Intermittent Fasting for Twelve Weeks Leads to Increases in Fat Mass and Hyperinsulinemia in Young Female Wistar Rats
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethical Approval
2.2. Animals
2.3. Indexes Calculation
2.4. Dual Energy x-Ray Absorptiometry (DEXA)
2.5. Glycogen Measurement
2.6. Oral Glucose Tolerance Test
2.7. Intraperitoneal Insulin Tolerance Test
2.8. Blood Analysis
2.9. Isolation of Pancreatic Islets
2.10. Static Insulin Secretion
2.11. Pancreas Histological Assessment
2.12. Cell Viability
2.13. Measurement of Net ROS Production
2.14. Measurement of Net Hydrogen Peroxide Production
2.15. Western Blot Analysis
2.16. Statistical Analysis
3. Results
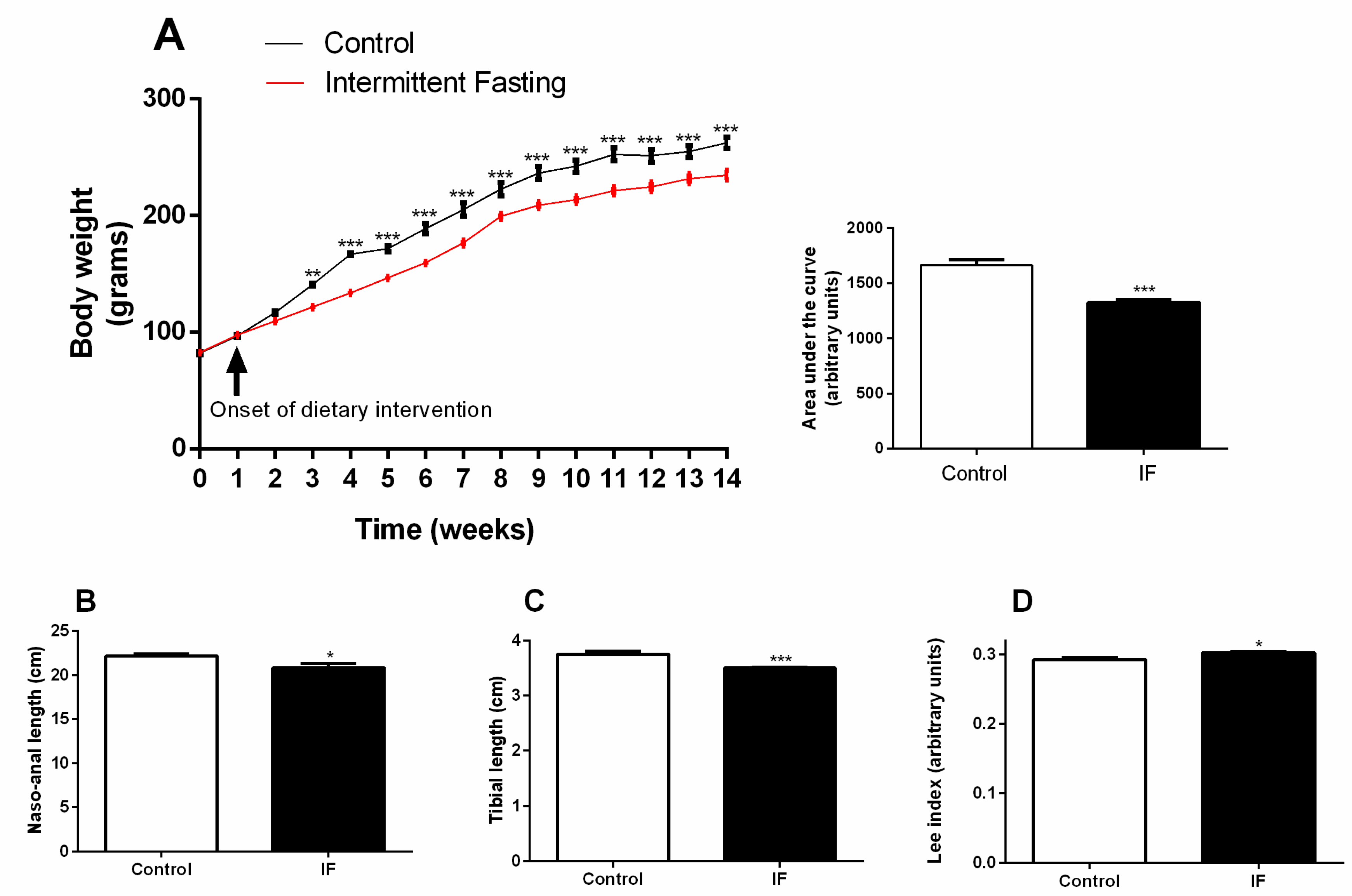
3.1. Body Weight Gain and Development
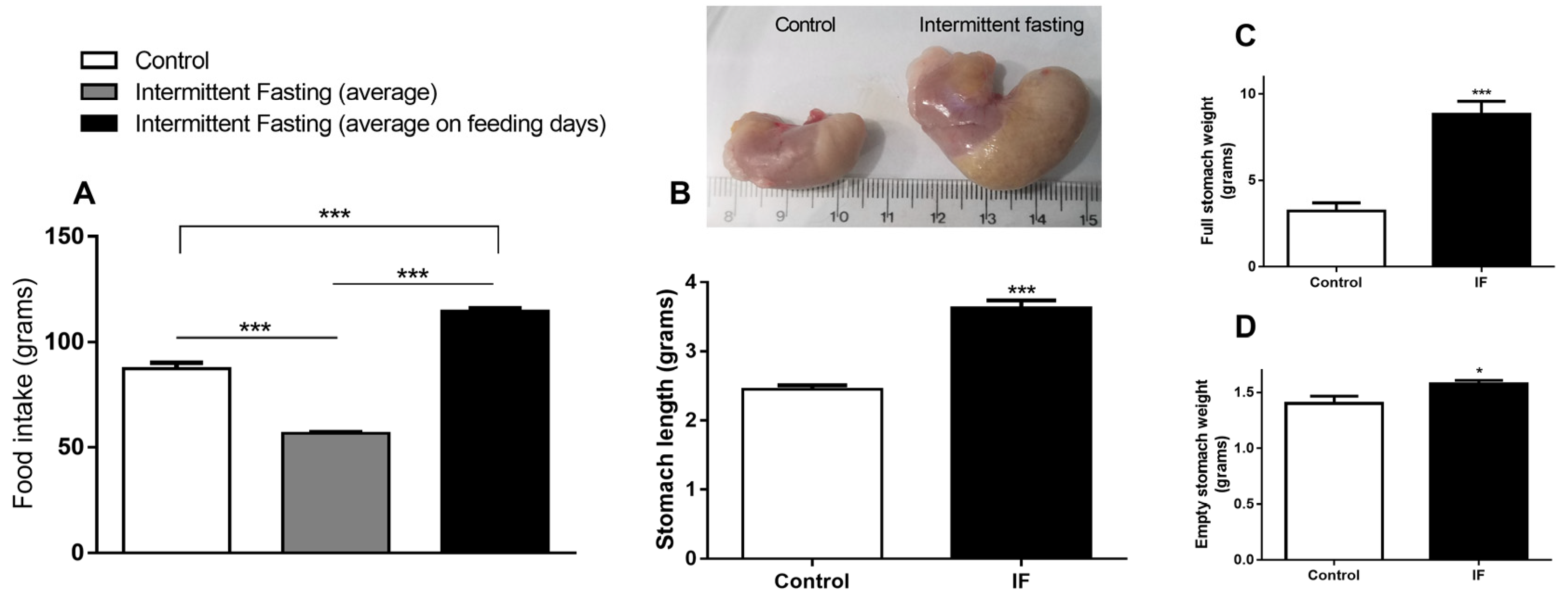
3.2. Food Intake and Stomach Disturbances
3.3. Body Composition
3.4. Liver Alterations
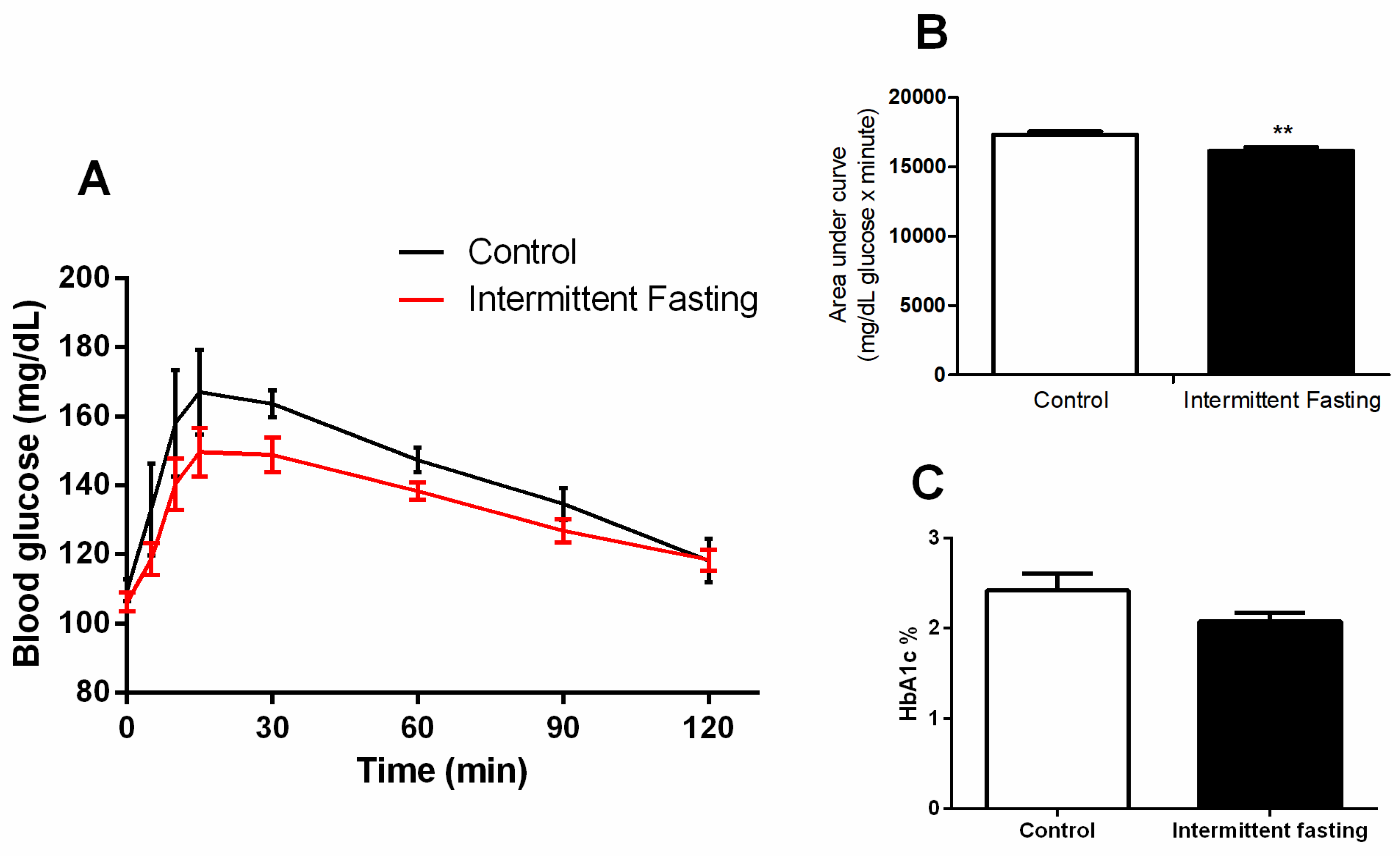
3.5. Glucose Homeostasis
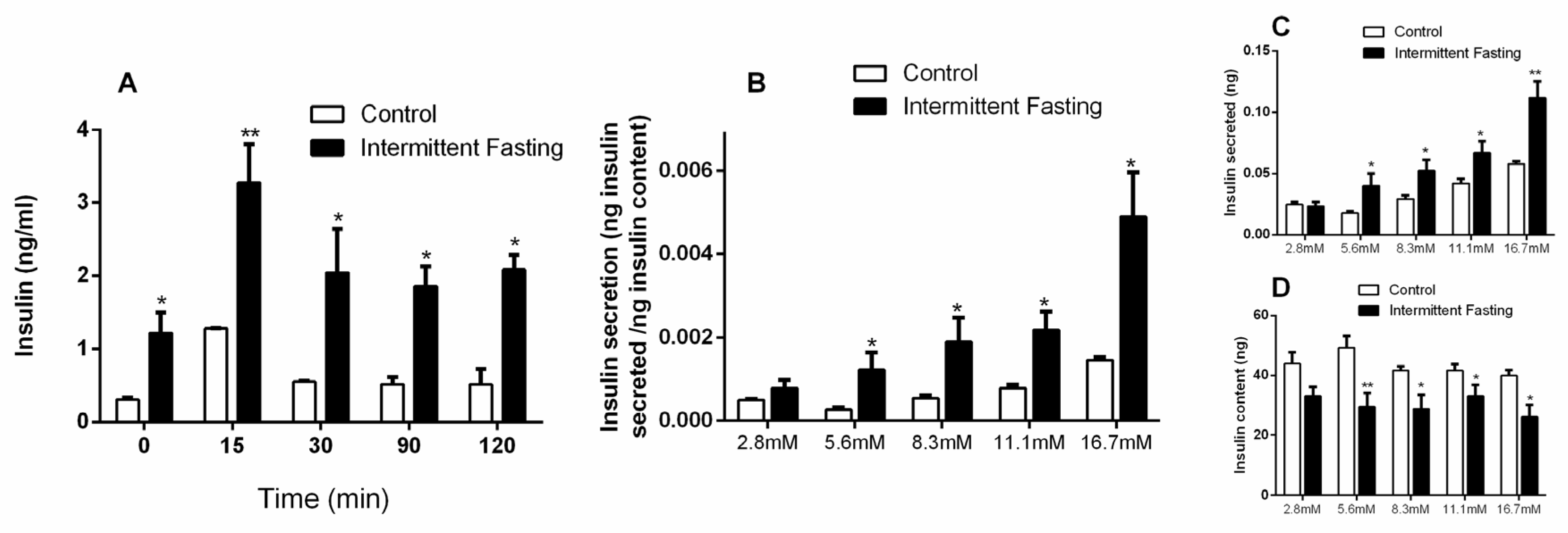
3.6. Insulin Concentrations
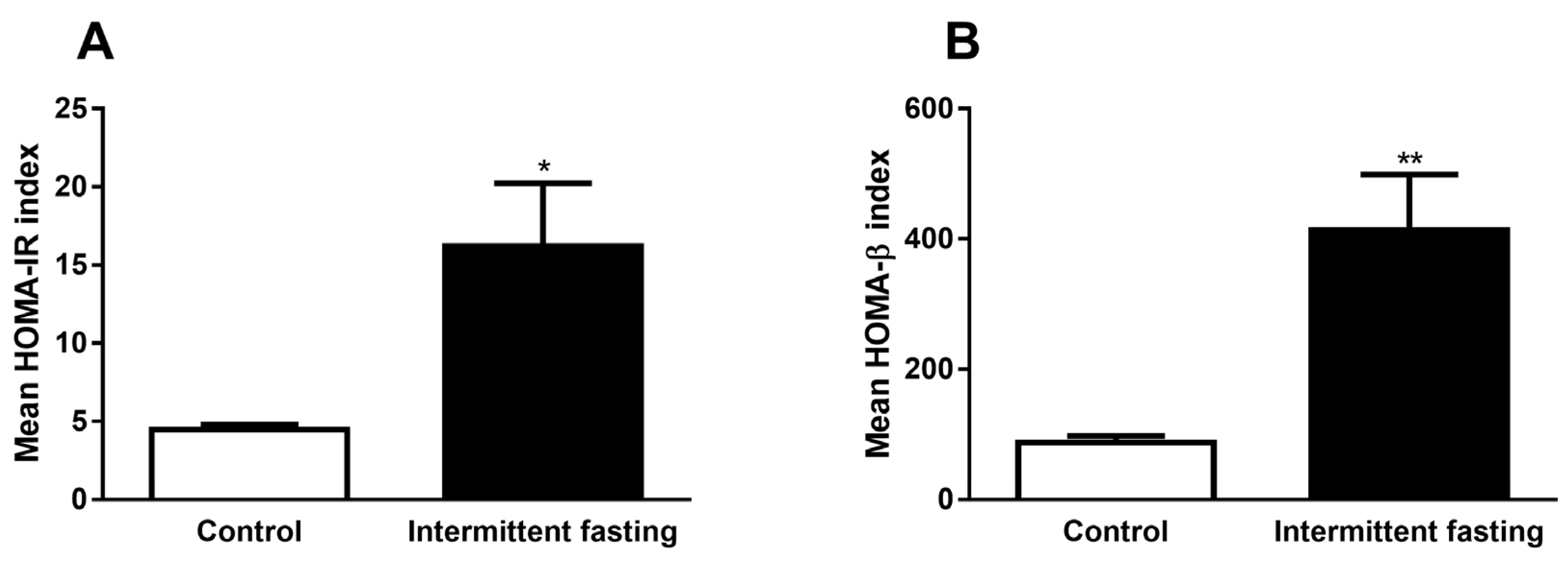
3.7. Homeostasis Model Assessment (HOMA) Indexes
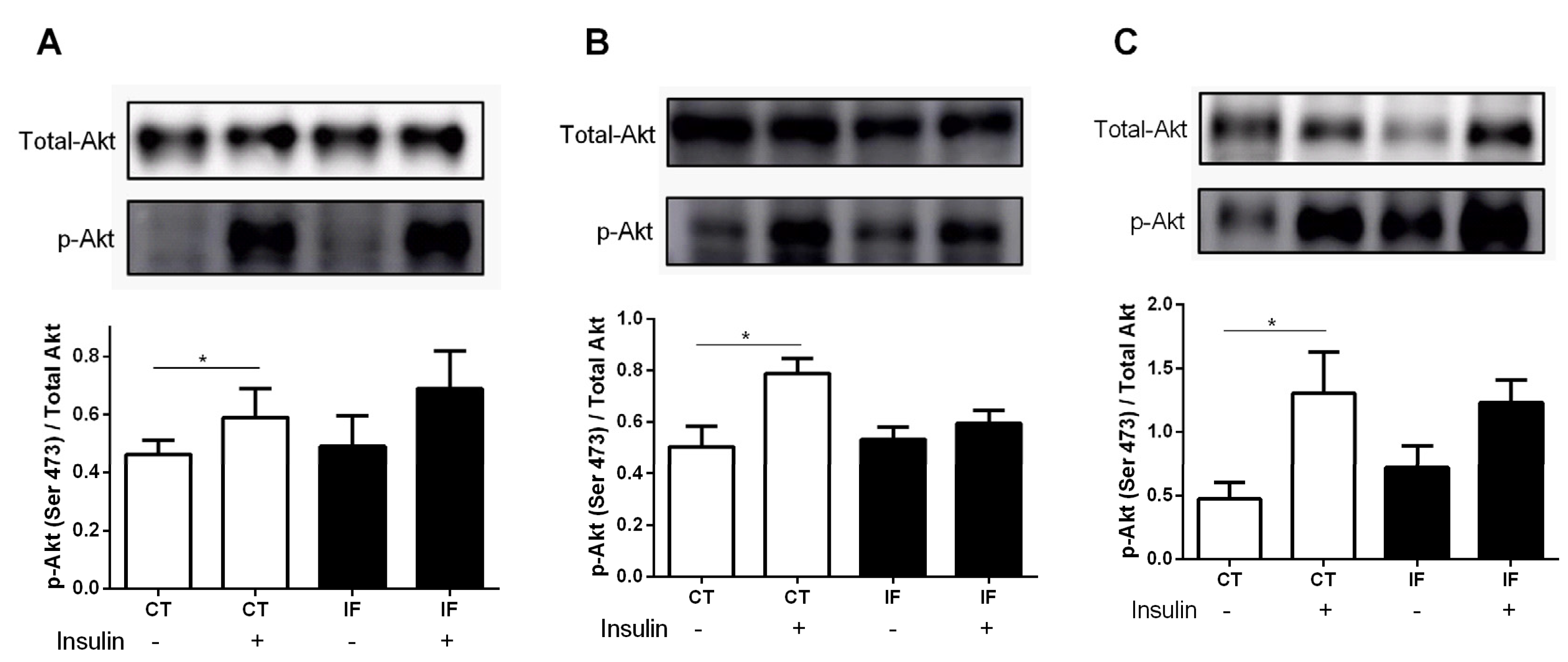
3.8. AKT Phosphorylation
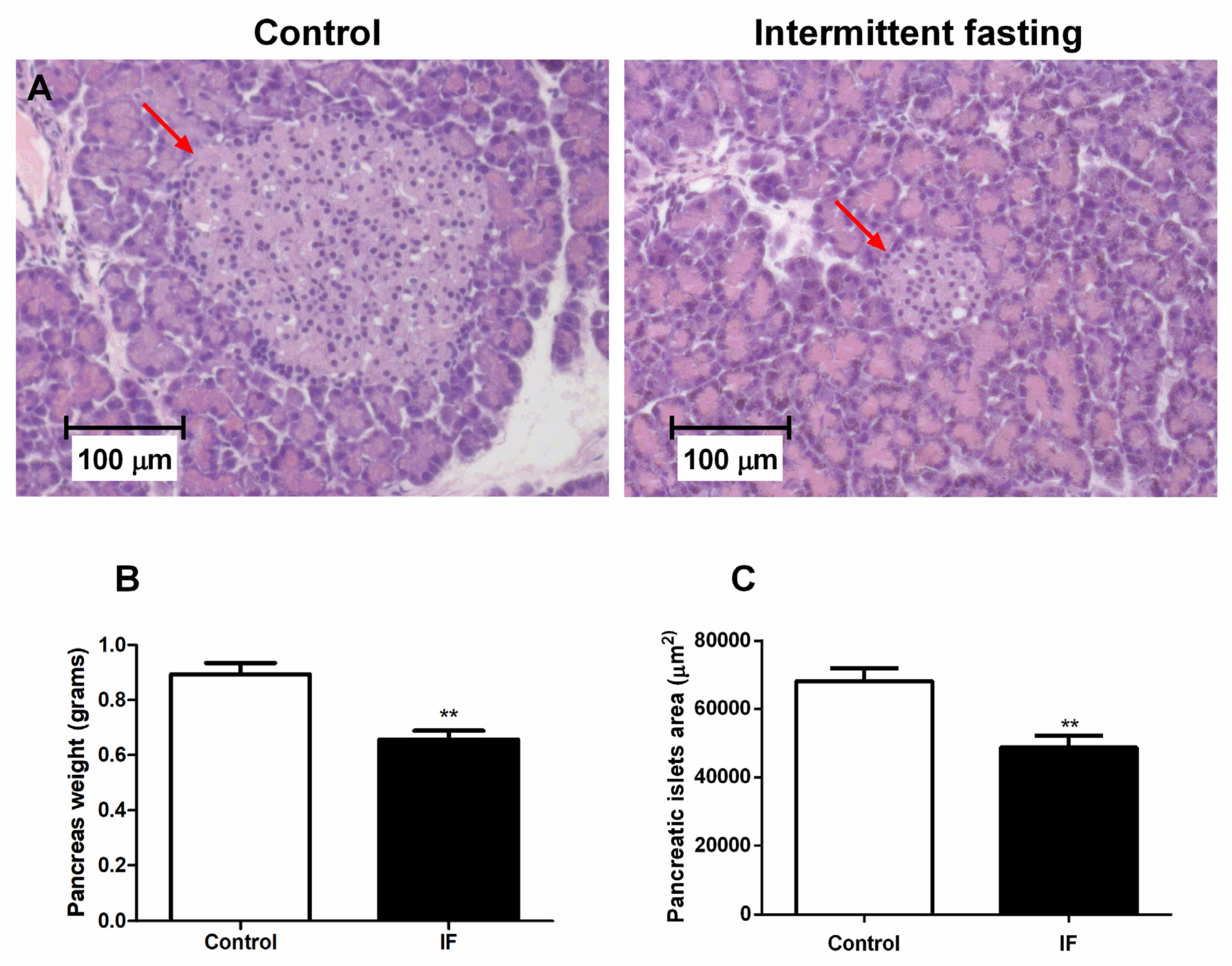
3.9. Pancreatic Islet Area and Viability of Cells from Pancreatic Islets
3.10. ROS Production of Dispersed Cells from Pancreatic Islets
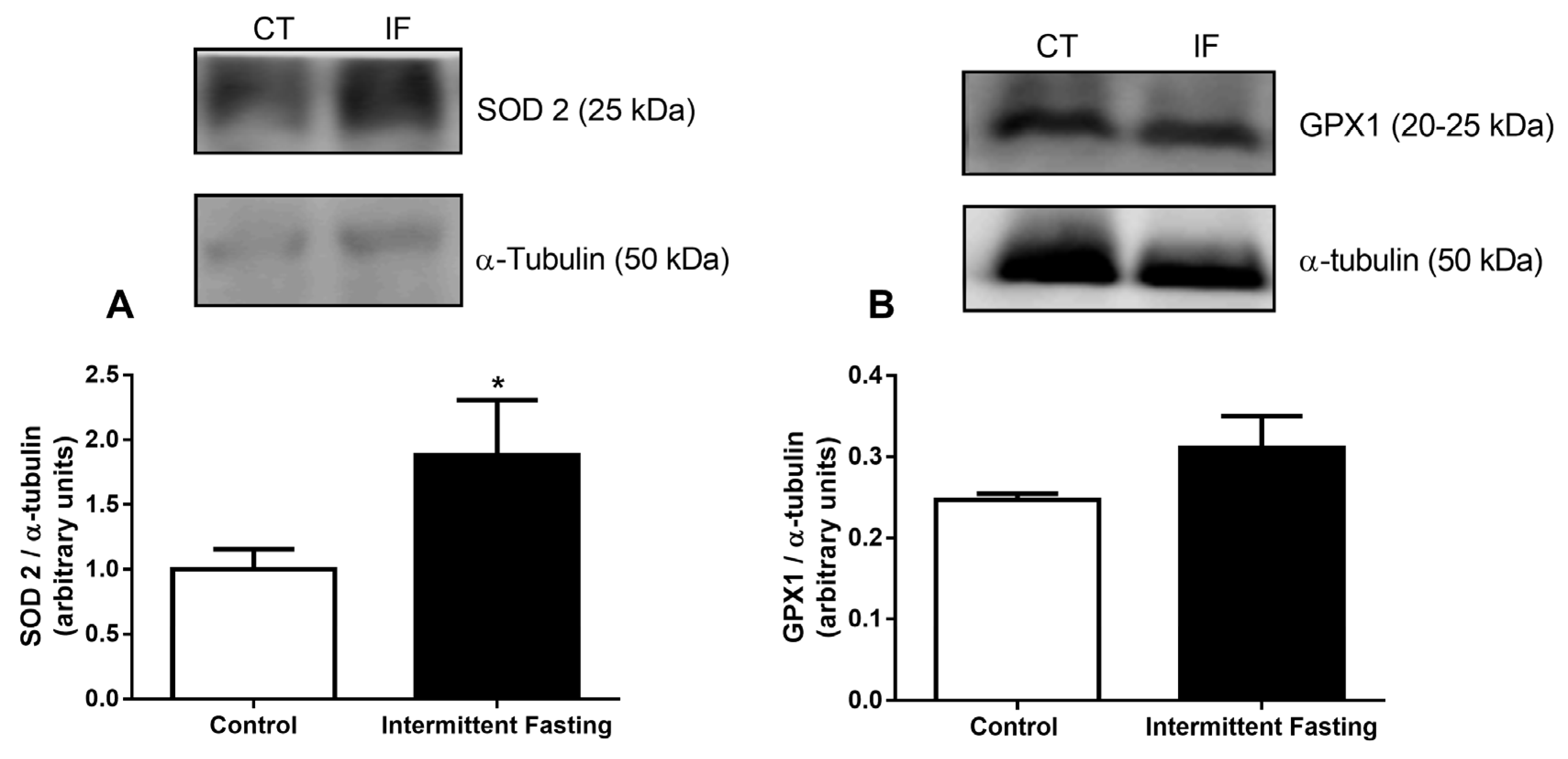
3.11. Antioxidant Systems
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Thent, Z.C.; Das, S.; Henry, L.J. Role of exercise in the management of diabetes mellitus: the global scenario. PLoS ONE 2013, 8, e80436. [Google Scholar] [CrossRef]
- Pilon, B. Eat Stop Eat; Medwin House Inc.: Ontario, Canada, 2007. [Google Scholar]
- Mosley, M.; Spencer, M. A Dieta dos 2 Dias—The Fast Diet; Sextante: Belo Horizonte, Brazil, 2013. [Google Scholar]
- Harris, L.; Hamilton, S.; Azevedo, L.B.; Olajide, J.; De Brún, C.; Waller, G.; Whittaker, V.; Sharp, T.; Lean, M.; Hankey, C.; et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: A systematic review and meta-analysis. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 507–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.E3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corley, B.T.; Carroll, R.W.; Hall, R.M.; Weatherall, M.; Parry-Strong, A.; Krebs, J.D. Intermittent fasting in Type 2 diabetes mellitus and the risk of hypoglycaemia: A randomized controlled trial. Diabet. Med. 2018. [Google Scholar] [CrossRef]
- Marosi, K.; Moehl, K.; Navas-Enamorado, I.; Mitchell, S.J.; Zhang, Y.; Lehrmann, E.; Aon, M.A.; Cortassa, S.; Becker, K.G.; Mattson, M.P. Metabolic and molecular framework for the enhancement of endurance by intermittent food deprivation. FASEB J. 2018. [Google Scholar] [CrossRef] [Green Version]
- Almeneessier, A.S.; Alzoghaibi, M.; BaHammam, A.A.; Ibrahim, M.G.; Olaish, A.H.; Nashwan, S.Z.; BaHammam, A.S. The effects of diurnal intermittent fasting on the wake-promoting neurotransmitter orexin-A. Ann. Thorac. Med. 2018, 13, 48–54. [Google Scholar] [CrossRef]
- Goodrick, C.L.; Ingram, D.K.; Reynolds, M.A.; Freeman, J.R.; Cider, N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: Interaction of genotype and age. Mech. Ageing Dev. 1990, 55, 69–87. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Smith, S.R.; Martin, C.K.; Anton, S.D.; Ravussin, E. Alternate-day fasting in nonobese subjects: Effects on body weight, body composition, and energy metabolism. Am. J. Clin. Nutr. 2005, 81, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Laub, D.R.; John, S. The effect on health of alternate day calorie restriction: eating less and more than needed on alternate days prolongs life. Med. Hypotheses 2006, 67, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Caviezel, F.; Margonato, A.; Slaviero, G.; Bonetti, F.; Vicedomini, G.; Cattaneo, A.G.; Pozza, G. Early improvement of left ventricular function during caloric restriction in obesity. Int. J. Obes. 1986, 10, 421–426. [Google Scholar] [PubMed]
- Bruce-Keller, A.J.; Umberger, G.; McFall, R.; Mattson, M.P. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann. Neurol. 1999, 45, 8–15. [Google Scholar] [CrossRef]
- Duan, W.; Guo, Z.; Mattson, M.P. Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J. Neurochem. 2001, 76, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kang, S.W.; Mallilankaraman, K.; Baik, S.H.; Lim, J.C.; Balaganapathy, P.; She, D.T.; Lok, K.Z.; Fann, D.Y.; Thambiayah, U.; et al. Transcriptome Analysis Reveals Intermittent Fasting-Induced Genetic Changes in Ischemic Stroke. Hum. Mol. Genet. 2018. [Google Scholar] [CrossRef]
- Wan, R.; Camandola, S.; Mattson, M.P. Intermittent fasting and dietary supplementation with 2-deoxy-D-glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J. 2003, 17, 1133–1134. [Google Scholar] [CrossRef]
- Anson, R.M.; Guo, Z.; de Cabo, R.; Iyun, T.; Rios, M.; Hagepanos, A.; Ingram, D.K.; Lane, M.A.; Mattson, M.P. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl. Acad. Sci. USA 2003, 100, 6216–6220. [Google Scholar] [CrossRef] [Green Version]
- Bouguerra, R.; Jabrane, J.; Maâtki, C.; Ben Salem, L.; Hamzaoui, J.; El Kadhi, A.; Ben Rayana, C.; Ben Slama, C. Ramadan fasting in type 2 diabetes mellitus. Ann. Endocrinol. (Paris) 2006, 67, 54–59. [Google Scholar] [CrossRef]
- Khaled, B.M.; Bendahmane, M.; Belbraouet, S. Ramadan fasting induces modifications of certain serum components in obese women with type 2 diabetes. Saudi Med. J. 2006, 27, 23–26. [Google Scholar] [PubMed]
- Shariatpanahi, Z.V.; Shariatpanahi, M.V.; Shahbazi, S.; Hossaini, A.; Abadi, A. Effect of Ramadan fasting on some indices of insulin resistance and components of the metabolic syndrome in healthy male adults. Br. J. Nutr. 2008, 100, 147–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belkacemi, L.; Selselet-Attou, G.; Bulur, N.; Louchami, K.; Sener, A.; Malaisse, W.J. Intermittent fasting modulation of the diabetic syndrome in sand rats. III. Post-mortem investigations. Int. J. Mol. Med. 2011, 27, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Teng, N.I.; Shahar, S.; Rajab, N.F.; Manaf, Z.A.; Johari, M.H.; Ngah, W.Z. Improvement of metabolic parameters in healthy older adult men following a fasting calorie restriction intervention. Aging Male 2013, 16, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Gnanou, J.V.; Caszo, B.A.; Khalil, K.M.; Abdullah, S.L.; Knight, V.F.; Bidin, M.Z. Effects of Ramadan fasting on glucose homeostasis and adiponectin levels in healthy adult males. J. Diabetes Metab. Disord. 2015, 14, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinsley, G.M.; La Bounty, P.M. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr. Rev. 2015, 73, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Li Volti, G.; Sanfilippo, C.; Tibullo, D.; Galvano, F.; Vecchio, M.; Avola, R.; Barbagallo, I.; Malaguarnera, L.; Castorina, S.; et al. Fasting and Fast Food Diet Play an Opposite Role in Mice Brain Aging. Mol. Neurobiol. 2018. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, M.; Chen, Y.; Yang, Y.; Zhang, J.J. Postoperative intermittent fasting prevents hippocampal oxidative stress and memory deficits in a rat model of chronic cerebral hypoperfusion. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef]
- Chentli, F.; Azzoug, S.; Amani, M.l.A.; Elgradechi, A. Diabetes mellitus and Ramadan in Algeria. Indian J. Endocrinol. Metab. 2013, 17, S295–S298. [Google Scholar] [CrossRef]
- Stote, K.S.; Baer, D.J.; Spears, K.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Strycula, P.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr. 2007, 85, 981–988. [Google Scholar] [CrossRef]
- al-Hadramy, M.S.; Zawawi, T.H.; Abdelwahab, S.M. Altered cortisol levels in relation to Ramadan. Eur. J. Clin. Nutr. 1988, 42, 359–362. [Google Scholar] [PubMed]
- Bogdan, A.; Bouchareb, B.; Touitou, Y. Ramadan fasting alters endocrine and neuroendocrine circadian patterns. Meal-time as a synchronizer in humans? Life Sci. 2001, 68, 1607–1615. [Google Scholar] [CrossRef]
- Ben Salem, L.; Bchir, S.; Bouguerra, R.; Ben Slama, C. Cortisol rhythm during the month of Ramadan. East Mediterr. Health J. 2003, 9, 1093–1098. [Google Scholar] [PubMed]
- Roky, R.; Houti, I.; Moussamih, S.; Qotbi, S.; Aadil, N. Physiological and chronobiological changes during Ramadan intermittent fasting. Ann. Nutr. Metab. 2004, 48, 296–303. [Google Scholar] [CrossRef]
- Haouari, M.; Haouari-Oukerro, F.; Sfaxi, A.; Ben Rayana, M.C.; Kâabachi, N.; Mbazâa, A. How Ramadan fasting affects caloric consumption, body weight, and circadian evolution of cortisol serum levels in young, healthy male volunteers. Horm. Metab. Res. 2008, 40, 575–577. [Google Scholar] [CrossRef]
- Bahijri, S.; Borai, A.; Ajabnoor, G.; Abdul Khaliq, A.; AlQassas, I.; Al-Shehri, D.; Chrousos, G. Relative metabolic stability, but disrupted circadian cortisol secretion during the fasting month of Ramadan. PLoS ONE 2013, 8, e60917. [Google Scholar] [CrossRef]
- Guerrero-Morilla, R.; Ramírez-Rodrigo, J.; Ruiz-Villaverde, G.; Sánchez-Caravaca, M.A.; Pérez-Moreno, B.A.; Villaverde-Gutiérrez, C. Endocrine-metabolic adjustments during Ramadan fasting in young athletes. Arch. Latinoam. Nutr. 2013, 63, 14–20. [Google Scholar]
- Ajabnoor, G.M.; Bahijri, S.; Borai, A.; Abdulkhaliq, A.A.; Al-Aama, J.Y.; Chrousos, G.P. Health impact of fasting in Saudi Arabia during Ramadan: association with disturbed circadian rhythm and metabolic and sleeping patterns. PLoS ONE 2014, 9, e96500. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Civitarese, A.E.; Bogacka, I.; Smith, S.R.; Hulver, M.; Ravussin, E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes. Res. 2005, 13, 574–581. [Google Scholar] [CrossRef]
- Reiches, M.W.; Moore, S.E.; Prentice, A.M.; Ellison, P.T. Endocrine responses, weight change, and energy sparing mechanisms during Ramadan among Gambian adolescent women. Am. J. Hum. Biol. 2014, 26, 395–400. [Google Scholar] [CrossRef]
- Munsters, M.J.; Saris, W.H. Effects of meal frequency on metabolic profiles and substrate partitioning in lean healthy males. PLoS ONE 2012, 7, e38632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosboom, R.S.; Zweens, J.; Bouman, P.R. Effects of feeding and fasting on the insulin secretory response to glucose and sulfonylureas in intact rats and isolated perfused rat pancreas. Diabetologia 1973, 9, 243–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svenningsen, A.; Bonnevie-Nielsen, V. Effects of fasting on beta-cell function, body fat, islet volume, and total pancreatic insulin content. Metabolism 1984, 33, 612–616. [Google Scholar] [CrossRef]
- Gasa, R.; Sener, A.; Malaisse, W.J.; Gomis, R. Apparent starvation-induced repression of pancreatic islet glucokinase. Biochem. Mol. Med. 1995, 56, 99–103. [Google Scholar] [CrossRef]
- Bone, A.J.; Howell, S.L. Alterations in regulation of insulin biosynthesis in pregnancy and starvation studied in isolated rat islets of langerhans. Biochem. J. 1977, 166, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Hedeskov, C.J.; Capito, K. The effect of starvation on insulin secretion and glucose metabolism in mouse pancreatic islets. Biochem. J. 1974, 140, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Tadayyon, M.; Bonney, R.C.; Green, I.C. Starvation decreases insulin secretion, prostaglandin E2 production and phospholipase A2 activity in rat pancreatic islets. J. Endocrinol. 1990, 124, 455–461. [Google Scholar] [CrossRef]
- Hedeskov, C.J.; Capito, K. The pentose cycle and insulin release in isolated mouse pancreatic islets during starvation. Biochem. J. 1975, 152, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Munhoz, A.C.; Riva, P.; Simões, D.; Curi, R.; Carpinelli, A.R. Control of Insulin Secretion by Production of Reactive Oxygen Species: Study Performed in Pancreatic Islets from Fed and 48-Hour Fasted Wistar Rats. PLoS ONE 2016, 11, e0158166. [Google Scholar] [CrossRef]
- Bernardis, L.L.; Patterson, B.D. Correlation between “Lee index” and carcass fat content in weanling and adult female rats with hypothalamic lesions. J. Endocrinol. 1968, 40, 527–528. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacy, P.E.; Kostianovsky, M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 1967, 16, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Atwater, I.; Rojas, E. A method for the simultaneous measurement of insulin release and B cell membrane potential in single mouse islets of Langerhans. Diabetologia 1981, 21, 470–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef]
- Sundfør, T.M.; Svendsen, M.; Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef] [Green Version]
- Chausse, B.; Solon, C.; Caldeira da Silva, C.C.; Masselli Dos Reis, I.G.; Manchado-Gobatto, F.B.; Gobatto, C.A.; Velloso, L.A.; Kowaltowski, A.J. Intermittent fasting induces hypothalamic modifications resulting in low feeding efficiency, low body mass and overeating. Endocrinology 2014, 155, 2456–2466. [Google Scholar] [CrossRef] [Green Version]
- Chausse, B.; Vieira-Lara, M.A.; Sanchez, A.B.; Medeiros, M.H.; Kowaltowski, A.J. Intermittent fasting results in tissue-specific changes in bioenergetics and redox state. PLoS ONE 2015, 10, e0120413. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, S.R.; Halset, E.H.; Gåsbakk, S.; Rehfeld, J.F.; Kulseng, B.; Truby, H.; Martins, C. Compensatory mechanisms activated with intermittent energy restriction: A randomized control trial. Clin. Nutr. 2017. [Google Scholar] [CrossRef]
- López-Bueno, M.; González-Jiménez, E.; Navarro-Prado, S.; Montero-Alonso, M.A.; Schmidt-RioValle, J. Influence of age and religious fasting on the body composition of Muslim women living in a westernized context. Nutr. Hosp. 2014, 31, 1067–1073. [Google Scholar] [CrossRef]
- Sakamoto, K.; Grunewald, K.K. Beneficial effects of exercise on growth of rats during intermittent fasting. J. Nutr. 1987, 117, 390–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Soeters, M.R.; Lammers, N.M.; Dubbelhuis, P.F.; Ackermans, M.; Jonkers-Schuitema, C.F.; Fliers, E.; Sauerwein, H.P.; Aerts, J.M.; Serlie, M.J. Intermittent fasting does not affect whole-body glucose, lipid, or protein metabolism. Am. J. Clin. Nutr. 2009, 90, 1244–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- el Ati, J.; Beji, C.; Danguir, J. Increased fat oxidation during Ramadan fasting in healthy women: An adaptative mechanism for body-weight maintenance. Am. J. Clin. Nutr. 1995, 62, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Gotthardt, J.D.; Verpeut, J.L.; Yeomans, B.L.; Yang, J.A.; Yasrebi, A.; Roepke, T.A.; Bello, N.T. Intermittent Fasting Promotes Fat Loss with Lean Mass Retention, Increased Hypothalamic Norepinephrine Content, and Increased Neuropeptide Y Gene Expression in Diet-Induced Obese Male Mice. Endocrinology 2016, 157, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Mitchell, S.E.; Hambly, C.; Morgan, D.G.; Clapham, J.C.; Speakman, J.R. Physiological and behavioral responses to intermittent starvation in C57BL/6J mice. Physiol. Behav. 2012, 105, 376–387. [Google Scholar] [CrossRef]
- Kalra, S.P.; Kalra, P.S. Neuropeptide Y: A physiological orexigen modulated by the feedback action of ghrelin and leptin. Endocrine 2003, 22, 49–56. [Google Scholar] [CrossRef]
- Finch, G.M.; Day, J.E.; Razak; Welch, D.A.; Rogers, P.J. Appetite changes under free-living conditions during Ramadan fasting. Appetite 1998, 31, 159–170. [Google Scholar] [CrossRef]
- Thivel, D.; Finlayson, G.; Miguet, M.; Pereira, B.; Duclos, M.; Boirie, Y.; Doucet, E.; Blundell, J.E.; Metz, L. Energy depletion by 24-h fast leads to compensatory appetite responses compared with matched energy depletion by exercise in healthy young males. Br. J. Nutr. 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Clayton, D.J.; Burrell, K.; Mynott, G.; Creese, M.; Skidmore, N.; Stensel, D.J.; James, L.J. Effect of 24-h severe energy restriction on appetite regulation and ad libitum energy intake in lean men and women. Am. J. Clin. Nutr. 2016, 104, 1545–1553. [Google Scholar] [CrossRef] [Green Version]
- Karbowska, J.; Kochan, Z. Intermittent fasting up-regulates Fsp27/Cidec gene expression in white adipose tissue. Nutrition 2012, 28, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Malafaia, A.B.; Nassif, P.A.; Ribas, C.A.; Ariede, B.L.; Sue, K.N.; Cruz, M.A. Obesity induction with high fat sucrose in rats. Arq. Bras. Cir. Dig. 2013, 26, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Lasheen, N.N. Pancreatic functions in high salt fed female rats. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res. Clin. Pract. 2016, 122, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Joslin, P.M.N.; Bell, R.K.; Swoap, S.J. Obese mice on a high-fat alternate-day fasting regimen lose weight and improve glucose tolerance. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Costill, D.L.; Hargreaves, M. Carbohydrate nutrition and fatigue. Sports Med. 1992, 13, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Alsubheen, S.A.; Ismail, M.; Baker, A.; Blair, J.; Adebayo, A.; Kelly, L.; Chandurkar, V.; Cheema, S.; Joanisse, D.R.; Basset, F.A. The effects of diurnal Ramadan fasting on energy expenditure and substrate oxidation in healthy men. Br. J. Nutr. 2017, 118, 1023–1030. [Google Scholar] [CrossRef] [Green Version]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Norouzy, A.; Salehi, M.; Philippou, E.; Arabi, H.; Shiva, F.; Mehrnoosh, S.; Mohajeri, S.M.; Mohajeri, S.A.; Motaghedi Larijani, A.; Nematy, M. Effect of fasting in Ramadan on body composition and nutritional intake: A prospective study. J. Hum. Nutr. Diet 2013, 97–104. [Google Scholar] [CrossRef]
- Yarahmadi, S.; Larijani, B.; Bastanhagh, M.H.; Pajouhi, M.; Baradar Jalili, R.; Zahedi, F.; Zendehdel, K.; Akrami, S.M. Metabolic and clinical effects of Ramadan fasting in patients with type II diabetes. J. Coll. Physicians Surg. Pak. 2003, 13, 329–332. [Google Scholar]
- Liu, H.; Javaheri, A.; Godar, R.J.; Murphy, J.; Ma, X.; Rohatgi, N.; Mahadevan, J.; Hyrc, K.; Saftig, P.; Marshall, C.; et al. Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway. Autophagy 2017, 13, 1952–1968. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yoo, K.M.; Hyun, J.S.; Kang, S. Intermittent fasting reduces body fat but exacerbates hepatic insulin resistance in young rats regardless of high protein and fat diets. J. Nutr. Biochem. 2017, 40, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.J.; Liang, G.; Li, R.L.; Xie, X.; Sleeman, M.W.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Goldstein, J.L.; Brown, M.S. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. USA 2010, 107, 7467–7472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangiao-Alvarellos, S.; Cordido, F. Effect of ghrelin on glucose-insulin homeostasis: Therapeutic implications. Int. J. Pept. 2010, 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes, L.A.C.; Jornada, M.N.; Elkfury, J.L.; Foletto, K.C.; Bertoluci, M.C. Fasting ghrelin but not PYY(3-36) is associated with insulin-resistance independently of body weight in Wistar rats. Arq. Bras. Endocrinol. Metabol. 2014, 58, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barazzoni, R.; Zanetti, M.; Cattin, M.R.; Visintin, L.; Vinci, P.; Cattin, L.; Stebel, M.; Guarnieri, G. Ghrelin enhances in vivo skeletal muscle but not liver AKT signaling in rats. Obesity 2007, 15, 2614–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerqueira, F.M.; da Cunha, F.M.; Caldeira da Silva, C.C.; Chausse, B.; Romano, R.L.; Garcia, C.C.; Colepicolo, P.; Medeiros, M.H.; Kowaltowski, A.J. Long-term intermittent feeding, but not caloric restriction, leads to redox imbalance, insulin receptor nitration, and glucose intolerance. Free Radic. Biol. Med. 2011, 51, 1454–1460. [Google Scholar] [CrossRef]
- Rosas Fernández, M.A.; Concha Vilca, C.M.; Batista, L.O.; Ramos, V.W.; Cinelli, L.P.; Tibau de Albuquerque, K. Intermittent food restriction in female rats induces SREBP high expression in hypothalamus and immediately postfasting hyperphagia. Nutrition 2017, 48, 122–126. [Google Scholar] [CrossRef]
- Marangou, A.G.; Weber, K.M.; Boston, R.C.; Aitken, P.M.; Heggie, J.C.; Kirsner, R.L.; Best, J.D.; Alford, F.P. Metabolic consequences of prolonged hyperinsulinemia in humans. Evidence for induction of insulin insensitivity. Diabetes 1986, 35, 1383–1389. [Google Scholar] [CrossRef]
- Del Prato, S.; Leonetti, F.; Simonson, D.C.; Sheehan, P.; Matsuda, M.; DeFronzo, R.A. Effect of sustained physiologic hyperinsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia 1994, 37, 1025–1035. [Google Scholar] [CrossRef]
- Koopmans, S.J.; Ohman, L.; Haywood, J.R.; Mandarino, L.J.; DeFronzo, R.A. Seven days of euglycemic hyperinsulinemia induces insulin resistance for glucose metabolism but not hypertension, elevated catecholamine levels, or increased sodium retention in conscious normal rats. Diabetes 1997, 46, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Bertacca, A.; Ciccarone, A.; Cecchetti, P.; Vianello, B.; Laurenza, I.; Maffei, M.; Chiellini, C.; Del Prato, S.; Benzi, L. Continually high insulin levels impair Akt phosphorylation and glucose transport in human myoblasts. Metabolism 2005, 54, 1687–1693. [Google Scholar] [CrossRef]
- Bertacca, A.; Ciccarone, A.; Cecchetti, P.; Vianello, B.; Laurenza, I.; Del Prato, S.; Benzi, L. High insulin levels impair intracellular receptor trafficking in human cultured myoblasts. Diabetes Res. Clin. Pract. 2007, 78, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; McLean, L.L.; Philip, S.S.; Feldman, E.L. Hyperinsulinemia induces insulin resistance in dorsal root ganglion neurons. Endocrinology 2011, 152, 3638–3647. [Google Scholar] [CrossRef] [PubMed]
- Catalano, K.J.; Maddux, B.A.; Szary, J.; Youngren, J.F.; Goldfine, I.D.; Schaufele, F. Insulin resistance induced by hyperinsulinemia coincides with a persistent alteration at the insulin receptor tyrosine kinase domain. PLoS ONE 2014, 9, e108693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genuth, S.M.; Przybylski, R.J.; Rosenberg, D.M. Insulin resistance in genetically obese, hyperglycemic mice. Endocrinology 1971, 88, 1230–1238. [Google Scholar] [CrossRef]
- Odeleye, O.E.; de Courten, M.; Pettitt, D.J.; Ravussin, E. Fasting hyperinsulinemia is a predictor of increased body weight gain and obesity in Pima Indian children. Diabetes 1997, 46, 1341–1345. [Google Scholar] [CrossRef]
- Sigal, R.J.; El-Hashimy, M.; Martin, B.C.; Soeldner, J.S.; Krolewski, A.S.; Warram, J.H. Acute postchallenge hyperinsulinemia predicts weight gain: A prospective study. Diabetes 1997, 46, 1025–1029. [Google Scholar] [CrossRef]
- Ishikawa, M.; Pruneda, M.L.; Adams-Huet, B.; Raskin, P. Obesity-independent hyperinsulinemia in nondiabetic first-degree relatives of individuals with type 2 diabetes. Diabetes 1998, 47, 788–792. [Google Scholar] [CrossRef]
- Mehran, A.E.; Templeman, N.M.; Brigidi, G.S.; Lim, G.E.; Chu, K.Y.; Hu, X.; Botezelli, J.D.; Asadi, A.; Hoffman, B.G.; Kieffer, T.J.; et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012, 16, 723–737. [Google Scholar] [CrossRef] [Green Version]
- Templeman, N.M.; Clee, S.M.; Johnson, J.D. Suppression of hyperinsulinaemia in growing female mice provides long-term protection against obesity. Diabetologia 2015, 58, 2392–2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reaven, G.M.; Hoffman, B.B. A role for insulin in the aetiology and course of hypertension? Lancet 1987, 2, 435–437. [Google Scholar] [CrossRef]
- Takatori, S.; Zamami, Y.; Mio, M.; Kurosaki, Y.; Kawasaki, H. Chronic hyperinsulinemia enhances adrenergic vasoconstriction and decreases calcitonin gene-related peptide-containing nerve-mediated vasodilation in pithed rats. Hypertens. Res. 2006, 29, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamami, Y.; Takatori, S.; Hobara, N.; Yabumae, N.; Tangsucharit, P.; Jin, X.; Hashikawa, N.; Kitamura, Y.; Sasaki, K.; Kawasaki, H. Hyperinsulinemia induces hypertension associated with neurogenic vascular dysfunction resulting from abnormal perivascular innervations in rat mesenteric resistance arteries. Hypertens. Res. 2011, 34, 1190–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, A.; Monma, Y.; Kajitani, S.; Noda, K.; Nakajima, S.; Endo, H.; Takahashi, T.; Nozaki, E. Effect of glycemic state on postprandial hyperlipidemia and hyperinsulinemia in patients with coronary artery disease. Heart Vessels 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zidi, W.; Allal-Elasmi, M.; Zayani, Y.; Zaroui, A.; Guizani, I.; Feki, M.; Mourali, M.S.; Mechmeche, R.; Kaabachi, N. Metabolic Syndrome, Independent Predictor for Coronary Artery Disease. Clin. Lab. 2015, 61, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; De Caterina, R. Prolonged exposure to high insulin impairs the endothelial PI3-kinase/Akt/nitric oxide signalling. Thromb. Haemost. 2009, 101, 345–350. [Google Scholar] [CrossRef]
- Steneberg, P.; Rubins, N.; Bartoov-Shifman, R.; Walker, M.D.; Edlund, H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 2005, 1, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Bril, F.; Lomonaco, R.; Orsak, B.; Ortiz-Lopez, C.; Webb, A.; Tio, F.; Hecht, J.; Cusi, K. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 2178–2187. [Google Scholar] [CrossRef]
- Westermark, P.; Wilander, E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia 1978, 15, 417–421. [Google Scholar] [CrossRef] [Green Version]
- Bonner-Weir, S. Regulation of pancreatic beta-cell mass in vivo. Recent Prog. Horm. Res. 1994, 49, 91–104. [Google Scholar] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasuga, M. Insulin resistance and pancreatic beta cell failure. J. Clin. Investig. 2006, 116, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Király, M.A.; Bates, H.E.; Kaniuk, N.A.; Yue, J.T.; Brumell, J.H.; Matthews, S.G.; Riddell, M.C.; Vranic, M. Swim training prevents hyperglycemia in ZDF rats: Mechanisms involved in the partial maintenance of beta-cell function. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E271–E283. [Google Scholar] [CrossRef] [PubMed]
- Karaca, M.; Magnan, C.; Kargar, C. Functional pancreatic beta-cell mass: Involvement in type 2 diabetes and therapeutic intervention. Diabetes Metab. 2009, 35, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E. Beta cell dysfunction and insulin resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, 31S–38S. [Google Scholar] [CrossRef]
- Halliwell, B.; Cross, C.E. Oxygen-derived species: Their relation to human disease and environmental stress. Environ. Health Perspect. 1994, 102, 5–12. [Google Scholar]
- Vincent, A.M.; Brownlee, M.; Russell, J.W. Oxidative stress and programmed cell death in diabetic neuropathy. Ann. N. Y. Acad. Sci. 2002, 959, 368–383. [Google Scholar] [CrossRef]
- Loh, K.P.; Huang, S.H.; De Silva, R.; Tan, B.K.; Zhu, Y.Z. Oxidative stress: Apoptosis in neuronal injury. Curr. Alzheimer Res. 2006, 3, 327–337. [Google Scholar] [CrossRef]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007, 9, 49–89. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kim, J.; Kim, G.W.; Choi, C. Oxidative stress-induced necrotic cell death via mitochondira-dependent burst of reactive oxygen species. Curr. Neurovasc. Res. 2009, 6, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Yepes, J.; Burns, M.; Anandhan, A.; Khalimonchuk, O.; del Razo, L.M.; Quintanilla-Vega, B.; Pappa, A.; Panayiotidis, M.I.; Franco, R. Oxidative stress, redox signaling, and autophagy: Cell death versus survival. Antioxid. Redox Signal. 2014, 21, 66–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zraika, S.; Hull, R.L.; Udayasankar, J.; Aston-Mourney, K.; Subramanian, S.L.; Kisilevsky, R.; Szarek, W.A.; Kahn, S.E. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia 2009, 52, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.W.; Chae, H.Y.; Kwon, S.J.; Park, S.Y.; Ihm, J.; Ihm, S.H. RAGE ligands induce apoptotic cell death of pancreatic β-cells via oxidative stress. Int. J. Mol. Med. 2010, 26, 813–818. [Google Scholar]
- Lu, T.H.; Su, C.C.; Chen, Y.W.; Yang, C.Y.; Wu, C.C.; Hung, D.Z.; Chen, C.H.; Cheng, P.W.; Liu, S.H.; Huang, C.F. Arsenic induces pancreatic β-cell apoptosis via the oxidative stress-regulated mitochondria-dependent and endoplasmic reticulum stress-triggered signaling pathways. Toxicol. Lett. 2011, 201, 15–26. [Google Scholar] [CrossRef]
- Chen, K.L.; Liu, S.H.; Su, C.C.; Yen, C.C.; Yang, C.Y.; Lee, K.I.; Tang, F.C.; Chen, Y.W.; Lu, T.H.; Su, Y.C.; et al. Mercuric compounds induce pancreatic islets dysfunction and apoptosis in vivo. Int. J. Mol. Sci. 2012, 13, 12349–12366. [Google Scholar] [CrossRef] [Green Version]
- Du, S.C.; Ge, Q.M.; Lin, N.; Dong, Y.; Su, Q. ROS-mediated lipopolysaccharide-induced apoptosis in INS-1 cells by modulation of Bcl-2 and Bax. Cell Mol. Biol. 2012, 58, OL1654-9. [Google Scholar]
- Chang, K.C.; Hsu, C.C.; Liu, S.H.; Su, C.C.; Yen, C.C.; Lee, M.J.; Chen, K.L.; Ho, T.J.; Hung, D.Z.; Wu, C.C.; et al. Cadmium induces apoptosis in pancreatic β-cells through a mitochondria-dependent pathway: The role of oxidative stress-mediated c-Jun N-terminal kinase activation. PLoS ONE 2013, 8, e54374. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, K.; Sakurai, K.; Yokoh, H.; Ishibashi, T.; Ishikawa, K.; Shirasawa, T.; Yokote, K. Mutation in insulin receptor attenuates oxidative stress and apoptosis in pancreatic beta-cells induced by nutrition excess: Reduced insulin signaling and ROS. Horm. Metab. Res. 2015, 47, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D.; Bernal-Mizrachi, E.; Alejandro, E.U.; Han, Z.; Kalynyak, T.B.; Li, H.; Beith, J.L.; Gross, J.; Warnock, G.L.; Townsend, R.R.; et al. Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc. Natl. Acad. Sci. USA 2006, 103, 19575–19580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beith, J.L.; Alejandro, E.U.; Johnson, J.D. Insulin stimulates primary beta-cell proliferation via Raf-1 kinase. Endocrinology 2008, 149, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Carlson, O.; Martin, B.; Stote, K.S.; Golden, E.; Maudsley, S.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; Longo, D.L.; Rumpler, W.V.; et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism 2007, 56, 1729–1734. [Google Scholar] [CrossRef] [Green Version]
- Oishi, K.; Hashimoto, C. Short-term time-restricted feeding during the resting phase is sufficient to induce leptin resistance that contributes to development of obesity and metabolic disorders in mice. Chronobiol. Int. 2018, 1–19. [Google Scholar] [CrossRef]
- Cote, I.; Toklu, H.Z.; Green, S.M.; Morgan, D.; Carter, C.S.; Tumer, N.; Scarpace, P.J. Limiting feeding to the active phase reduces blood pressure without the necessity of caloric reduction or fat mass loss. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018. [Google Scholar] [CrossRef]
- Woodie, L.N.; Luo, Y.; Wayne, M.J.; Graff, E.C.; Ahmed, B.; O’Neill, A.M.; Greene, M.W. Restricted feeding for 9h in the active period partially abrogates the detrimental metabolic effects of a Western diet with liquid sugar consumption in mice. Metabolism 2018, 82, 1–13. [Google Scholar] [CrossRef]
- Bi, H.; Gan, Y.; Yang, C.; Chen, Y.; Tong, X.; Lu, Z. Breakfast skipping and the risk of type 2 diabetes: A meta-analysis of observational studies. Public Health Nutr. 2015, 18, 3013–3019. [Google Scholar] [CrossRef]
- Reutrakul, S.; Hood, M.M.; Crowley, S.J.; Morgan, M.K.; Teodori, M.; Knutson, K.L. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol. Int. 2014, 31, 64–71. [Google Scholar] [CrossRef]
- Azami, Y.; Funakoshi, M.; Matsumoto, H.; Ikota, A.; Ito, K.; Okimoto, H.; Shimizu, N.; Tsujimura, F.; Fukuda, H.; Miyagi, C.; et al. Long working hours and skipping breakfast concomitant with late evening meals are associated with suboptimal glycemic control among young male Japanese patients with type 2 diabetes. J. Diabetes Investig. 2018. [Google Scholar] [CrossRef]
- Watanabe, Y.; Saito, I.; Henmi, I.; Yoshimura, K.; Maruyama, K.; Yamauchi, K.; Matsuo, T.; Kato, T.; Tanigawa, T.; Kishida, T.; et al. Skipping Breakfast is Correlated with Obesity. J. Rural Med. 2014, 9, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, M.; Yoshita, K.; Nakamura, K.; Miura, K.; Takamura, T.; Nagasawa, S.Y.; Morikawa, Y.; Kido, T.; Naruse, Y.; Nogawa, K.; et al. Skipping breakfast and 5-year changes in body mass index and waist circumference in Japanese men and women. Obes. Sci. Pract. 2017, 3, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Yatsuya, H.; Hilawe, E.H.; Li, Y.; Wang, C.; Chiang, C.; Otsuka, R.; Toyoshima, H.; Tamakoshi, K.; Aoyama, A. Breakfast Skipping is Positively Associated with Incidence of Type 2 Diabetes Mellitus: Evidence From the Aichi Workers’ Cohort Study. J. Epidemiol. 2015, 25, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Møller, N.; Jørgensen, J.O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munhoz, A.C.; Vilas-Boas, E.A.; Panveloski-Costa, A.C.; Leite, J.S.M.; Lucena, C.F.; Riva, P.; Emilio, H.; Carpinelli, A.R. Intermittent Fasting for Twelve Weeks Leads to Increases in Fat Mass and Hyperinsulinemia in Young Female Wistar Rats. Nutrients 2020, 12, 1029. https://doi.org/10.3390/nu12041029
Munhoz AC, Vilas-Boas EA, Panveloski-Costa AC, Leite JSM, Lucena CF, Riva P, Emilio H, Carpinelli AR. Intermittent Fasting for Twelve Weeks Leads to Increases in Fat Mass and Hyperinsulinemia in Young Female Wistar Rats. Nutrients. 2020; 12(4):1029. https://doi.org/10.3390/nu12041029
Chicago/Turabian StyleMunhoz, Ana Cláudia, Eloisa Aparecida Vilas-Boas, Ana Carolina Panveloski-Costa, Jaqueline Santos Moreira Leite, Camila Ferraz Lucena, Patrícia Riva, Henriette Emilio, and Angelo R. Carpinelli. 2020. "Intermittent Fasting for Twelve Weeks Leads to Increases in Fat Mass and Hyperinsulinemia in Young Female Wistar Rats" Nutrients 12, no. 4: 1029. https://doi.org/10.3390/nu12041029
APA StyleMunhoz, A. C., Vilas-Boas, E. A., Panveloski-Costa, A. C., Leite, J. S. M., Lucena, C. F., Riva, P., Emilio, H., & Carpinelli, A. R. (2020). Intermittent Fasting for Twelve Weeks Leads to Increases in Fat Mass and Hyperinsulinemia in Young Female Wistar Rats. Nutrients, 12(4), 1029. https://doi.org/10.3390/nu12041029




