The Disruption of Liver Metabolic Circadian Rhythms by a Cafeteria Diet Is Sex-Dependent in Fischer 344 Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experimental Procedure
2.2. Biometric Parameters and Serum Analysis
2.3. Liver Extraction Procedure for 1H NMR-Based Metabolomics Assays
2.4. NMR Analyses
2.5. NMR Data Analysis
2.6. Data Processing and Multivariate Analysis
3. Results
3.1. Biometric Parameters Validated the Obesogenic Effect of the Diet
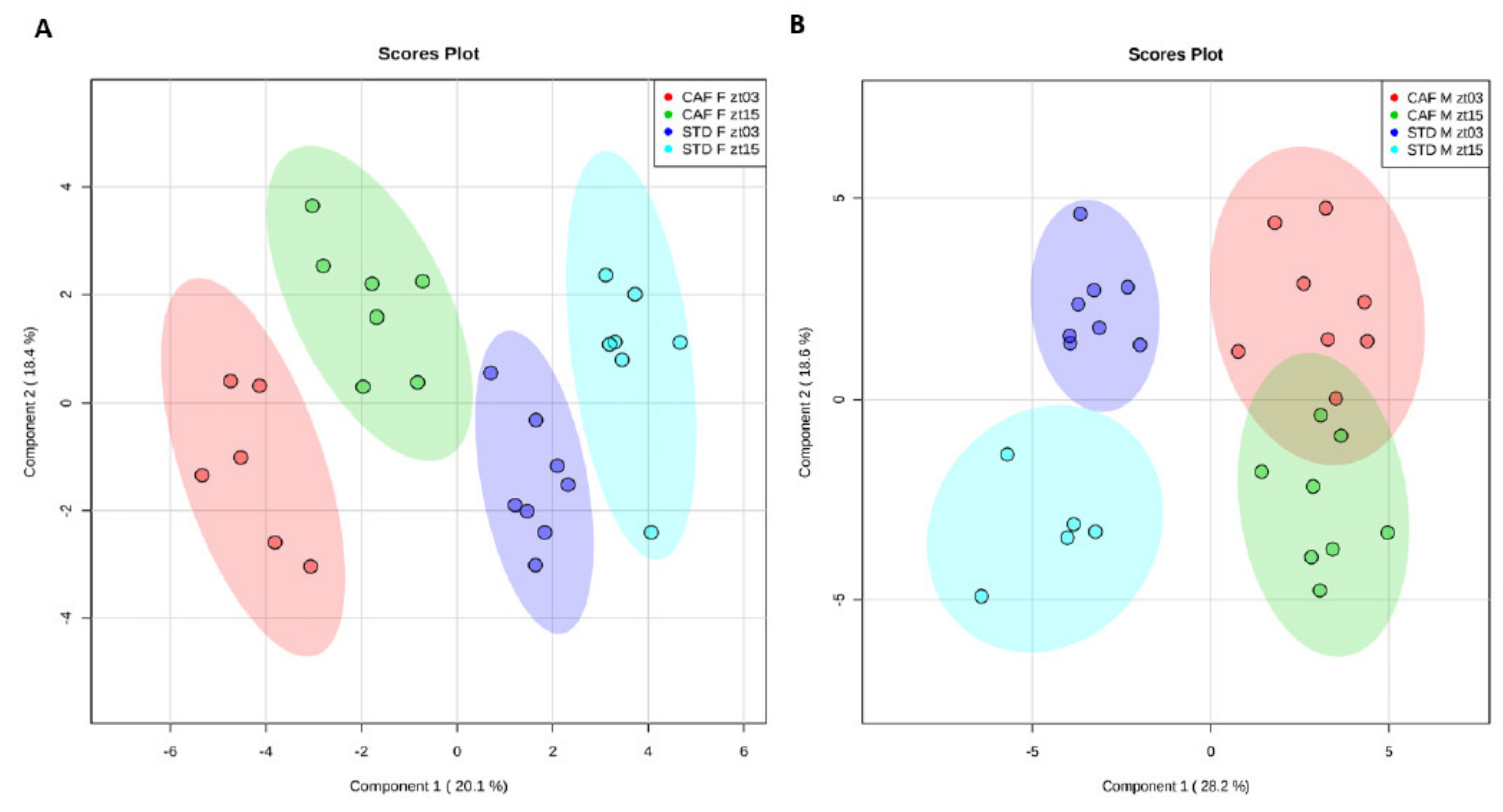
3.2. Multivariate Chemometric Analysis of NMR Data
3.3. Multivariant Analyses Showed a Gender-Dependent Dysregulation of the Homeostatic Equilibrium of the Hepatic Metabolism Induced by a Cafeteria Diet
3.4. The Differences between both Times are Gender-Dependent
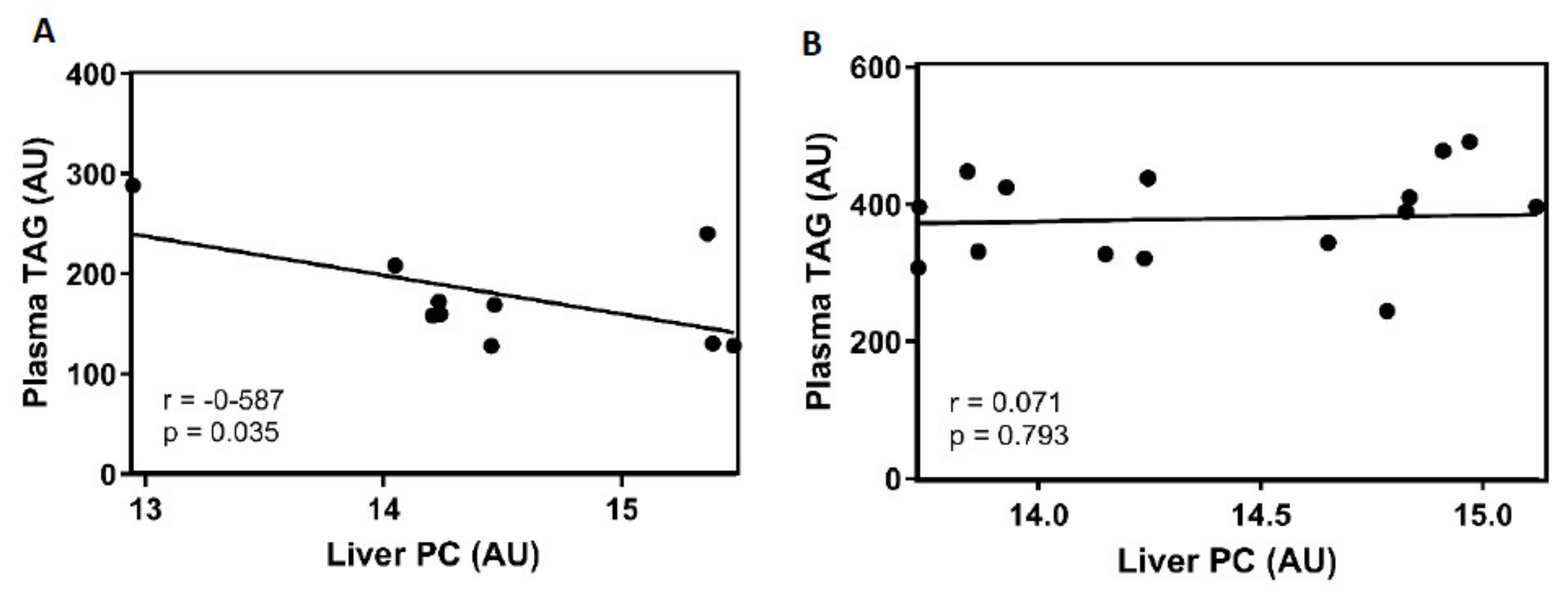
3.5. PC Changes in the Livers of Female Rats Fed a Cafeteria Diet were Negatively Correlated with Plasma TAG
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Highlights
References
- Bass, J.; Takahashi, J.S. Circadian Integration of Metabolism and Energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinke, H.; Asher, G. Circadian Clock Control of Liver Metabolic Functions. Gastroenterology 2016, 150, 574–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalsbeek, A.; la Fleur, S.; Fliers, E. Circadian control of glucose metabolism. Mol. Metab. 2014, 3, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Eggink, H.M.; Oosterman, J.E.; de Goede, P.; de Vries, E.M.; Foppen, E.; Koehorst, M. Complex interaction between circadian rhythm and diet on bile acid homeostasis in male rats. Chronobiol. Int. 2017, 34, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J. Circadian regulation of lipid metabolism. Proc. Nutr. Soc. 2016, 75, 440–450. [Google Scholar] [CrossRef]
- Ruiter, M.; La Fleur, S.E.; van Heijningen, C.; van der Vliet, J.; Kalsbeek, A.; Buijs, R.M. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes 2003, 52, 1709–1715. [Google Scholar] [CrossRef] [Green Version]
- Gnocchi, D.; Pedrelli, M.; Hurt-Camejo, E.; Parini, P. Lipids around the Clock: Focus on Circadian Rhythms and Lipid Metabolism. Biology 2015, 4, 104–132. [Google Scholar] [CrossRef] [Green Version]
- Kovár, J.; Lenícek, M.; Zimolová, M.; Vítek, L.; Jirsa, M.; Pitha, J. Regulation of diurnal variation of cholesterol 7alpha-hydroxylase (CYP7A1) activity in healthy subjects. Physiol. Res. 2010, 59, 233–238. [Google Scholar]
- Noshiro, M.; Usui, E.; Kawamoto, T.; Kubo, H.; Fujimoto, K.; Furukawa, M. Multiple Mechanisms Regulate Circadian Expression of the Gene for Cholesterol 7α-Hydroxylase (Cyp7a), a Key Enzyme in Hepatic Bile Acid Biosynthesis. J. Biol. Rhythms 2007, 22, 299–311. [Google Scholar] [CrossRef]
- Le Martelot, G.; Claudel, T.; Gatfield, D.; Schaad, O.; Kornmann, B.; Sasso, G.L. REV-ERBα Participates in Circadian SREBP Signaling and Bile Acid Homeostasis. PLoS Biol. 2009, 7, e1000181. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.E.; Liu, Y.; Dentin, R.; Pongsawakul, P.Y.; Liu, A.C.; Hirota, T. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 2010, 16, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Kasukawa, T.; Kakazu, Y.; Iigo, M.; Sugimoto, M.; Ikeda, S. Measurement of internal body time by blood metabolomics. Proc. Natl. Acad. Sci. USA 2009, 106, 9890–9895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, J.; Pévet, P.; Challet, E. High-fat feeding alters the clock synchronization to light. J. Physiol. 2008, 586, 5901–5910. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.J.; Moffitt, T.E.; Gregory, A.M.; Goldman-Mellor, S.; Nolan, P.M.; Poulton, R. Social jetlag, obesity and metabolic disorder: Investigation in a cohort study. Int. J. Obes. 2015, 39, 842–848. [Google Scholar] [CrossRef] [Green Version]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Barnea, M.; Madar, Z.; Froy, O. High-Fat Diet Delays and Fasting Advances the Circadian Expression of Adiponectin Signaling Components in Mouse Liver. Endocrinology 2009, 150, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Barnea, M.; Madar, Z.; Froy, O. High-fat Diet Followed by Fasting Disrupts Circadian Expression of Adiponectin Signaling Pathway in Muscle and Adipose Tissue. Obesity 2010, 18, 230–238. [Google Scholar] [CrossRef]
- Shi, D.; Chen, J.; Wang, J.; Yao, J.; Huang, Y.; Zhang, G. Circadian Clock Genes in the Metabolism of Non-alcoholic Fatty Liver Disease. Front. Physiol. 2019, 10, 423. [Google Scholar] [CrossRef] [Green Version]
- Power, M.L.; Schulkin, J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolutionary origins. Br. J. Nutr. 2008, 99, 931–940. [Google Scholar] [CrossRef]
- Van Nas, A.; GuhaThakurta, D.; Wang, S.S.; Yehya, N.; Horvath, S.; Zhang, B. Elucidating the Role of Gonadal Hormones in Sexually Dimorphic Gene Coexpression Networks. Endocrinology 2009, 150, 1235–1249. [Google Scholar] [CrossRef] [Green Version]
- Carmona-Alcocer, V.; Fuentes-Granados, C.; Carmona-Castro, A.; Aguilar-González, I.; Cárdenas-Vázquez, R.; Miranda-Anaya, M. Obesity alters circadian behavior and metabolism in sex dependent manner in the volcano mouse Neotomodon alstoni. Physiol. Behav. 2012, 105, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Viscarra, J.; Kim, S.-J.; Sul, H.S. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 2015, 16, 678–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillaumond, F.; Gréchez-Cassiau, A.; Subramaniam, M.; Brangolo, S.; Peteri-Brünback, B.; Staels, B. Kruppel-like factor KLF10 is a link between the circadian clock and metabolism in liver. Mol. Cell. Biol. 2010, 30, 3059–3070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Mendoza, M.; Rivera-Zavala, J.B.; Rodríguez-Guadarrama, A.H.; Montoya-Gomez, L.M.; Carmona-Castro, A.; Díaz-Muñoz, M. Daily cycle in hepatic lipid metabolism in obese mice, Neotomodon alstoni: Sex differences. Chronobiol. Int. 2018, 35, 643–657. [Google Scholar] [CrossRef]
- Vinaixa, M.; Ángel Rodríguez, M.; Rull, A.; Beltrán, R.; Bladé, C.; Brezmes, J. Metabolomic Assessment of the Effect of Dietary Cholesterol in the Progressive Development of Fatty Liver Disease. J. Proteome Res. 2010, 9, 2527–2538. [Google Scholar] [CrossRef]
- Corbin, K.D.; Zeisel, S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2012, 28, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Cole, L.K.; Vance, J.E.; Vance, D.E. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 754–761. [Google Scholar] [CrossRef]
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian rhythms, sleep, and metabolism. J. Clin. Investig. 2011, 121, 2133–2141. [Google Scholar] [CrossRef]
- Maury, E.; Ramsey, K.M.; Bass, J. Circadian Rhythms and Metabolic Syndrome. Circ. Res. 2010, 106, 447–462. [Google Scholar] [CrossRef] [Green Version]
- Engin, A. Circadian Rhythms in Diet-Induced Obesity. Adv. Exp. Med. Biol. 2017, 960, 19–52. [Google Scholar]
- Blancas-Velazquez, A.; Mendoza, J.; Garcia, A.N.; la Fleur, S.E. Diet-Induced Obesity and Circadian Disruption of Feeding Behavior. Front. Neurosci. 2017, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Honma, K.; Hikosaka, M.; Mochizuki, K.; Goda, T. Loss of circadian rhythm of circulating insulin concentration induced by high-fat diet intake is associated with disrupted rhythmic expression of circadian clock genes in the liver. Metabolism 2016, 65, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, M.; Handgraaf, S.; Fabre, A.; Raymond-Letron, I.; Riant, E.; Montagner, A. Selective Activation of Estrogen Receptor α Activation Function-1 Is Sufficient to Prevent Obesity, Steatosis, and Insulin Resistance in Mouse. Am. J. Pathol. 2017, 187, 1273–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Zou, F.; Yang, Y.; Xu, P.; Saito, K.; Othrell Hinton, A. Estrogens Prevent Metabolic Dysfunctions Induced by Circadian Disruptions in Female Mice. Endocrinology 2015, 156, 2114–2123. [Google Scholar] [CrossRef]
- Ågren, J.J.; Kurvinen, J.-P.; Kuksis, A. Isolation of very low density lipoprotein phospholipids enriched in ethanolamine phospholipids from rats injected with Triton WR 1339. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2005, 1734, 34–43. [Google Scholar] [CrossRef]
- Fast, D.G.; Vance, D.E. Nascent VLDL phospholipid composition is altered when phosphatidylcholine biosynthesis is inhibited: Evidence for a novel mechanism that regulates VLDL secretion. Biochim. Biophys. Acta Lipids Lipid Metab. 1995, 1258, 159–168. [Google Scholar] [CrossRef]
- Sherriff, J.L.; O’Sullivan, T.A.; Properzi, C.; Oddo, J.-L.; Adams, L.A. Choline, Its Potential Role in Nonalcoholic Fatty Liver Disease, and the Case for Human and Bacterial Genes. Adv. Nutr. 2016, 7, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Fagone, P.; Jackowski, S. Phosphatidylcholine and the CDP–choline cycle. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Bremer, J.; Greenberg, D.M. Methyl transfering enzyme system of microsomes in the biosynthesis of lecithin (phosphatidylcholine). Biochim. Biophys. Acta 1961, 46, 205–216. [Google Scholar] [CrossRef]
- Craig, S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Miguelsanz, J.; Vallecillo, N.; Garrido, F.; Reytor, E.; Pérez-Sala, D.; Pajares, M.A. Betaine homocysteine S-methyltransferase emerges as a new player of the nuclear methionine cycle. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1165–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Y.-W.; Mehedint, M.G.; Garrow, T.A.; Zeisel, S.H. Deletion of Betaine-Homocysteine S -Methyltransferase in Mice Perturbs Choline and 1-Carbon Metabolism, Resulting in Fatty Liver and Hepatocellular Carcinomas. J. Biol. Chem. 2011, 286, 36258–36267. [Google Scholar] [CrossRef] [Green Version]
- Mason, T.M. The Role of Factors that Regulate the Synthesis and Secretion of Very-Low-Density Lipoprotein by Hepatocytes. Crit. Rev. Clin. Lab. Sci. 1998, 35, 461–487. [Google Scholar] [CrossRef] [PubMed]
- Vedala, A.; Wang, W.; Neese, R.A.; Christiansen, M.P.; Hellerstein, M.K. Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humans. J. Lipid Res. 2006, 47, 2562–2574. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Ginsberg, H.N. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol. Metab. 2011, 22, 353–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruffat, D.; Durand, D.; Graulet, B.; Bauchart, D. Regulation of VLDL synthesis and secretion in the liver. Reprod. Nutr. Dev. 1996, 36, 375–389. [Google Scholar] [CrossRef]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidlo, J.; Zaviacic, M.; Kvasnicka, P. Night and day differences in the food-intake of laboratory rats Wistar and Koletsky strains. Bratisl. Lek. Listy 1995, 96, 655–657. [Google Scholar] [PubMed]
- Cohn, J.S.; Patterson, B.W.; Uffelman, K.D.; Davignon, J.; Steiner, G. Rate of Production of Plasma and Very-Low-Density Lipoprotein (VLDL) Apolipoprotein C-III Is Strongly Related to the Concentration and Level of Production of VLDL Triglyceride in Male Subjects with Different Body Weights and Levels of Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2004, 89, 3949–3955. [Google Scholar] [CrossRef]
- Santhi, N.; Lazar, A.S.; McCabe, P.J.; Lo, J.C.; Groeger, J.A.; Dijk, D.-J. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E2730–E2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, J.F.; Cain, S.W.; Chang, A.-M.; Phillips, A.J.K.; Munch, M.Y.; Gronfier, C. Sex difference in the near-24-h intrinsic period of the human circadian timing system. Proc. Natl. Acad. Sci. USA 2011, 108, 15602–15608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santosa, S.; Jensen, M.D. The sexual dimorphism of lipid kinetics in humans. Front. Endocrinol. 2015, 6, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios-Jordan, H.; Martín-González, M.Z.; Suárez, M.; Aragonès, G.; Muguerza, B.; Rodríguez, M.A.; Bladé, C. The Disruption of Liver Metabolic Circadian Rhythms by a Cafeteria Diet Is Sex-Dependent in Fischer 344 Rats. Nutrients 2020, 12, 1085. https://doi.org/10.3390/nu12041085
Palacios-Jordan H, Martín-González MZ, Suárez M, Aragonès G, Muguerza B, Rodríguez MA, Bladé C. The Disruption of Liver Metabolic Circadian Rhythms by a Cafeteria Diet Is Sex-Dependent in Fischer 344 Rats. Nutrients. 2020; 12(4):1085. https://doi.org/10.3390/nu12041085
Chicago/Turabian StylePalacios-Jordan, Héctor, Miguel Z. Martín-González, Manuel Suárez, Gerard Aragonès, Begoña Muguerza, Miguel A. Rodríguez, and Cinta Bladé. 2020. "The Disruption of Liver Metabolic Circadian Rhythms by a Cafeteria Diet Is Sex-Dependent in Fischer 344 Rats" Nutrients 12, no. 4: 1085. https://doi.org/10.3390/nu12041085
APA StylePalacios-Jordan, H., Martín-González, M. Z., Suárez, M., Aragonès, G., Muguerza, B., Rodríguez, M. A., & Bladé, C. (2020). The Disruption of Liver Metabolic Circadian Rhythms by a Cafeteria Diet Is Sex-Dependent in Fischer 344 Rats. Nutrients, 12(4), 1085. https://doi.org/10.3390/nu12041085






