Vitamin B12 Status and Optimal Range for Hemoglobin Formation in Elite Athletes
Abstract
:1. Introduction
2. Materials and methods
Statistics
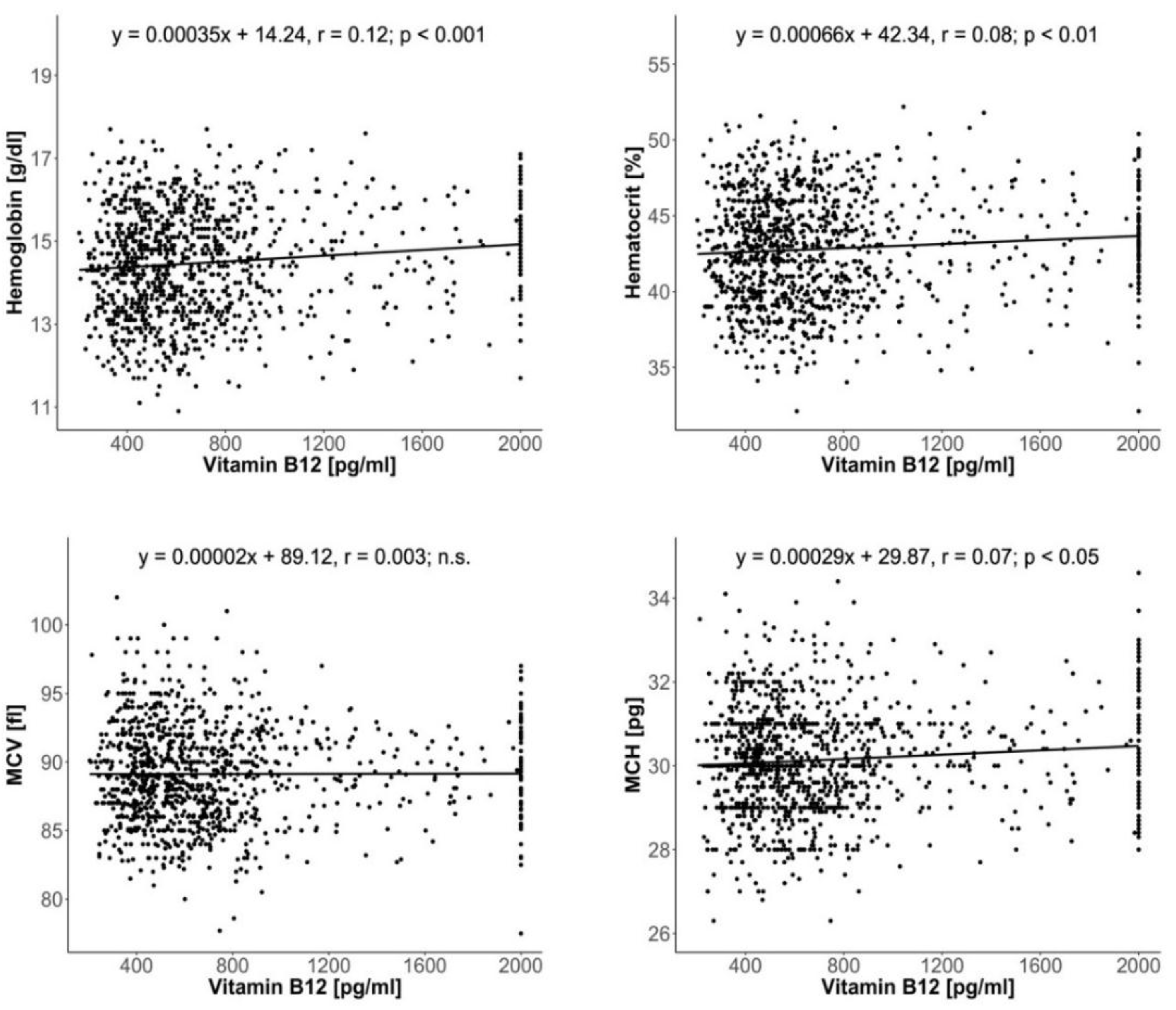
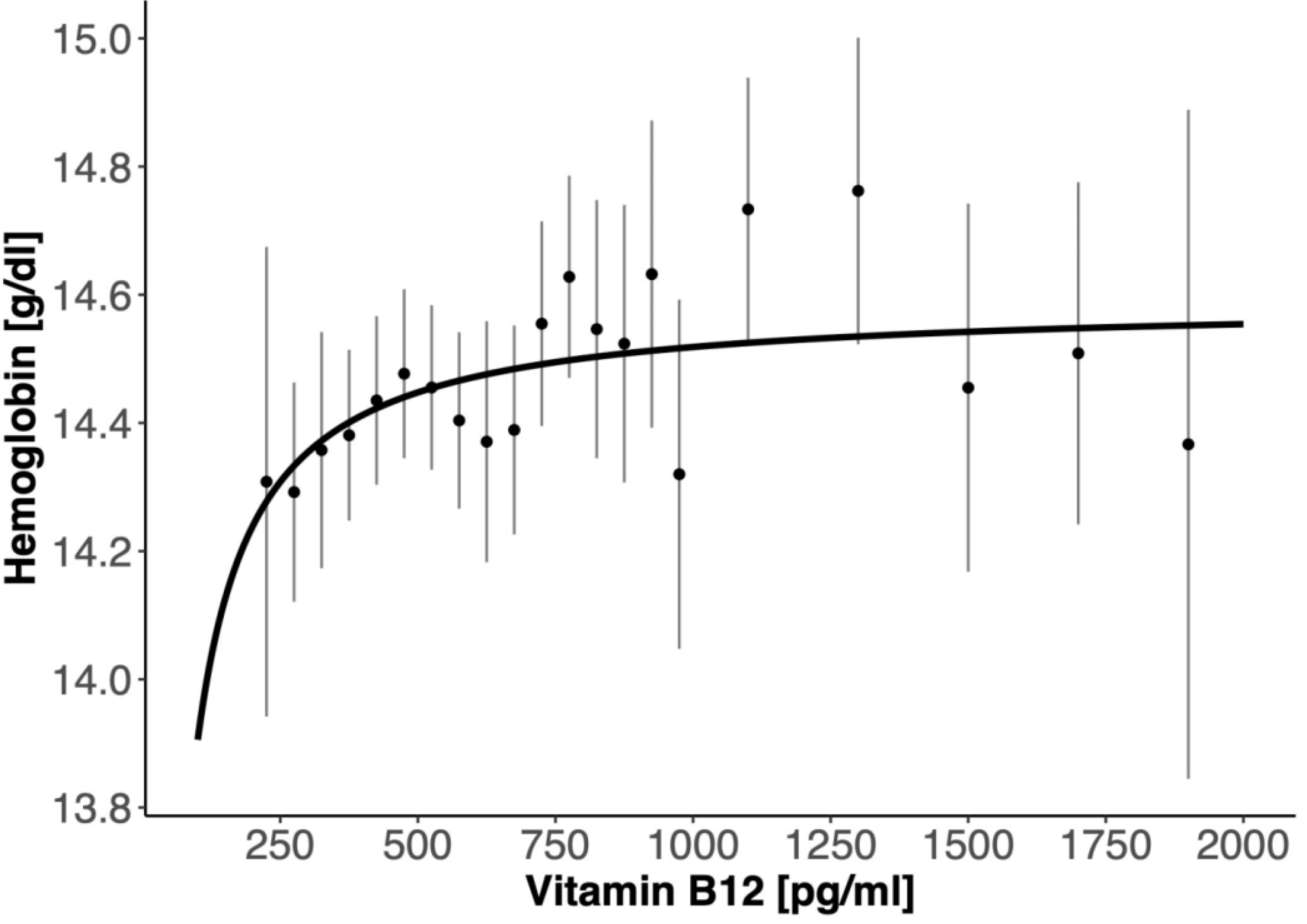
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Allen, L.H.; Miller, J.W.; de Groot, L.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148 (Suppl. 4), 1995S–2027S. [Google Scholar] [CrossRef] [Green Version]
- Beck, W.S. Cobalamin (Vitamin B12). In Handbook of Vitamins, 3rd ed.; Rucker, R.B., Suttie, J.W., McCormick, D.B., Machlin, L.J., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2001; pp. 466–476. [Google Scholar]
- Herrmann, W.; Obeid, R. Cobalamin deficiency. Subcell. Biochem. 2012, 56, 301–322. [Google Scholar] [PubMed]
- O’Leary, F.; Samman, S. Vitamin B12 in health and disease. Nutrients 2010, 2, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Williams, C. Intense exercise training and immune function. Nestle Nutr. Inst. Workshop Ser. 2013, 76, 39–50. [Google Scholar] [PubMed]
- Pyne, D.B.; Verhagen, E.A.; Mountjoy, M. Nutrition, illness, and injury in aquatic sports. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 460–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, M.H. Dietary supplements and sports performance: Introduction and vitamins. J. Int. Soc. Sports Nutr. 2004, 1, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolf, K.; Manore, M.M. B-vitamins and exercise: Does exercise alter requirements? Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 453–484. [Google Scholar] [CrossRef]
- Beals, K.A.; Manore, M.M. Nutritional status of female athletes with subclinical eating disorders. J. Am. Diet. Assoc. 1998, 98, 419–425. [Google Scholar] [CrossRef]
- Braun, H.; Koehler, K.; Geyer, H.; Kleiner, J.; Mester, J.; Schanzer, W. Dietary supplement use among elite young German athletes. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 97–109. [Google Scholar] [CrossRef]
- Eskici, G.; Ersoy, G. An evaluation of wheelchair basketball players’ nutritional status and nutritional knowledge levels. J. Sports Med. Phys. Fitness 2016, 56, 259–268. [Google Scholar]
- Froiland, K.; Koszewski, W.; Hingst, J.; Kopecky, L. Nutritional supplement use among college athletes and their sources of information. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Obeid, R.; Scharhag, J.; Kindermann, W.; Herrmann, W. Altered vitamin B12 status in recreational endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 433–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, M.; Schorr, H.; Obeid, R.; Scharhag, J.; Urhausen, A.; Kindermann, W.; Herrmann, W. Homocysteine increases during endurance exercise. Clin. Chem. Lab. Med. 2003, 41, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Keen, C.L. Patterns of vitamin/mineral supplement usage by adolescents attending athletic high schools in Korea. Int. J. Sport Nutr. 1999, 9, 391–405. [Google Scholar] [CrossRef]
- Krumbach, C.J.; Ellis, D.R.; Driskell, J.A. A report of vitamin and mineral supplement use among university athletes in a division I institution. Int. J. Sport Nutr. 1999, 9, 416–425. [Google Scholar] [CrossRef]
- Aparicio-Ugarriza, R.; Palacios, G.; Alder, M.; González-Gross, M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin. Chem. Lab. Med. 2014, 53, 1149–1159. [Google Scholar] [CrossRef]
- Eussen, S.J.; de Groot, L.C.; Clarke, R.; Schneede, J.; Ueland, P.M.; Hoefnagels, W.H.; van Staveren, W.A. Oral cyanocobalamin supplementation in older people with vitamin B12 deficiency: A dose-finding trial. Arch. Intern. Med. 2005, 165, 1167–1172. [Google Scholar] [CrossRef]
- Sobczynska-Malefora, A.; Gorska, R.; Pelisser, M.; Ruwona, P.; Witchlow, B.; Harrington, D.J. An audit of holotranscobalamin (“Active” B12) and methylmalonic acid assays for the assessment of vitamin B12 status: Application in a mixed patient population. Clin. Biochem. 2014, 47, 82–86. [Google Scholar] [CrossRef]
- Zappacosta, B.; Persichilli, S.; Iacoviello, L.; Di Castelnuovo, A.; Graziano, M.; Gervasoni, J.; Leoncini, E.; Cimino, G.; Mastroiacovo, P. Folate, vitamin B12 and homocysteine status in an Italian blood donor population. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 473–480. [Google Scholar] [CrossRef]
- Parmar, V.; Shah, P.; Khubchandani, A.; Solanki, V.; Vaghela, V.; Jadav, J. Vitamin B12 Status in different Age Groups. Int. J. Res. Med. 2015, 4, 113–115. [Google Scholar]
- Palacios, G.; Sola, R.; Barrios, L.; Pietrzik, K.; Castillo, M.J.; González-Gross, M. Algorithm for the early diagnosis of vitamin B12 deficiency in elderly people. Nutr. Hosp. 2013, 28, 1447–1452. [Google Scholar] [PubMed]
- Battat, R.; Kopylov, U.; Szilagyi, A.; Saxena, A.; Rosenblatt, D.S.; Warner, M.; Bessissow, T.; Seidman, E.; Bitton, A. Vitamin B12 deficiency in inflammatory bowel disease: Prevalence, risk factors, evaluation, and management. Inflamm. Bowel Dis. 2014, 20, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Salinas, M.; Flores, E.; López-Garrigós, M.; Leiva-Salinas, C. Vitamin B12 deficiency and clinical laboratory: Lessons revisited and clarified in seven questions. Int. J. Lab. Hematol. 2018, 40 (Suppl. 1), 83–88. [Google Scholar] [CrossRef] [Green Version]
- Lukaski, H.C. Vitamin and mineral status: Effects on physical performance. Nutrition 2004, 20, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Read, M.H.; McGuffin, S.L. The effect of B-complex supplementation on endurance performance. J. Sports Med. Phys. Fitness 1983, 23, 178–184. [Google Scholar]
- Than, T.M.; May, M.W.; Aung, K.S.; Mya-Tu, M. The effect of vitamin B12 on physical performance capacity. Br. J. Nutr. 1978, 40, 269–273. [Google Scholar] [CrossRef]
- Habte, K.; Adish, A.; Zerfu, D.; Kebede, A.; Moges, T.; Tesfaye, B.; Challa, F.; Baye, K. Iron, folate and vitamin B12 status of Ethiopian professional runners. Nutr. Metab. (Lond.) 2015, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Woolf, K.; Hahn, N.L.; Christensen, M.M.; Carlson-Phillips, A.; Hansen, C.M. Nutrition Assessment of B-Vitamins in Highly Active and Sedentary Women. Nutrients 2017, 9, E329. [Google Scholar] [CrossRef]
- Telford, R.D.; Catchpole, E.A.; Deakin, V.; McLeay, A.C.; Plank, A.W. The effect of 7 to 8 months of vitamin/mineral supplementation on the vitamin and mineral status of athletes. Int. J. Sport Nutr. 1992, 2, 123–134. [Google Scholar] [CrossRef]
- Pawlak, R.; Lester, S.E.; Babatunde, T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: A review of literature. Eur. J. Clin. Nutr. 2016, 70, 866. [Google Scholar] [CrossRef] [Green Version]
- Otten, J.J.; Hellwig, J.P.; Meyers, L.D. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academic Press: Washington, DC, USA, 2006. [Google Scholar]
- Beshgetoor, D.; Nichols, J.F. Dietary intake and supplement use in female master cyclists and runners. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.D.; Zaltas, E.S.; Whittam, J.H. Dietary intakes of male endurance cyclists during training and racing. J. Am. Diet. Assoc. 1992, 92, 986–988. [Google Scholar] [PubMed]
- Manore, M.M. Nutritional needs of the female athlete. Clin. Sports Med. 1999, 18, 549–563. [Google Scholar] [CrossRef]
- Nieman, D.C.; Butler, J.V.; Pollett, L.M.; Dietrich, S.J.; Lutz, R.D. Nutrient intake of marathon runners. J. Am. Diet. Assoc. 1989, 89, 1273–1278. [Google Scholar]
- Wierniuk, A.; Włodarek, D. Estimation of energy and nutritional intake of young men practicing aerobic sports. Rocz. Panstw. Zakl. Hig. 2013, 64, 143–148. [Google Scholar]
- Fraczek, B.; Tyrala, F.; Warzecha, M.; Pieta, A. Consumption of vitamin B12 among professional athletes in Poland. Nauki Przyrodnicze 2016, 3, 41–54. [Google Scholar]
- Chan, C.Q.; Low, L.L.; Lee, K.H. Oral Vitamin B12 Replacement for the Treatment of Pernicious Anemia. Front. Med. (Lausanne) 2016, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Haiden, N.; Klebermass, K.; Cardona, F.; Schwindt, J.; Berger, A.; Kohlhauser-Vollmuth, C.; Jilma, B.; Pollak, A. A randomized, controlled trial of the effects of adding vitamin B12 and folate to erythropoietin for the treatment of anemia of prematurity. Pediatrics 2006, 118, 180–188. [Google Scholar] [CrossRef]
- Nyholm, E.; Turpin, P.; Swain, D.; Cunningham, B.; Daly, S.; Nightingale, P.; Fegan, C. Oral vitamin B12 can change our practice. Postgrad. Med. J. 2003, 79, 218–220. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, B.K.; Davis, R.E.; Nicol, D.J.; van Merwyk, A.J.; Larwood, C.J. Hematological, vitamin B 12, and folate studies on Seventh-day Adventist vegetarians. Am. J. Clin. Nutr. 1974, 27, 712–718. [Google Scholar] [CrossRef]
- Bentley, S.; Hermes, A.; Phillips, D.; Daoud, Y.A.; Hanna, S. Comparative effectiveness of a prenatal medical food to prenatal vitamins on hemoglobin levels and adverse outcomes: A retrospective analysis. Clin. Ther. 2011, 33, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Shamim, A.A.; Kabir, A.; Merrill, R.D.; Ali, H.; Rashid, M.; Schulze, K.; Labrique, A.; West, K.P., Jr.; Christian, P. Plasma zinc, vitamin B(12) and α-tocopherol are positively and plasma γ-tocopherol is negatively associated with Hb concentration in early pregnancy in north-west Bangladesh. Public Health Nutr. 2013, 16, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Solomon, L.R. Cobalamin-responsive disorders in the ambulatory care setting: Unreliability of cobalamin, methylmalonic acid, and homocysteine testing. Blood 2005, 105, 978–985. [Google Scholar] [CrossRef]
- Bansal, P.G.; Toteja, G.S.; Bhatia, N.; Vikram, N.K.; Siddhu, A. Impact of weekly iron folic acid supplementation with and without vitamin B12 on anaemic adolescent girls: A randomised clinical trial. Eur. J. Clin. Nutr. 2016, 70, 730–737. [Google Scholar] [CrossRef] [PubMed]
- den Elzen, W.P.; van der Weele, G.M.; Gussekloo, J.; Westendorp, R.G.; Assendelft, W.J. Subnormal vitamin B12 concentrations and anaemia in older people: A systematic review. BMC Geriatr. 2010, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Jenssen, H.B.; Torsvik, I.; Ueland, P.M.; Midttun, Ø.; Bjørke-Monsen, A.L. Biochemical signs of impaired cobalamin function do not affect hematological parameters in young infants: Results from a double-blind randomized controlled trial. Pediatr. Res. 2013, 74, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Kumar, T.; Taneja, S.; Sachdev, H.P.S.; Refsum, H.; Yajnik, C.S.; Bhandari, N.; Strand, T.A.; Study Group. Supplementation of vitamin B12 or folic acid on hemoglobin concentration in children 6-36 months of age: A randomized placebo controlled trial. Clin. Nutr. 2017, 36, 986–991. [Google Scholar] [CrossRef]
- Smelt, A.F.; Gussekloo, J.; Bermingham, L.W.; Allen, E.; Dangour, A.D.; Eussen, S.J.; Favrat, B.; De Groot, L.C.; Kok, F.J.; Kwok, T.; et al. The effect of vitamin B12 and folic acid supplementation on routine haematological parameters in older people: An individual participant data meta-analysis. Eur. J. Clin. Nutr. 2018, 72, 785–795. [Google Scholar] [CrossRef]
- Convertino, V.A. Blood volume response to physical activity and inactivity. Am. J. Med. Sci. 2007, 334, 72–79. [Google Scholar] [CrossRef]
- Schmidt, W.; Prommer, N. Effects of various training modalities on blood volume. Scand. J. Med. Sci. Sports 2008, 18 (Suppl. 1), 57–69. [Google Scholar] [CrossRef]
- Garvican-Lewis, L.A.; Schumacher, Y.O.; Clark, S.A.; Christian, R.; Menaspà, P.; Plowman, J.; Stephens, B.; Qi, J.; Fan, R.; He, Y.; et al. Stage racing at altitude induces hemodilution despite an increase in hemoglobin mass. J. Appl. Physiol. (1985) 2014, 117, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, W.; Prommer, N. The optimised CO-rebreathing method: A new tool to determine total haemoglobin mass routinely. Eur. J. Appl. Physiol. 2005, 95, 486–495. [Google Scholar] [CrossRef]
- Sitkowski, D.; Szygula, Z.; Pokrywka, A.; Turowski, D.; Malczewska-Lenczowska, J. Interrelationships between changes in erythropoietin, plasma volume, haemoglobin concentration, and total haemoglobin mass in endurance athletes. Res. Sports Med. 2018, 26, 381–389. [Google Scholar] [CrossRef]
- Heil, S.G.; de Jonge, R.; de Rotte, M.C.; van Wijnen, M.; Heiner-Fokkema, R.M.; Kobold, A.C.; Pekelharing, J.M.; Adriaansen, H.J.; Sanders, E.; Trienekens, P.H.; et al. Screening for metabolic vitamin B12 deficiency by holotranscobalamin in patients suspected of vitamin B12 deficiency: A multicentre study. Ann. Clin. Biochem. 2012, 49, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Holleland, G.; Schneede, J.; Ueland, P.M.; Lund, P.K.; Refsum, H.; Sandberg, S. Cobalamin deficiency in general practice. Assessment of the diagnostic utility and cost-benefit analysis of methylmalonic acid determination in relation to current diagnostic strategies. Clin. Chem. 1999, 45, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Lindenbaum, J.; Savage, D.G.; Stabler, S.P.; Allen, R.H. Diagnosis of cobalamin deficiency II. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am. J. Hematol. 1990, 34, 99–107. [Google Scholar] [CrossRef]
- Valente, E.; Scott, J.M.; Ueland, P.M.; Cunningham, C.; Casey, M.; Molloy, A.M. Diagnostic accuracy of holotranscobalamin, methylmalonic acid, serum cobalamin, and other indicators of tissue vitamin B12 status in the elderly. Clin. Chem. 2011, 57, 856–863. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, W.; Obeid, R.; Schorr, H.; Geisel, J. The usefulness of holotranscobalamin in predicting vitamin B12 status in different clinical settings. Curr. Drug Metab. 2005, 6, 47–53. [Google Scholar] [CrossRef]
- Oh, R.; Brown, D.L. Vitamin B12 deficiency. Am. Fam. Physician 2003, 67, 979–986. [Google Scholar]
- Axis-Shield Clinical utility—Correlation between Active-B12 and Total B12. Available online: http://www.active-b12.com/Clinical-Utility (accessed on 15 October 2019).
- Golding, P.H. Holotranscobalamin (HoloTC, Active-B12) and Herbert’s model for the development of vitamin B12 deficiency: A review and alternative hypothesis. Springerplus 2016, 5, 668. [Google Scholar] [CrossRef] [Green Version]
- Clarke, R.; Sherliker, P.; Hin, H.; Nexo, E.; Hvas, A.M.; Schneede, J.; Birks, J.; Ueland, P.M.; Emmens, K.; Scott, J.M.; et al. Detection of vitamin B12 deficiency in older people by measuring vitamin B12 or the active fraction of vitamin B12, holotranscobalamin. Clin. Chem. 2007, 53, 963–970. [Google Scholar] [CrossRef]
- Herbert, V. The 1986 Herman award lecture. Nutrition science as a continually unfolding story: The folate and vitamin B-12 paradigm. Am. J. Clin. Nutr. 1987, 46, 387–402. [Google Scholar] [CrossRef]
- Herbert, V. Staging vitamin B-12 (cobalamin) status in vegetarians. Am. J. Clin. Nutr. 1994, 59 (Suppl. 5), 1213S–1222S. [Google Scholar] [CrossRef]
- Herrmann, W.; Obeid, R.; Green, R.; Jacobson, D.; Harrington, D.; Ueland, P.M.; Lindemans, J.; Nexo, E.; Molloy, A. Expert’s Consensus Statement; Axis-Shield Diagnostics Ltd.: Dunde, UK, 2012; Available online: https://www.active-b12.com/wp-ontent/uploads/2015/08/Experts-Consensus-Flyer_PRINT-FILE.pdf (accessed on 13 August 2019).
- Yetley, E.A.; Pfeiffer, C.M.; Phinney, K.W.; Bailey, R.L.; Blackmore, S.; Bock, J.L.; Brody, L.C.; Carmel, R.; Curtin, L.R.; Durazo-Arvizu, R.A.; et al. Biomarkers of vitamin B-12 status in NHANES: A roundtable summary. Am. J. Clin. Nutr. 2011, 94, 313S–321S. [Google Scholar] [CrossRef] [Green Version]
- Ebenbichler, C.F.; Kaser, S.; Bodner, J.; Gander, R.; Lechleitner, M.; Herold, M.; Patsch, J.R. Hyperhomocysteinemia in bodybuilders taking anabolic steroids. Eur. J. Intern. Med. 2001, 12, 43–47. [Google Scholar] [CrossRef]
- Lane, L.A.; Rojas-Fernandez, C. Treatment of vitamin B12-deficiency anemia: Oral versus parenteral therapy. Ann. Pharmacother. 2002, 36, 1268–1272. [Google Scholar] [CrossRef]
- Andrès, E.; Dali-Youcef, N.; Vogel, T.; Serraj, K.; Zimmer, J. Oral cobalamin (vitamin B12) treatment. An update. Int. J. Lab. Hematol. 2009, 31, 1–8. [Google Scholar] [CrossRef]
- Langan, R.C.; Zawistoski, K.J. Update on vitamin B12 deficiency. Am. Fam. Physician 2011, 83, 1425–1430. [Google Scholar]
- Vidal-Alaball, J.; Butler, C.C.; Cannings-John, R.; Goringe, A.; Hood, K.; McCaddon, A.; McDowell, I.; Papaioannou, A. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst. Rev. 2005, 3, CD004655. [Google Scholar] [CrossRef] [Green Version]
- Andrès, E.; Zulfiqar, A.A.; Vogel, T. State of the Art Review: Oral and nasal vitamin B12 therapy in the elderly. QJM 2019. [Google Scholar] [CrossRef]
- Metaxas, C.; Mathis, D.; Jeger, C.; Hersberger, K.E.; Arnet, I.; Walter, P. Early biomarker response and patient prefer- ences to oral and intramuscular vitamin B12 substitution in primary care: A randomised parallel-group trial. Swiss Med. Wkly. 2017, 147, w14421. [Google Scholar] [PubMed] [Green Version]



| Vitamin B12 | <300 pg/mL | <350 pg/mL | <400 pg/mL | >700 pg/mL |
|---|---|---|---|---|
| Strength N (%) | 48 (5.3%) | 103 (11.4%) | 186 (20.6%) | 296 (32.7%) |
| Endurance N (%) | 2 (0.9%) ** | 7 (3.1%) *** | 19 (8.4%) *** | 128 (56.4%) *** |
| Total N (%) | 50 (4.4%) | 110 (9.7%) | 205 (18.1%) | 424 (7.5%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzywański, J.; Mikulski, T.; Pokrywka, A.; Młyńczak, M.; Krysztofiak, H.; Frączek, B.; Ziemba, A. Vitamin B12 Status and Optimal Range for Hemoglobin Formation in Elite Athletes. Nutrients 2020, 12, 1038. https://doi.org/10.3390/nu12041038
Krzywański J, Mikulski T, Pokrywka A, Młyńczak M, Krysztofiak H, Frączek B, Ziemba A. Vitamin B12 Status and Optimal Range for Hemoglobin Formation in Elite Athletes. Nutrients. 2020; 12(4):1038. https://doi.org/10.3390/nu12041038
Chicago/Turabian StyleKrzywański, Jarosław, Tomasz Mikulski, Andrzej Pokrywka, Marcel Młyńczak, Hubert Krysztofiak, Barbara Frączek, and Andrzej Ziemba. 2020. "Vitamin B12 Status and Optimal Range for Hemoglobin Formation in Elite Athletes" Nutrients 12, no. 4: 1038. https://doi.org/10.3390/nu12041038
APA StyleKrzywański, J., Mikulski, T., Pokrywka, A., Młyńczak, M., Krysztofiak, H., Frączek, B., & Ziemba, A. (2020). Vitamin B12 Status and Optimal Range for Hemoglobin Formation in Elite Athletes. Nutrients, 12(4), 1038. https://doi.org/10.3390/nu12041038





