In Vitro Bioaccessibility and Bioavailability of Iron from Mature and Microgreen Fenugreek, Rocket and Broccoli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Plant Samples
2.3. Moisture Analysis
2.4. Determination of Mineral Content in Plant Products
2.5. Bioaccessibility Studies: Peptic-Pancreatic in Vitro Digestion
2.6. Cell Culture
2.7. Cell Viability Studies
2.8. Iron Bioavailability Studies
2.9. Phytic Acid Analysis
2.10. Statistical Analysis
3. Results
3.1. Mineral Content and Moisture in Plant Products
3.2. The Bioaccessible and Fractional Low-Molecular-Weight Iron Content of the Digested Extracts of the Vegetables
3.3. Cell Viability of Caco-2 cells after Exposure to Digested Vegetable Samples
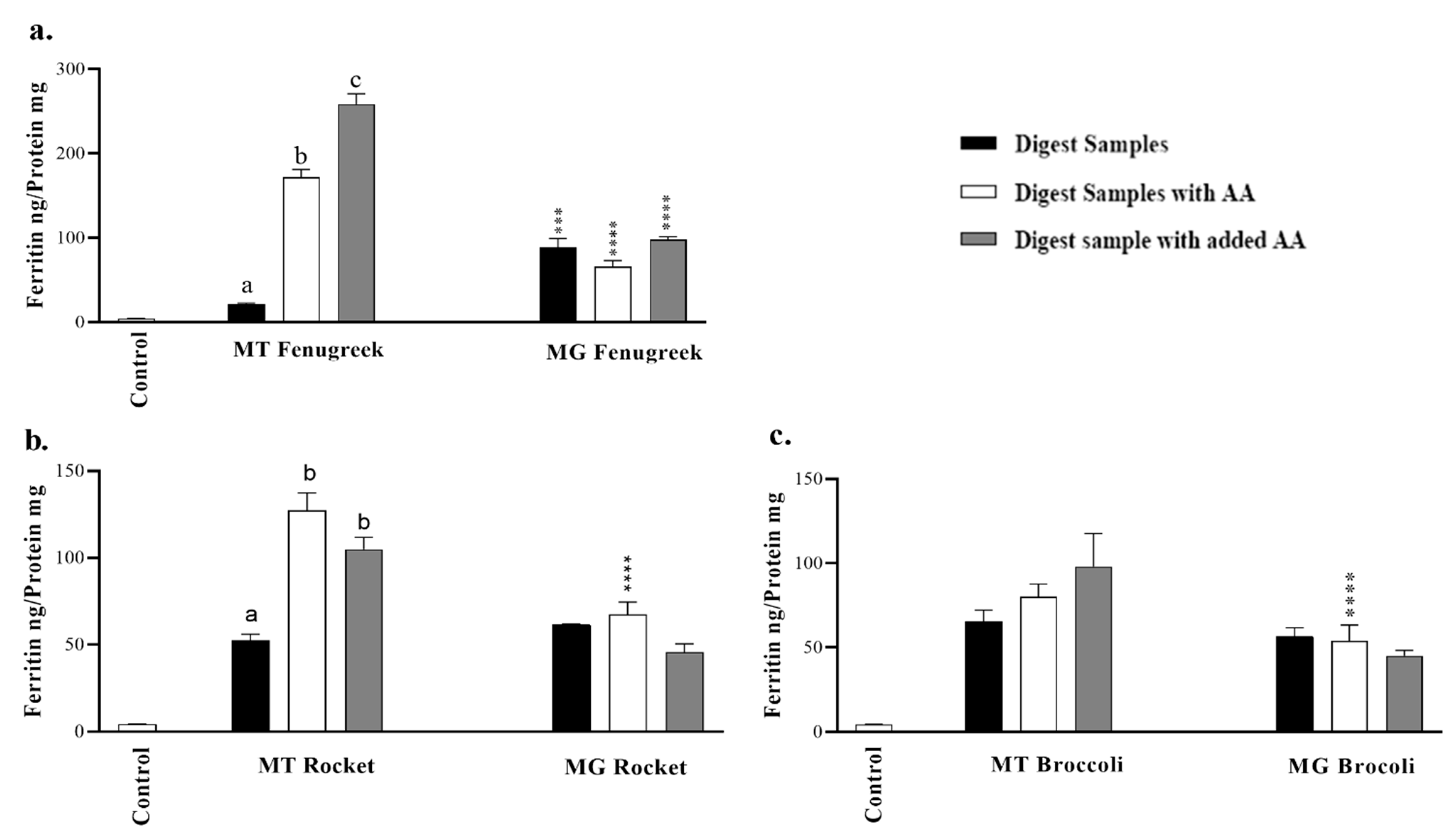
3.4. In Vitro Bioavailability of Iron from Fenugreek, Rocket or Broccoli in Caco-2 Cells
3.5. Modulating Effects of Fenugreek, Rocket and Broccoli on Iron Bioavailability from Iron Salts in Caco-2 Cells
3.6. Phytic Acid Levels in Fenugreek, Rocket and Broccoli
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Heath Organization. The Global Prevalence of Anaemia in 2011; WHO: Geneva, Switzerland, 2015; ISBN 9789241564960. [Google Scholar]
- Lozoff, B.; Jimenez, E.; Hagen, J.; Mollen, E.; Wolf, A.W. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. J. Pediatr. 2000, 105, e51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, D.L.; Camaschella, C. Iron-deficiency anemia. J. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar]
- Muñoz, C.; Rios, E.; Olivos, J.; Brunser, O.; Olivares, M. Iron, copper and immunocompetence. Br. J. Nutr. 2007, 98, S24–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UNICEF. Vitamin and Mineral Deficiency: A Global Progress Report; UNICEF: Ottawa, ON, Canada, 2004. [Google Scholar]
- World Health Organization. Global Nutrition Targets 2025: Anaemia Policy Brief; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Xiao, Z.; Codling, E.E.; Luo, Y.; Nou, X.; Lester, G.E.; Wang, Q. Microgreens of Brassicaceae: Mineral composition and content of 30 varieties. J. Food Compos. Anal. 2016, 49, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Treadwell, D.; Hochmuth, R.; Landrum, L.; Laughlin, W. Microgreens: A New Specialty Crop; UF/IFAS Extension HS1164, No. 3; University of Florida: Gainesville, FL, USA, 2010.
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, shelf life, and bioactive components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef]
- Weber, C.F. Broccoli microgreens: A mineral-rich crop that can diversify food systems. Front. Nutr. 2017, 4, 7. [Google Scholar] [CrossRef]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.; Ferreira, I.M. Comparison between the mineral profile and nitrate content of microgreens and mature lettuces. J. Food Compos. Anal. 2015, 37, 38–43. [Google Scholar] [CrossRef]
- Moore, K.L.; Rodríguez-Ramiro, I.; Jones, E.R.; Jones, E.J.; Rodríguez-Celma, J.; Halsey, K.; Domoney, C.; Shewry, P.R.; Fairweather-Tait, S.; Balk, J. The stage of seed development influences iron bioavailability in pea (Pisum sativum L.). Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, Z.; Lin, L.Z.; Lester, G.E.; Wang, Q.; Harnly, J.M.; Chen, P. Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMS n. J. Agric. Food Chem. 2013, 61, 10960–10970. [Google Scholar] [CrossRef] [Green Version]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones, flavanones, and human health: Epidemiological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F.; Reddy, M.; Cook, J.D. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br. J. Nutr. 1999, 81, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Feldsine, P.; Abeyta, C.; Andrews, W.H. AOAC International methods committee guidelines for validation of qualitative and quantitative food microbiological official methods of analysis. J. AOAC Int. 2002, 85, 1187–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, J.J.; Bruggraber, S.F.; Faria, N.; Poots, L.K.; Hondow, N.; Pennycook, T.J.; Latunde-Dada, G.O.; Simpson, R.J.; Brown, A.P.; Pereira, D.I. A nano-disperse ferritin-core mimetic that efficiently corrects anemia without luminal iron redox activity. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1529–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glahn, R.P.; Lee, O.A.; Yeung, A.; Goldman, M.I.; Miller, D.D. Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/Caco-2 cell culture model. J. Nutr. 1998, 128, 1555–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKie, V.A.; MccleAry, B.V. A novel and rapid colorimetric method for measuring total phosphorus and phytic acid in foods and animal feeds. J. AOAC Int. 2016, 99, 738–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franks, E. Microgreens: A Guide to Growing Nutrient-Packed Greens; Gibbs Smith: Layton, UT, USA, 2009. [Google Scholar]
- McCance, R.A.; Widdowson, E.M. McCance and Widdowson’s the Composition of Foods; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Diego Quintaes, K.; Barbera, R.; Cilla, A. Iron bioavailability in iron-fortified cereal foods: The contribution of in vitro studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 2028–2041. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O.; Yang, W.; Vera Aviles, M. In Vitro Iron Availability from Insects and Sirloin Beef. J. Agric. Food Chem. 2016, 64, 8420–8424. [Google Scholar] [CrossRef]
- Das, P.; Raghuramulu, N.; Rao, K. Determination of in vitro availability of iron from common foods. J. Hum. Ecol. 2005, 18, 13–20. [Google Scholar] [CrossRef]
- Hallberg, L.; Brune, M.; Erlandsson, M.; Sandberg, A.-S.; Rossander-Hulten, L. Calcium: Effect of different amounts on nonheme-and heme-iron absorption in humans. Am. J. Clin. Nutr. 1991, 53, 112–119. [Google Scholar] [CrossRef]
- Mamiro, P.S.; Kolsteren, P.W.; van Camp, J.H.; Roberfroid, D.A.; Tatala, S.; Opsomer, A.S. Processed complementary food does not improve growth or hemoglobin status of rural Tanzanian infants from 6–12 months of age in Kilosa district, Tanzania. J. Nutr. 2004, 134, 1084–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glahn, R.P.; Wortley, G.M.; South, P.K.; Miller, D.D. Inhibition of iron uptake by phytic acid, tannic acid, and ZnCl2: Studies using an in vitro digestion/Caco-2 cell model. J. Agric. Food Chem. 2002, 50, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Benito, P.; Miller, D. Iron absorption and bioavailability: An updated review. J. Nutr. Res. 1998, 18, 581–603. [Google Scholar] [CrossRef]
- Gillooly, M.; Bothwell, T.H.; Torrance, J.D.; MacPhail, A.P.; Derman, D.P.; Bezwoda, W.R.; Mills, W.; Charlton, R.W.; Mayet, F. The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br. J. Nutr. 1983, 49, 331–342. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Srividya, N. Storage effects on phytochemicals, antioxidant activity and sensory quality of fenugreek (Trigonella foenum-graecum L.) microgreens and mature leaves. Int. J. Food Nutr. Sci. 2017, 6, 59. [Google Scholar]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Siegenberg, D.; Baynes, R.D.; Bothwell, T.H.; Macfarlane, B.J.; Lamparelli, R.D.; Car, N.G.; MacPhail, P.; Schmidt, U.; Tal, A.; Mayet, F. Ascorbic acid prevents the dose-dependent inhibitory effects of polyphenols and phytates on nonheme-iron absorption. Am. J. Clin. Nutr. 1991, 53, 537–541. [Google Scholar] [CrossRef]
- Chiplonkar, S.; Tarwadi, K.; Kavedia, R.; Mengale, S.; Paknikar, K.; Agte, V. Fortification of vegetarian diets for increasing bioavailable iron density using green leafy vegetables. J. Food Res. Int. 1999, 32, 169–174. [Google Scholar] [CrossRef]
- Hotz, C.; Gibson, R.S. Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. J. Nutr. 2007, 137, 1097–1100. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, S.; Lakshmi, A.J.; Prakash, J. Iron bioavailability in green leafy vegetables cooked in different utensils. J. Food Chem. 2004, 86, 217–222. [Google Scholar] [CrossRef]
- Rodriguez-Ramiro, I.; Dell’Aquila, C.; Ward, J.L.; Neal, A.L.; Bruggraber, S.F.A.; Shewry, P.R.; Fairweather-Tait, S. Estimation of the iron bioavailability in green vegetables using an in vitro digestion/Caco-2 cell model. Food Chem. 2019, 301, 125292. [Google Scholar] [CrossRef] [PubMed]
- Glahn, R.; Tako, E.; Hart, J.; Haas, J.; Lung’aho, M.; Beebe, S. Iron Bioavailability Studies of the First Generation of Iron-Biofortified Beans Released in Rwanda. Nutrients 2017, 9, 787. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Samples | Fenugreek | Rocket | Broccoli | |||
|---|---|---|---|---|---|---|
| Mature | Microgreen | Mature | Microgreen | Mature | Microgreen | |
| Moisture (%) | 86.97 | 90.52 | 92.6 | 92.28 | 89.89 | 92.12 |
| Fe | 108.5 ± 0.76 | 82.32 ± 1.22 **** | 130.82 ± 82.3 | 74.41 ± 1.21 **** | 54.37 ± 0.48 | 55.90 ± 1.03 |
| Ca | 26834.2 ± 139.56 | 7658.93 ± 79.58 **** | 36753.4 ± 319.08 | 22475.13 ±137.14 **** | 3911.91 ± 35.9 | 25601.7 ± 136.28 **** |
| Cu | 6.72 ± 0.22 | 9.15 ± 0.11 **** | 11.65 ± 0.32 | 9.93 ± 0.40 ** | 8.26 ± 0.42 | 6.40 ± 0.12 *** |
| Mg | 2687.4 ± 18.31 | 1645.0 ± 24.92 **** | 3886.85 ± 45.66 | 3472.46 ± 140.1 *** | 1752.92 ± 9.52 | 3702.53 ± 52.35 **** |
| Mn | 41.24 ± 1.4 | 24.94 ± 0.37 **** | 119.93 ± 1.22 | 92.30 ± 1.10 **** | 25.67 ± 0.20 | 115.0 ± 1.15 * |
| Mo | 6.18 ± 0.025 | 2.88 ± 0.05 **** | 3.23 ± 0.036 | 7.91 ± 0.29 **** | 1.67 ± 0.007 | 2.76 ± 0.046 **** |
| Zn | 23.27 ± 0.32 | 34.92 ± 0.52 **** | 80.29 ± 0.03 | 90.34 ± 1.58 **** | 52.22 ± 0.22 | 39.65 ± 0.67 **** |
| Samples | Fenugreek | Rocket | Broccoli | ||||
|---|---|---|---|---|---|---|---|
| Mature | Microgreens | Mature | Microgreens | Mature | Microgreens | ||
| Total Bioaccessible Iron (TBF) | µg/g | 61.07 ± 0.11 | 34.27 ± 0.11 **** | 25.02 ± 0.07 | 28.15 ± 0.64 **** | 24.73 ± 0.05 | 20.10 ± 0.02 **** |
| % | 56.26 ± 0.10 | 41.63 ± 0.13 **** | 19.13 ± 0.06 | 37.83 ± 0.87 **** | 45.49 ± 0.10 | 35.96 ± 0.31 **** | |
| Low-Molecular-Weight Iron (LMW) | µg/g | 6.32 ± 0.11 | 5.76 ± 0.11 | 7.29 ± 0.16 | 5.44 ± 0.08 **** | 6.00 ± 0.11 | 4.41 ± 0.06 **** |
| % | 5.82 ± 0.10 | 7.00 ± 0.14 | 5.58 ± 0.13 | 7.32 ± 0.12 | 11.04 ± 0.20 | 7.88 ± 0.11 ** | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
K. Khoja, K.; Buckley, A.; F. Aslam, M.; A. Sharp, P.; Latunde-Dada, G.O. In Vitro Bioaccessibility and Bioavailability of Iron from Mature and Microgreen Fenugreek, Rocket and Broccoli. Nutrients 2020, 12, 1057. https://doi.org/10.3390/nu12041057
K. Khoja K, Buckley A, F. Aslam M, A. Sharp P, Latunde-Dada GO. In Vitro Bioaccessibility and Bioavailability of Iron from Mature and Microgreen Fenugreek, Rocket and Broccoli. Nutrients. 2020; 12(4):1057. https://doi.org/10.3390/nu12041057
Chicago/Turabian StyleK. Khoja, Kholoud, Amy Buckley, Mohamad F. Aslam, Paul A. Sharp, and Gladys O. Latunde-Dada. 2020. "In Vitro Bioaccessibility and Bioavailability of Iron from Mature and Microgreen Fenugreek, Rocket and Broccoli" Nutrients 12, no. 4: 1057. https://doi.org/10.3390/nu12041057
APA StyleK. Khoja, K., Buckley, A., F. Aslam, M., A. Sharp, P., & Latunde-Dada, G. O. (2020). In Vitro Bioaccessibility and Bioavailability of Iron from Mature and Microgreen Fenugreek, Rocket and Broccoli. Nutrients, 12(4), 1057. https://doi.org/10.3390/nu12041057







