constructed predictive models using univariate and multivariate logistic regression tests. To construct the model, all the variables included on Table 2 were included first in the univariate analysis, and only those variables that presented statistically significant differences or a trend (p < 0.15) in the univariate analysis, along with the variables that, based on the theoretical or empirical knowledge, were considered related to the dependent variable and were included on the MV on Table 3. We measured the magnitude of the association between the model’s predictive variables and the dependent variable with the odds ratio (OR) and its corresponding 95% confidence interval (CI). Accepting an alpha risk of 0.05 in a two-sided test with 1307 participants in the first group (2) and 222 in the second, a statistical power of 85% was needed to recognize as statistically significant the difference between 0.74 in the first group and 0.83 in the second group.
2.2. Ethical Issues
The study (Code 0623-M2-14) and protocols for recruitment were initially approved by the Ethics Committee of the Hospital Regional Universitario de Málaga. Later it was approved by the rest of the ethics committees of the participating centers.
3. Results
We received data from 235 patients; of these, we analysed a total of 222 patients from 35 hospital centres and discarded 13 due to a lack of availability of all requested information. Only 11.2% (25/222) of the patients came from rural areas, 37 (17%) patients had a family history of IBD (14% CD, 2.5% UC and 0.5% IBD), 29% were born by caesarean section, and 64% had been breastfed for a median of 3 months from birth. Some 26.7% had their initial presentation in winter, and only 3% had received an appendectomy. The time to diagnosis did not differ significantly between those who had a family history of IBD and those who did not (4.7 vs. 4.4 months, p = 0.977). The clinical characteristics are listed in Table 1.

Table 1.
Baseline characteristics of the patients treated with exclusive enteral nutrition (n = 222).
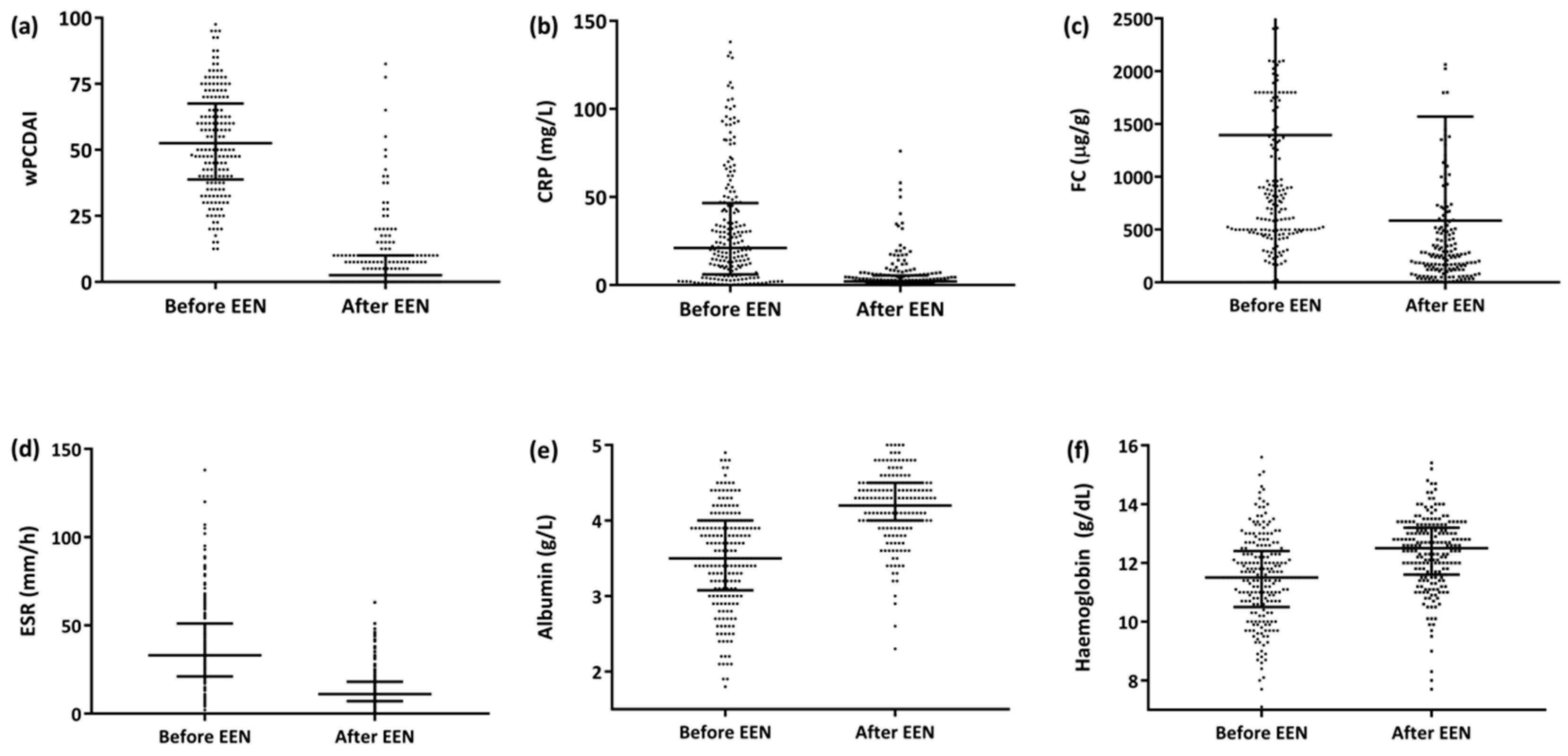
The median EEN duration for the entire series was 8 weeks (IQR 6.7–8.5) and was significantly longer for the patient group that achieved remission than for the groups that did not (8 [IQR 7.0–8.7] weeks for the remission group vs. 7.7 [IQR 4.6–8.1] weeks for the non-remission responders, p = 0.019]). The median time to response was 15 days (IQR 11–27). Of the 222 patients, 178 (80%) were administered Modulen IBD®/Resource IBD® (Nestlé Health Science, Vevey, Switzerland), while the rest (20%) were administered other polymeric formulas; 18 (8.1%) patients required a nasogastric tube (NGT) to administer the enteral formula. By the end of the EEN period, 83% of the patients (184/222) had achieved clinical remission (ΔwPCDAI, −47 ± 18), and an additional 12.3% (27/222) responded but did not achieve clinical remission (ΔwPCDAI, −41 ± 16) (Figure 1a). A significant reduction in C-reactive protein (CRP), faecal calprotectin (FC) and erythrocyte sedimentation rate readings was also observed (Figure 1b–d), as well as a significant increase in albumin and haemoglobin values (Figure 1e,f).
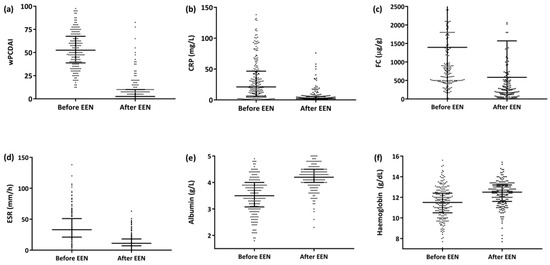
Figure 1.
Progression of the activity index and biochemical parameters after the EEN period (n = 222). (a) wPCDAI [52.5 (IQR 39–67) vs. 2.5 (IQR 0–10), (ΔwPCDAI −44 ± 20 p < 0.0001]. (b) CRP [21 (IQR 6–47) vs. 2 (IQR 1–5), p < 0.0001]. (c) FC [830 (IQR 500-1800) vs. 256 (IQR 120–585), p < 0.0001]. (d) SS [33 (IQR 21–51) vs. 11 (IQR 7–18), p < 0.0001]. (e) Albumin [3.5 (IQR 3–4) vs. 4.2 (IQR 4–4.5), p < 0.0001. (f) Haemoglobin (11.5 [IQR 10.5–12.4] vs. 12.5 [IQR 11.6–13.2], p = 0.0001). Abbreviations: CRP, C-reactive protein; FC, faecal calprotectin; wPCDAI, weighted Paediatric Crohn’s Disease Activity Index; EEN: exclusive enteral nutrition.
After the EEN period, all patients started on a normal diet. In addition, 82.9% of the patients who had not achieved clinical remission and 85.9% of those who had were supplemented every day with 500 ml of polymeric formula over the following months. Of the 38 (17.1%) patients who did not achieve clinical remission after EEN, nine had been administered steroids (eight received prednisone, and one budesonide) for 4 weeks (IQR 4–7.5) from the start of the EEN, and 29 had been administered anti-TNF (16 infliximab, and 23 adalimumab) for a median of 1.4 months (IQR 0–5) since EEN was initiated. The time to the start of anti-TNF treatment was longer for those who responded but did not achieve remission than for those who did not respond (3.2 months [IQR 1.4–7.4] vs. 0 months [IQR 0–1.2], p = 0.009). Of the nine patients who were started on steroids due to lack of response to EEN, five achieved clinical remission (55.5%), but all started treatment with anti-TNF during the first year of follow-up (8 months, IQR 5–10).
3.1. Predictors of Response to Exclusive Enteral Nutrition
Table 2 shows the differences in baseline clinical activity and laboratory biomarkers in responders (wPCDAI < 12.5) versus nonresponders. In terms of predictors of response (Table 3), those patients with a mild–moderate disease (wPCDAI < 57.5), with FC < 500 μg/g, ileal involvement, and CRP > 15 mg/L showed a better response to EEN.

Table 2.
Baseline clinical activity and laboratory biomarkers in both groups: Responders (wPCDAI < 12.5) and Nonresponders.

Table 3.
Predictive variables of response to EEN. Multivariate analysis. Dependent variable: wPCDAI < 12.5. n = 222 patients.
3.2. Time to Biological Therapy
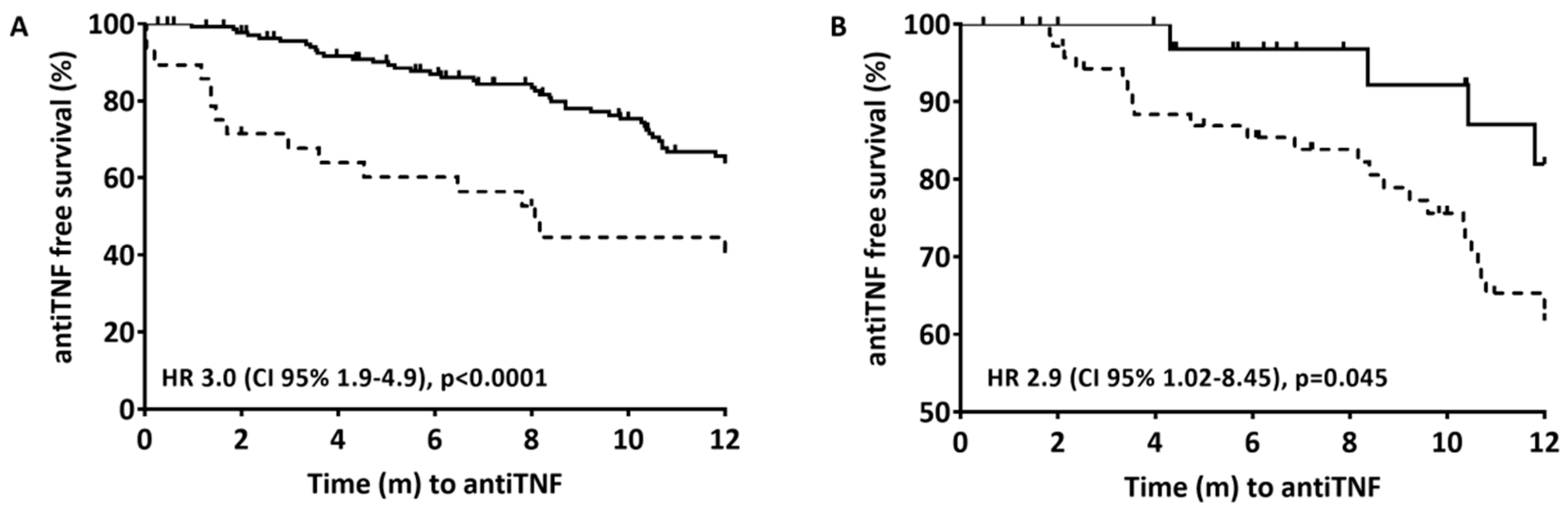
At 12 months of follow-up, 84/222 (37%) of the patients had been started on therapy with anti-TNF (36 on infliximab and 48 on adalimumab) after a median of 5 months (IQR 1.7–8.7) from finishing EEN. The time taken to the start of anti-TNF therapy was longer for those with clinical remission (6.8 months [IQR 3.5–10.2] vs. 1.3 months [IQR 0–4.3], p < 0.0001) and with biological remission (7.1 months [IQR 4.1–10.5] vs. 3.4 months [IQR 0.6–8.2], p = 0.056). Clinical remission after EEN (wPCDAI < 12.5) (Figure 2A) and biological remission (wPCDAI < 12.5, ESR < 20 mm/h, CRP < 5 mg/L and FC 300 μg/g) (Figure 2B) were associated with a significantly reduced risk of starting anti-TNF therapy in the following 12 months. At the start of anti-TNF therapy, the patients’ mean wPCDAI, CRP, and FC were 33.7 (IQR 17.5–42.5), 13.8 (IQR 2.7–30.1), and 992 (IQR 376–2213), respectively.
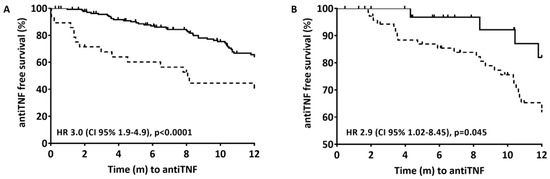
Figure 2.
Time from the end of induction with EEN to the start of anti-TNF therapy during the first 12 months after diagnosis. Cox proportional hazard models; only the stratification variables were included (clinical remission in 2A and biological remission in 2B). (A) Solid line is clinical remission (wPCDAI <12.5) after EEN; Dashed line is no clinical remission after EEN. Stratified by clinical remission (6.8 months [IRQ 3.5–10.2] vs 1.3 months [IQR 0–4.3], p < 0.0001). (B) Solid line is biological remission (wPCDAI <12.5, ESR <20 mm/h, CRP <5 mg/L and FC <300 μg/g); Dashed line is clinical remission (wPCDAI <12.5). Stratified by biological remission (7.1 months [IQR 4.1–10.5] vs 3.4 months [IQR 0.6–8.2], p = 0.056).
4. Discussion
The present study reinforces the literature’s efficacy data for EEN in newly diagnosed CD children regardless of age. Our results for the clinical remission rates are similar to the previously published data [2]. EEN induces clinical remission [8,9] in the first flare-up or during relapses [10,11], induces mucosal [12] and transmural healing [13], has a positive effect on growth [14], on bone health [15], on the nutritional state [16], and on the health-related quality of life [17], and is a therapeutic option for decreasing the risk of relapses during follow-up [18], to avoid treatment with steroids [14] and to update these patients’ vaccination schedules before starting immunosuppressive therapy [19].
EEN should therefore be the first option when treating paediatric patients after diagnosing luminal CD, given its demonstrated effects in the short, medium, and long term (growth, bone health, and vaccinations) in the absence of the adverse effects of steroids. In our patient group, all those who started steroids after EEN failure required a shift to biological therapy during the first year of follow-up (8 months, IQR 5–10). It therefore makes sense not to subject patients to steroids and instead directly start therapy with anti-TNF. A recent clinical trial conducted with a paediatric population showed that the top-down (TD) strategy was superior to the step-up strategy for achieving endoscopic remission at week 10 and sustained clinical remission at 52 weeks without requiring other treatments or surgery [20]. This TD strategy is standard practice in Europe, the United States, and Canada [21]. A meta-analysis of studies conducted with adult populations revealed that the use of enteral nutrition in combination with infliximab was more effective for inducing and maintaining clinical remission among patients with CD than monotherapy with infliximab [22], which is based on an etiopathogenic standpoint [23] and by the capacity of EEN to change the proinflammatory cytokine profile in the intestinal mucosa [24]. This strategy, which was not evaluated in our study, could be assessed in an ad hoc designed study.
Regarding the predictors to response, although our model is statistically significant, it only partially explains the dependent variable (Cox-Snell R2: 0.130 and Nagelkerke R2: 0.202), indicating that additional factors play a role in the response [25]. Unlike CRP, which is related to transmural involvement, FC is correlated with mucosal involvement [26]. In our case, CRP indicated the presence of an inflammatory pattern. Although EEN was initially thought to be more effective in patients with ileal involvement than in those with exclusively colonic involvement (L1 or L3 versus L2 according to Paris classification), there is no quality evidence to support this belief [27]. In our series, however, we did find that ileal involvement was a predictor of a response to EEN. This finding could be partly explained by the fact that the ileum is considered by a number of authors as the location from which all phenotypic forms of CD originate. The authors confer a predominant role to the ileal microbiota in the prognosis and treatment response [28]. Moreover, biological remission (PCDAI, CRP, and FC) correlates better with the degree of mucosal inflammation [29] than the routinely employed activity indices [4]. The growth study [30] revealed that biological remission at week 12 (PCDAI < 5, calprotectin < 400 μg/g and CRP < 20 mg/L) was the best predictor of relapses and complications during follow-up. In our case, biological remission (wPCDAI < 12.5, CRP < 5 mg/L, ESR < 20 mm/h and FC < 300 μg/g) after EEN predicted the need for anti-TNF therapy in the following 12 months. An important aspect here is the role of other drugs in maintaining the remission after induction therapy. In our series, more than 80% of the patients were treated with thiopurines concomitantly with the EEN. The results of the initial study by Markowitz et al. [31] were not reproduced in two multicentre studies conducted with adults [32,33]. A prospective cohort of patients with IBD showed a modest role for thiopurines in maintaining remission [34]. Similarly, a considerable majority of patients were supplemented with a polymeric formula once the EEN period had been completed, although the evidence regarding the effectiveness of the polymeric formula in this setting is scarce [35]. There were no complications resulting from the use of EEN.
In our study, 40% of the patients were started on anti-TNF therapy during the first year from the diagnosis, compared with 70% in the series published by Jongsma et al. [20]. This difference, in addition to being affected by the design of the two studies, could be due to the fact that despite the current tendency to start anti-TNF therapy earlier [36], there is still a certain reluctance in our setting to use anti-TNF in the initial phases of the disease, after the failure of induction with EEN.
One of the limitations of EEN is willingness on the part of the child and their family. Compliance with EEN was high in our study, unlike that reported in other studies, which attributed the low compliance to problems in the formulas’ taste and to monotony [37]. In our series, less than 10% of patients needed NGT (18 out of 222) and none of them declined the EEN, except for medical indication due to the lack of response. The development of the CD Exclusion Diet (CDED), which partly minimises the problems of EEN, has enabled a turnaround in the treatment of paediatric CD. The CDED has been shown to respond to a frequent demand by patients and their relatives regarding acceptability and compliance [38]. The CDED is as effective as EEN in achieving clinical and biochemical remission and mucosal healing but superior to EEN in tolerance and compliance. CDED, unlike EEN, constitutes a long-term strategy for maintaining remission and is nutritionally balanced. By including fibre, CDED corrects the bacterial dysbiosis present in these patients [39], and is therefore a much more realistic and advanced approach than EEN if complied with adequately.
The most relevant limitations of the present study are its retrospective and multicentered nature, which limits the quality of the data and their corresponding analysis. Another issue to consider is the inclusion bias, given that the researchers decided whether or not the patients were candidates for EEN. Moreover, the small sample size in some of the subgroups could explain the considerable variation in the confidence intervals in a number of the measurements. The results should therefore be interpreted with caution.
5. Conclusions
In conclusion, EEN administered for 6–8 weeks is effective for inducing clinical remission. Given the high response rate in our series, EEN should be employed as the first-line therapy for luminal paediatric CD, regardless of the location of the disease, the CRP and FC levels, and the wPCDAI. Some patients, however, will respond better than others to EEN.
Author Contributions
M.M. designed the data collection instruments, coordinated and supervised data collection, conducted the analyses, drafted the initial manuscript and reviewed and edited the manuscript; V.M.N.-L., J.M.-d.-C. and G.P.-M., conceptualized and designed the study, drafted the initial manuscript, and reviewed and edit the manuscript; R.M.-M. conducted the analyses, drafted the initial manuscript and reviewed and edit the manuscript; C.O.S. supported for methodological aspects, conducted the analyses and reviewed and edited the manuscript; S.J.T., O.S.C., L.P.Q., D.G.S., A.R.M., A.R.C., H.A., J.B., R.G.d.C., M.R.S., E.B.S., E.D.A., A.B.P., E.V.S., R.V.L., A.S.B., A.M.Á., C.S.S., M.T.H., C.G.J., N.M.T., M.R.L.T., F.J.E., M.G.P., E.M.B., B.F.C., A.M.V.Á., L.C.V., C.A.V., J.R.S., R.G.-M., R.G.-R., I.R.A., S.F.C., H.L.G., J.F.V.B., M.V.R.-B., J.M.B.P., M.B.R., P.B.G., G.B., F.J.C.M., E.L.O.I., E.C.-G. reviewed and edited the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Ruemmele, F.M.; Veres, G.; Kolho, K.L.; Griffiths, A.; Levine, A.; Escher, J.C.; Amil Dias, J.; Barabino, A.; Braegger, C.P.; Bronsky, J.; et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J. Crohn’s Colitis 2014, 8, 1179–1207. [Google Scholar] [CrossRef] [PubMed]
- Navas-López, V.M.; Van Limbergen, J.; Martín-de-Carpi, J. Nutrición enteral en el paciente pediátrico con enfermedad de Crohn. Enferm. Inflamatoria Intest. al Día 2016, 15, 112–122. [Google Scholar] [CrossRef]
- Navas-López, V.M.; Martín-de-Carpi, J.; Segarra, O.; García-Burriel, J.I.; Díaz-Martín, J.J.; Rodríguez, A.; Medina, E.; Juste, M. Present; prescription of enteral nutrition in pediatric crohn’s disease in spain | Present; prescripción de nutrición enteral en la enfermedad de crohn pediátrica en españa. Nutr. Hosp. 2014, 29, 537–546. [Google Scholar] [PubMed]
- Turner, D.; Levine, A.; Walters, T.D.; Focht, G.; Otley, A.; Lopez, V.N.; Koletzko, S.; Baldassano, R.; Mack, D.; Hyams, J.; et al. Which PCDAI Version Best Reflects Intestinal Inflammation in Pediatric Crohn Disease? J. Pediatr. Gastroenterol. Nutr. 2017, 64, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Griffiths, A.; Markowitz, J.; Wilson, D.C.; Turner, D.; Russell, R.K.; Fell, J.; Ruemmele, F.M.; Walters, T.; Sherlock, M.; et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: The Paris classification. Inflamm. Bowel Dis. 2011, 17, 1314–1321. [Google Scholar] [CrossRef]
- Levine, A.; Koletzko, S.; Turner, D.; Escher, J.C.; Cucchiara, S.; de Ridder, L.; Kolho, K.-L.; Veres, G.; Russell, R.K.; Paerregaard, A.; et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 795–806. [Google Scholar] [CrossRef]
- Carrascosa, A.; Fernández, J.; Fernández, M.; López-Siguero, J.; López, D.; Sánchez, E.; Colaborador, G. Estudios españoles de crecimiento 2010. Available online: http://www.estudiosdecrecimiento.es/estudio-transversal.html (accessed on 1 January 2020).
- Swaminath, A.; Feathers, A.; Ananthakrishnan, A.N.; Falzon, L.; Li Ferry, S. Systematic review with meta-Analysis: Enteral nutrition therapy for the induction of remission in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2017, 46, 645–656. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, K.-C.; Chen, J. Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn’s disease: A meta-Analysis. World J. Pediatr. 2019, 15, 26–36. [Google Scholar] [CrossRef]
- Frivolt, K.; Schwerd, T.; Werkstetter, K.J.; Schwarzer, A.; Schatz, S.B.; Bufler, P.; Koletzko, S. Repeated exclusive enteral nutrition in the treatment of paediatric Crohn’s disease: Predictors of efficacy and outcome. Aliment. Pharmacol. Ther. 2014, 39, 1398–1407. [Google Scholar] [CrossRef]
- Cameron, F.L.; Gerasimidis, K.; Papangelou, A.; Missiou, D.; Garrick, V.; Cardigan, T.; Buchanan, E.; Barclay, A.R.; McGrogan, P.; Russell, R.K. Clinical progress in the two years following a course of exclusive enteral nutrition in 109 paediatric patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2013, 37, 622–629. [Google Scholar] [CrossRef]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: A randomized controlled open-label trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Grover, Z.; Muir, R.; Lewindon, P. Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. J. Gastroenterol. 2014, 49, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.; Basseri, S.; Grant, A.; Giffin, N.; Mahdi, G.; Noble, A.; Rashid, M.; Otley, A.; Van Limbergena, J. Exclusive enteral nutrition therapy in paediatric Crohn’s disease results in long-Term avoidance of corticosteroids: Results of a propensity-Score matched cohort analysis. J. Crohn’s Colitis 2017, 11, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Werkstetter, K.J.; Schatz, S.B.; Alberer, M.; Filipiak-Pittroff, B.; Koletzko, S. Influence of exclusive enteral nutrition therapy on bone density and geometry in newly diagnosed pediatric Crohn’s disease patients. Ann. Nutr. Metab. 2013, 63, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Gerasimidis, K.; Talwar, D.; Duncan, A.; Moyes, P.; Buchanan, E.; Hassan, K.; O’Reilly, D.; McGrogan, P.; Edwards, C.A. Impact of exclusive enteral nutrition on body composition and circulating micronutrients in plasma and erythrocytes of children with active Crohn’s disease. Inflamm. Bowel Dis. 2012, 18, 1672–1681. [Google Scholar] [CrossRef]
- Afzal, N.A.; Van Der Zaag-Loonen, H.J.; Arnaud-Battandier, F.; Davies, S.; Murch, S.; Derkx, B.; Heuschkel, R.; Fell, J.M. Improvement in quality of life of children with acute Crohn’s disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Aliment. Pharmacol. Ther. 2004, 20, 167–172. [Google Scholar] [CrossRef]
- Hojsak, I.; Pavić, A.M.; Mišak, Z.; Kolaček, S. Risk factors for relapse and surgery rate in children with Crohn’s disease. Eur. J. Pediatr. 2014, 173, 617–621. [Google Scholar] [CrossRef]
- Martinelli, M.; Giugliano, F.P.; Strisciuglio, C.; Urbonas, V.; Serban, D.E.; Banaszkiewicz, A.; Assa, A.; Hojsak, I.; Lerchova, T.; Navas-Lopez, V.M.; et al. Vaccinations and Immunization Status in Pediatric Inflammatory Bowel Disease: A Multicenter Study From the Pediatric IBD Porto Group of the ESPGHAN. Inflamm. Bowel Dis. 2019. [Google Scholar] [CrossRef]
- Jongsma, M.; Aardoom, M.; Cozijnsen, M.; Van Pieterson, M.; De Meij, T.; Norbuis, O.; Groeneweg, M.; Wolters, V.; Van Wering, H.; Hojsak, I.; et al. Top-Down infliximab superior to step-Up in children with moderate-To-Severe Crohn’s disease-A multicenter randomized controlled trial. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 23–24. [Google Scholar]
- Bronsky, J.; de Ridder, L.; Ruemmele, F.M.; Griffiths, A.; Buderus, S.; Hradsky, O.; Hauer, A.C. Diagnostic and Therapeutic Approach in Paediatric Inflammatory Bowel Diseases: Results from a Clinical Practice Survey. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 676–683. [Google Scholar] [CrossRef]
- Nguyen, D.L.; Palmer, L.B.; Nguyen, E.T.; McClave, S.A.; Martindale, R.G.; Bechtold, M.L. Specialized enteral nutrition therapy in Crohn’s disease patients on maintenance infliximab therapy: A meta-Analysis. Therap. Adv. Gastroenterol. 2015, 8, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Boneh, R.S.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 66, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rolandsdotter, H.; Jonsson-Videsater, K.; Fagerberg, U.L.; Finkel, Y.; Eberhardson, M. Exclusive Enteral Nutrition: Clinical Effects and Changes in Mucosal Cytokine Profile in Pediatric New Inflammatory Bowel Disease. Nutrients 2019, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.; Dunn, K.A.; Allott, J.; Bandsma, R.; Rashid, M.; Otley, A.R.; Bielawski, J.P.; Van Limbergen, J. The relationship between fecal bile acids and microbiome community structure in pediatric Crohn’s disease. ISME J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Weinstein-Nakar, I.; Focht, G.; Church, P.; Walters, T.D.; Abitbol, G.; Anupindi, S.; Berteloot, L.; Hulst, J.M.; Ruemmele, F.; Lemberg, D.A.; et al. Associations Among Mucosal and Transmural Healing and Fecal Level of Calprotectin in Children With Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 1089.e4–1097.e4. [Google Scholar] [CrossRef]
- Buchanan, E.; Gaunt, W.W.; Cardigan, T.; Garrick, V.; McGrogan, P.; Russell, R.K. The use of exclusive enteral nutrition for induction of remission in children with Crohn’s disease demonstrates that disease phenotype does not influence clinical remission. Aliment. Pharmacol. Ther. 2009, 30, 501–507. [Google Scholar] [CrossRef]
- Haberman, Y.; Tickle, T.L.; Dexheimer, P.J.; Kim, M.-O.; Tang, D.; Karns, R.; Baldassano, R.N.; Noe, J.D.; Rosh, J.; Markowitz, J.; et al. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J. Clin. Invest. 2014, 124, 3617–3633. [Google Scholar] [CrossRef]
- Grover, Z.; Lewindon, P. Predicting Endoscopic Crohn’s Disease Activity Before and After Induction Therapy in Children: A Comprehensive Assessment of PCDAI, CRP, and Fecal Calprotectin. Inflamm. Bowel Dis. 2015, 21, 1386–1391. [Google Scholar]
- Ziv-Baran, T.; Hussey, S.; Sladek, M.; Amil Dias, J.; Martin de Carpi, J.; Miele, E.; Veres, G.; Lionetti, P.; Koletzko, S.; Nuti, F.; et al. Response to treatment is more important than disease severity at diagnosis for prediction of early relapse in new-Onset paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2018, 48, 1242–1250. [Google Scholar] [CrossRef]
- Markowitz, J.; Grancher, K.; Kohn, N.; Lesser, M.; Daum, F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn’s disease. Gastroenterology 2000, 119, 895–902. [Google Scholar] [CrossRef]
- Panés, J.; López–SanRomán, A.; Bermejo, F.; García–Sánchez, V.; Esteve, M.; Torres, Y.; Domènech, E.; Piqueras, M.; Gomez–García, M.; Gutiérrez, A.; et al. Early Azathioprine Therapy Is No More Effective Than Placebo for Newly Diagnosed Crohn’s Disease. Gastroenterology 2013, 145, 766.e1–774.e1. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Bourrier, A.; Laharie, D.; Nahon, S.; Bouhnik, Y.; Carbonnel, F.; Allez, M.; Dupas, J.; Reimund, J.; Savoye, G.; et al. Early Administration of Azathioprine vs Conventional Management of Crohn’s Disease: A Randomized Controlled Trial. Gastroenterology 2013, 145, 758.e2–765.e2. [Google Scholar] [CrossRef] [PubMed]
- Atia, O.; Ledder, O.; Ben-Moshe, T.; Abitbol, G.; Tzion, R.L.; Rachmen, Y.; Meyer, E.O.; Beeri, R.; Renbaum, P.; Algur, N.; et al. The Role of Thiopurines in Pediatric Inflammatory Bowel Diseases: A Real-Life Prospective Cohort Study. J. Pediatr. Gastroenterol. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.; Buchanan, E.; Cardigan, T.; Garrick, V.; Curtis, L.; McGrogan, P.; Barclay, A.; Russell, R.K. A retrospective study showing maintenance treatment options for paediatric CD in the first year following diagnosis after induction of remission with EEN: Supplemental enteral nutrition is better than nothing! BMC Gastroenterol. 2014, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- El-Matary, W.; Leung, S.; Tennakoon, A.; Benchimol, E.I.; Bernstein, C.N.; Targownik, L.E. Trends of Utilization of Tumor Necrosis Factor Antagonists in Children With Inflammatory Bowel Disease: A Canadian Population-Based Study. Inflamm. Bowel Dis. 2020, 26, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Lawley, M.; Wu, J.W.; Navas-Lopez, V.M.; Huynh, H.Q.; Carroll, M.W.; Chen, M.; Medvedev, P.; Day, A.S.; Hussey, S.; Sigall-Boneh, R.; et al. Global Variation in Use of Enteral Nutrition for Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2018, 67, e22–e29. [Google Scholar] [CrossRef] [PubMed]
- Svolos, V.; Gerasimidis, K.; Buchanan, E.; Curtis, L.; Garrick, V.; Hay, J.; Laird, S.; Munro, J.; Gaya, D.R.; Russell, R.K.; et al. Dietary treatment of Crohn’s disease: Perceptions of families with children treated by exclusive enteral nutrition, a questionnaire survey. BMC Gastroenterol. 2017, 17, 14. [Google Scholar] [CrossRef]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440.e8–450.e8. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).