Beneficial Effect of Mildly Pasteurized Whey Protein on Intestinal Integrity and Innate Defense in Preterm and Near-Term Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Piglet Study
2.2. Clinical Evaluation and Sample Collection
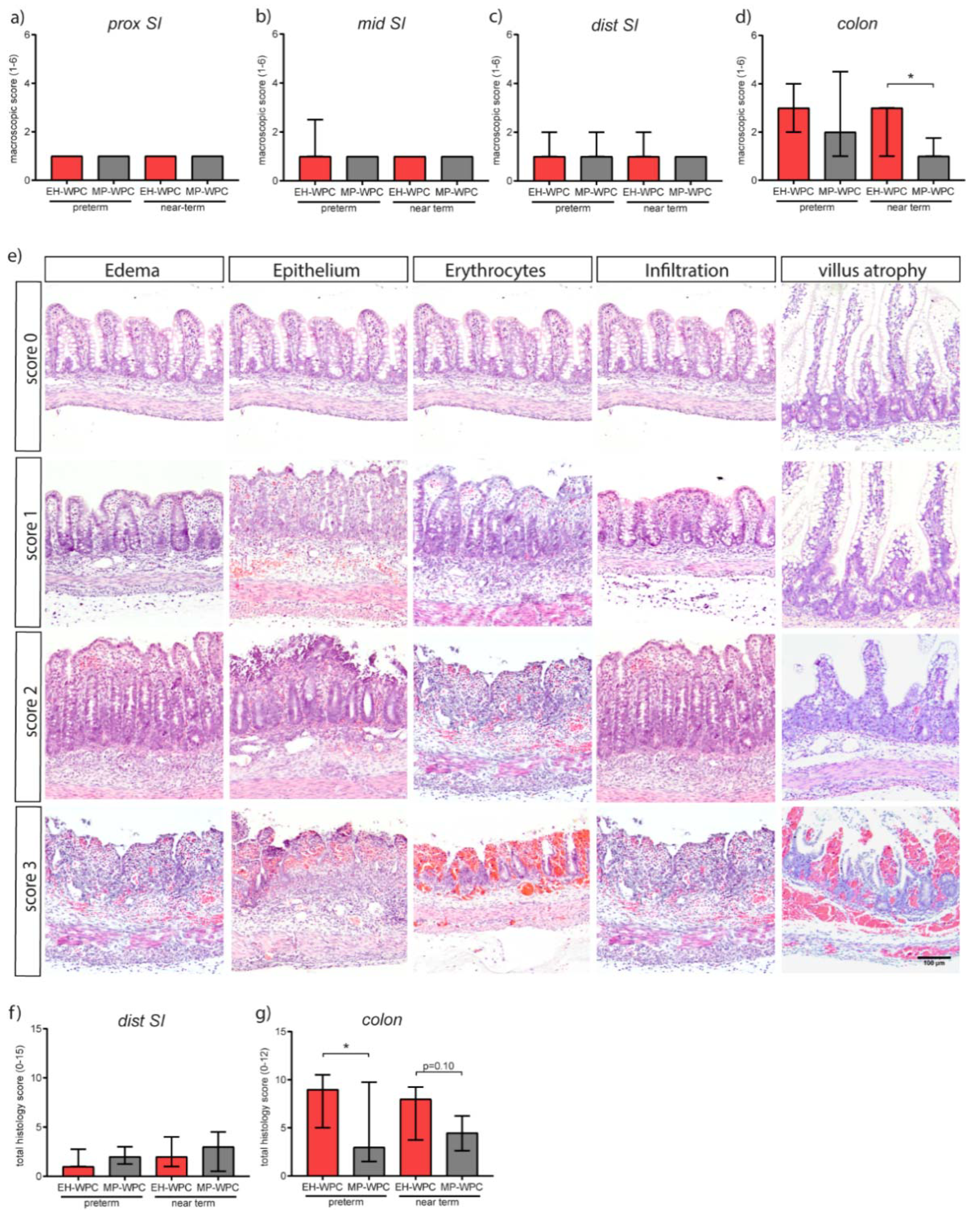
2.3. Microscopic Evaluation
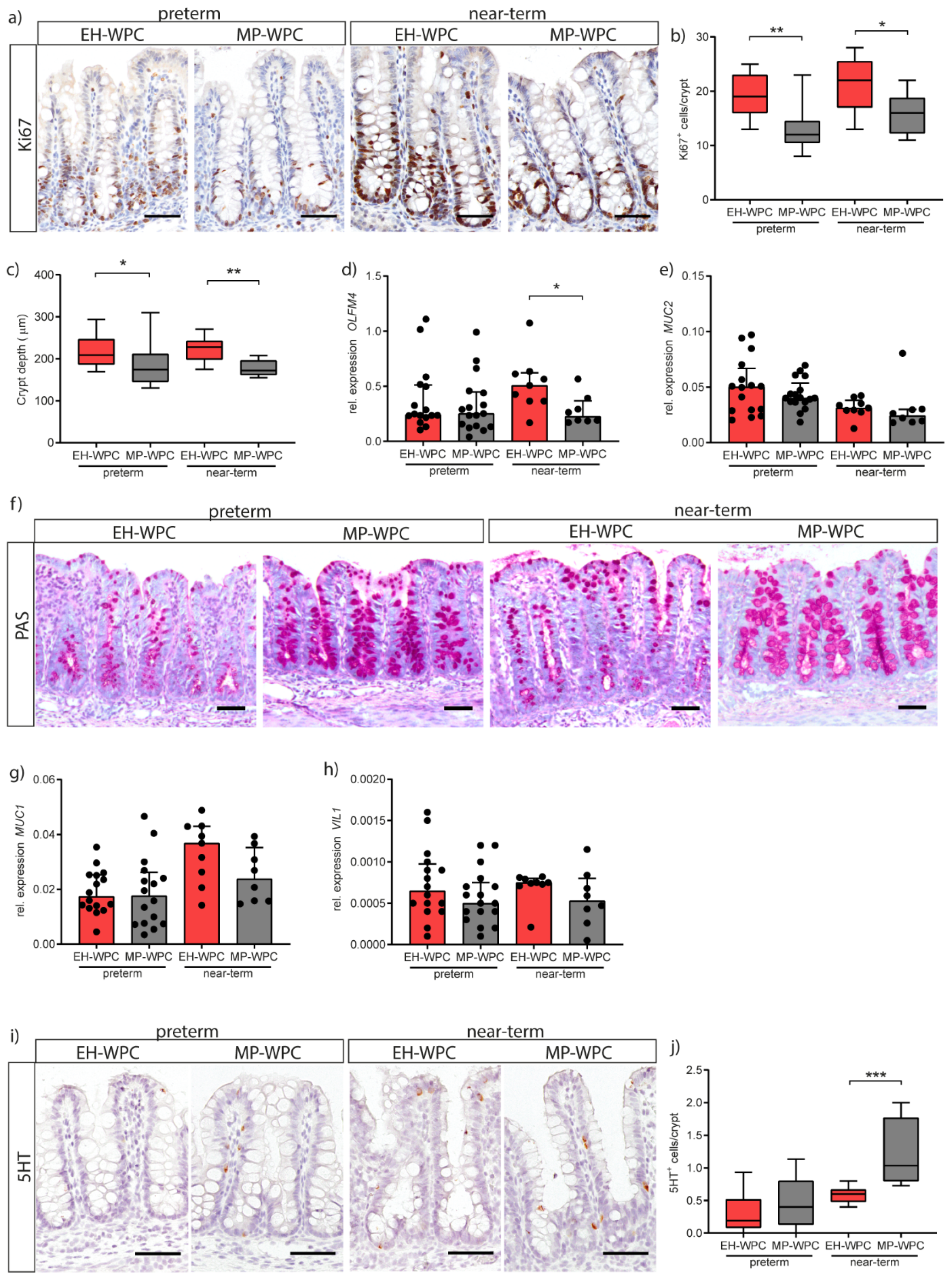
2.4. Immunohistochemistry
2.5. Gene Expression Analysis
2.6. Enzyme Activity Assay
2.7. Protein Analysis
2.8. Statistical Analysis
3. Results
3.1. Clinical Symptoms
3.2. Intestinal Morphology
3.3. Intestinal Inflammation
3.4. Innate Defense
3.5. Epithelial Functioning: Cell-Type Specific Differentiation and Proliferation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end products |
| EN | enteral nutrition |
| iALP | intestinal alkaline phosphatase |
| IMFs | infant milk formulas |
| LPS | lipopolysaccharide |
| NEC | necrotizing enterocolitis |
| PN | parenteral nutrition |
| SI | small intestine |
| WPC | whey protein concentrate |
| MP-WPC | mildly pasteurized WPC |
| EH-WPC | extensively heated WPC |
References
- Chin, A.M.; Hill, D.R.; Aurora, M.; Spence, J.R. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin. Cell Dev. Biol. 2017, 66, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Renz, H.; Brandtzaeg, P.; Hornef, M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat. Rev. Immunol. 2011, 12, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, E.; Minekus, M.; van Aken, G.A.; van de Heijning, B.; Knol, J.; Bartke, N.; Oozeer, R.; van der Beek, E.M.; Ludwig, T. Development of the Digestive System-Experimental Challenges and Approaches of Infant Lipid Digestion. Food Dig. 2012, 3, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.A. Development of the intestinal mucosal barrier. J. Pediatric Gastroenterol. Nutr. 2002, 34 (Suppl. 1), S33–S39. [Google Scholar] [CrossRef]
- Tourneur, E.; Chassin, C. Neonatal immune adaptation of the gut and its role during infections. Clin. Dev. Immunol. 2013, 2013, 270301. [Google Scholar] [CrossRef]
- Jacobi, S.K.; Odle, J. Nutritional factors influencing intestinal health of the neonate. Adv. Nutr. 2012, 3, 687–696. [Google Scholar] [CrossRef]
- Berman, L.; Moss, R.L. Necrotizing enterocolitis: An update. Semin. Fetal Neonatal Med. 2011, 16, 145–150. [Google Scholar] [CrossRef]
- Bazacliu, C.; Neu, J. Pathophysiology of Necrotizing Enterocolitis: An Update. Curr. Pediatric Rev. 2018. [Google Scholar] [CrossRef]
- Horta, B.L.; Bahl, R.; Martinés, J.C.; Victora, C.G.; World Health Organization. Evidence on the Long-Term Effects of Breastfeeding: Systematic Review and Meta-Analyses; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Reisinger, K.W.; de Vaan, L.; Kramer, B.W.; Wolfs, T.G.; van Heurn, L.W.; Derikx, J.P. Breast-feeding improves gut maturation compared with formula feeding in preterm babies. J. Pediatric Gastroenterol. Nutr. 2014, 59, 720–724. [Google Scholar] [CrossRef]
- Eldelman, A.I.; Schandler, R.J. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; Franca, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Hillman, M.; Westrom, B.; Aalaei, K.; Erlanson-Albertsson, C.; Wolinski, J.; Lozinska, L.; Sjoholm, I.; Rayner, M.; Landin-Olsson, M. Skim milk powder with high content of Maillard reaction products affect weight gain, organ development and intestinal inflammation in early life in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 125, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Kellow, N.J.; Coughlan, M.T. Effect of diet-derived advanced glycation end products on inflammation. Nutr. Rev. 2015, 73, 737–759. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ostergaard, M.V.; Jiang, P.; Chatterton, D.E.; Thymann, T.; Kvistgaard, A.S.; Sangild, P.T. Whey protein processing influences formula-induced gut maturation in preterm pigs. J. Nutr. 2013, 143, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nguyen, D.N.; Obelitz-Ryom, K.; Andersen, A.D.; Thymann, T.; Chatterton, D.E.W.; Purup, S.; Heckmann, A.B.; Bering, S.B.; Sangild, P.T. Bioactive Whey Protein Concentrate and Lactose Stimulate Gut Function in Formula-fed Preterm Pigs. J. Pediatric Gastroenterol. Nutr. 2018, 66, 128–134. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Wijayanti, H.B.; Crowley, S.V.; O’Mahony, J.A.; Fenelon, M.A. Pilot-scale ceramic membrane filtration of skim milk for the production of a protein base ingredient for use in infant milk formula. Int. Dairy J. 2017, 73, 57–62. [Google Scholar] [CrossRef]
- Miller, E.R.; Ullrey, D.E. The pig as a model for human nutrition. Annu. Rev. Nutr. 1987, 7, 361–382. [Google Scholar] [CrossRef]
- Alizadeh, A.; Akbari, P.; Difilippo, E.; Schols, H.A.; Ulfman, L.H.; Schoterman, M.H.; Garssen, J.; Fink-Gremmels, J.; Braber, S. The piglet as a model for studying dietary components in infant diets: Effects of galacto-oligosaccharides on intestinal functions. Br. J. Nutr. 2016, 115, 605–618. [Google Scholar] [CrossRef]
- Rasmussen, S.O.; Martin, L.; Ostergaard, M.V.; Rudloff, S.; Li, Y.; Roggenbuck, M.; Bering, S.B.; Sangild, P.T. Bovine colostrum improves neonatal growth, digestive function, and gut immunity relative to donor human milk and infant formula in preterm pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G480–G491. [Google Scholar] [CrossRef]
- Sangild, P.T.; Thymann, T.; Schmidt, M.; Stoll, B.; Burrin, D.G.; Buddington, R.K. Invited review: The preterm pig as a model in pediatric gastroenterology. J. Anim. Sci. 2013, 91, 4713–4729. [Google Scholar] [CrossRef]
- Cilieborg, M.S.; Boye, M.; Thymann, T.; Jensen, B.B.; Sangild, P.T. Diet-dependent effects of minimal enteral nutrition on intestinal function and necrotizing enterocolitis in preterm pigs. JPEN J. Parenter. Enter. Nutr. 2011, 35, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Thymann, T.; Moller, H.K.; Stoll, B.; Stoy, A.C.; Buddington, R.K.; Bering, S.B.; Jensen, B.B.; Olutoye, O.O.; Siggers, R.H.; Molbak, L.; et al. Carbohydrate maldigestion induces necrotizing enterocolitis in preterm pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G1115–G1125. [Google Scholar] [CrossRef] [PubMed]
- Moller, H.K.; Thymann, T.; Fink, L.N.; Frokiaer, H.; Kvistgaard, A.S.; Sangild, P.T. Bovine colostrum is superior to enriched formulas in stimulating intestinal function and necrotising enterocolitis resistance in preterm pigs. Br. J. Nutr. 2011, 105, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Bjornvad, C.R.; Thymann, T.; Deutz, N.E.; Burrin, D.G.; Jensen, S.K.; Jensen, B.B.; Molbak, L.; Boye, M.; Larsson, L.I.; Schmidt, M.; et al. Enteral feeding induces diet-dependent mucosal dysfunction, bacterial proliferation, and necrotizing enterocolitis in preterm pigs on parenteral nutrition. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1092–G1103. [Google Scholar] [CrossRef] [PubMed]
- Welch-Jernigan, R.J.; Abrahamse, E.; Stoll, B.; Smith, O.; Wierenga, P.A.; van de Heijning, B.J.M.; Renes, I.B.; Burrin, D.G. Postprandial Amino Acid Kinetics of Milk Protein Mixtures Are Affected by Composition, But Not Denaturation, in Neonatal Piglets. Curr. Dev. Nutr. 2019, 3, nzy102. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations; WHO. Enterobacter Sakazakii and Other Microorganisms in Powdered Infant Formula: Meeting Report; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Navis, M.; Martins Garcia, T.; Renes, I.B.; Vermeulen, J.L.; Meisner, S.; Wildenberg, M.E.; van den Brink, G.R.; van Elburg, R.M.; Muncan, V. Mouse fetal intestinal organoids: New model to study epithelial maturation from suckling to weaning. EMBO Rep. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Srivillibhuthur, M.; Warder, B.N.; Toke, N.H.; Shah, P.P.; Feng, Q.; Gao, N.; Bonder, E.M.; Verzi, M.P. TFAM is required for maturation of the fetal and adult intestinal epithelium. Dev. Biol. 2018, 439, 92–101. [Google Scholar] [CrossRef]
- Schneeberger, K.; Vogel, G.F.; Teunissen, H.; van Ommen, D.D.; Begthel, H.; El Bouazzaoui, L.; van Vugt, A.H.; Beekman, J.M.; Klumperman, J.; Muller, T.; et al. An inducible mouse model for microvillus inclusion disease reveals a role for myosin Vb in apical and basolateral trafficking. Proc. Natl. Acad. Sci. USA 2015, 112, 12408–12413. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, Research0034. [Google Scholar] [CrossRef]
- Arnal, M.E.; Zhang, J.; Erridge, C.; Smidt, H.; Lalles, J.P. Maternal antibiotic-induced early changes in microbial colonization selectively modulate colonic permeability and inducible heat shock proteins, and digesta concentrations of alkaline phosphatase and TLR-stimulants in swine offspring. PLoS ONE 2015, 10, e0118092. [Google Scholar] [CrossRef]
- Ghosh, N.K.; Fishman, W.H. On the mechanism of inhibition of intestinal alkaline phosphatase by L-phenylalanine. I. Kinetic studies. J. Biol. Chem. 1966, 241, 2516–2522. [Google Scholar] [PubMed]
- Tanner, S.M.; Berryhill, T.F.; Ellenburg, J.L.; Jilling, T.; Cleveland, D.S.; Lorenz, R.G.; Martin, C.A. Pathogenesis of necrotizing enterocolitis: Modeling the innate immune response. Am. J. Pathol. 2015, 185, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Siggers, J.; Sangild, P.T.; Jensen, T.K.; Siggers, R.H.; Skovgaard, K.; Stoy, A.C.; Jensen, B.B.; Thymann, T.; Bering, S.B.; Boye, M. Transition from parenteral to enteral nutrition induces immediate diet-dependent gut histological and immunological responses in preterm neonates. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G435–G445. [Google Scholar] [CrossRef] [PubMed]
- Koelink, P.J.; Wildenberg, M.E.; Stitt, L.W.; Feagan, B.G.; Koldijk, M.; van’t Wout, A.B.; Atreya, R.; Vieth, M.; Brandse, J.F.; Duijst, S.; et al. Development of Reliable, Valid and Responsive Scoring Systems for Endoscopy and Histology in Animal Models for Inflammatory Bowel Disease. J. Crohn’s Colitis 2018, 12, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, R.; Baumdick, M.E.; Sagebiel, A.F.; Kaufmann, M.; Mokry, M.; Klarenbeek, P.L.; Schaltenberg, N.; Steinert, F.L.; van Rijn, J.M.; Drewniak, A.; et al. Human Fetal TNF-alpha-Cytokine-Producing CD4(+) Effector Memory T Cells Promote Intestinal Development and Mediate Inflammation Early in Life. Immunity 2019, 50, 462–476. [Google Scholar] [CrossRef]
- Claud, E.C.; Lu, L.; Anton, P.M.; Savidge, T.; Walker, W.A.; Cherayil, B.J. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc. Natl. Acad. Sci. USA 2004, 101, 7404–7408. [Google Scholar] [CrossRef]
- Nanthakumar, N.N.; Fusunyan, R.D.; Sanderson, I.; Walker, W.A. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proc. Natl. Acad. Sci. USA 2000, 97, 6043–6048. [Google Scholar] [CrossRef]
- Benkoe, T.; Baumann, S.; Weninger, M.; Pones, M.; Reck, C.; Rebhandl, W.; Oehler, R. Comprehensive evaluation of 11 cytokines in premature infants with surgical necrotizing enterocolitis. PLoS ONE 2013, 8, e58720. [Google Scholar] [CrossRef]
- Benkoe, T.M.; Mechtler, T.P.; Weninger, M.; Pones, M.; Rebhandl, W.; Kasper, D.C. Serum levels of interleukin-8 and gut-associated biomarkers in diagnosing necrotizing enterocolitis in preterm infants. J. Pediatric Surg. 2014, 49, 1446–1451. [Google Scholar] [CrossRef]
- Chan, K.Y.; Leung, F.W.; Lam, H.S.; Tam, Y.H.; To, K.F.; Cheung, H.M.; Leung, K.T.; Poon, T.C.; Lee, K.H.; Li, K.; et al. Immunoregulatory protein profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in preterm infants. PLoS ONE 2012, 7, e36977. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.M.; Christensen, R.D. Incidence, neutrophil kinetics, and natural history of neonatal neutropenia associated with maternal hypertension. N. Engl. J. Med. 1989, 321, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Falconer, A.E.; Carr, R.; Edwards, S.W. Impaired neutrophil phagocytosis in preterm neonates: Lack of correlation with expression of immunoglobulin or complement receptors. Biol. Neonate 1995, 68, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Kallman, J.; Schollin, J.; Schalen, C.; Erlandsson, A.; Kihlstrom, E. Impaired phagocytosis and opsonisation towards group B streptococci in preterm neonates. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 78, F46–F50. [Google Scholar] [CrossRef]
- Yost, C.C.; Cody, M.J.; Harris, E.S.; Thornton, N.L.; McInturff, A.M.; Martinez, M.L.; Chandler, N.B.; Rodesch, C.K.; Albertine, K.H.; Petti, C.A.; et al. Impaired neutrophil extracellular trap (NET) formation: A novel innate immune deficiency of human neonates. Blood 2009, 113, 6419–6427. [Google Scholar] [CrossRef]
- Kapel, N.; Campeotto, F.; Kalach, N.; Baldassare, M.; Butel, M.J.; Dupont, C. Faecal calprotectin in term and preterm neonates. J. Pediatric Gastroenterol. Nutr. 2010, 51, 542–547. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Konstantopoulos, P.; Karampetsou, N.; Koutaki, D.; Gkioka, E.; Perrea, D.N.; Papantoniou, N. Calprotectin levels in necrotizing enterocolitis: A systematic review of the literature. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2016, 65, 847–852. [Google Scholar] [CrossRef]
- Lalles, J.P. Intestinal alkaline phosphatase: Novel functions and protective effects. Nutr. Rev. 2014, 72, 82–94. [Google Scholar] [CrossRef]
- Fawley, J.; Koehler, S.; Cabrera, S.; Lam, V.; Fredrich, K.; Hessner, M.; Salzman, N.; Gourlay, D. Intestinal alkaline phosphatase deficiency leads to dysbiosis and bacterial translocation in the newborn intestine. J. Surg. Res. 2017, 218, 35–42. [Google Scholar] [CrossRef]
- Lalles, J.P. Microbiota-host interplay at the gut epithelial level, health and nutrition. J. Anim. Sci. Biotechnol. 2016, 7, 66. [Google Scholar] [CrossRef]
- Fawley, J.; Gourlay, D.M. Intestinal alkaline phosphatase: A summary of its role in clinical disease. J. Surg. Res. 2016, 202, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Schaart, M.W.; de Bruijn, A.C.; Bouwman, D.M.; de Krijger, R.R.; van Goudoever, J.B.; Tibboel, D.; Renes, I.B. Epithelial functions of the residual bowel after surgery for necrotising enterocolitis in human infants. J. Pediatric Gastroenterol. Nutr. 2009, 49, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Birchenough, G.M.; Johansson, M.E.; Gustafsson, J.K.; Bergstrom, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Morampudi, V.; Crowley, S.M.; Stahl, M.; Yu, H.; Bhullar, K.; Knodler, L.A.; Bressler, B.; Jacobson, K.; Vallance, B.A. Frontline defenders: Goblet cell mediators dictate host-microbe interactions in the intestinal tract during health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G360–G377. [Google Scholar] [CrossRef]
- McElroy, S.J.; Prince, L.S.; Weitkamp, J.H.; Reese, J.; Slaughter, J.C.; Polk, D.B. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: A potential role in neonatal necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G656–G666. [Google Scholar] [CrossRef]
- Zhao, H.; Sovadinova, I.; Swope, V.M.; Swain, G.M.; Kadrofske, M.M.; Bian, X. Postnatal development of the serotonin signaling system in the mucosa of the guinea pig ileum. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2011, 23, 161.e40–168.e40. [Google Scholar] [CrossRef][Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navis, M.; Muncan, V.; Sangild, P.T.; Møller Willumsen, L.; Koelink, P.J.; Wildenberg, M.E.; Abrahamse, E.; Thymann, T.; van Elburg, R.M.; Renes, I.B. Beneficial Effect of Mildly Pasteurized Whey Protein on Intestinal Integrity and Innate Defense in Preterm and Near-Term Piglets. Nutrients 2020, 12, 1125. https://doi.org/10.3390/nu12041125
Navis M, Muncan V, Sangild PT, Møller Willumsen L, Koelink PJ, Wildenberg ME, Abrahamse E, Thymann T, van Elburg RM, Renes IB. Beneficial Effect of Mildly Pasteurized Whey Protein on Intestinal Integrity and Innate Defense in Preterm and Near-Term Piglets. Nutrients. 2020; 12(4):1125. https://doi.org/10.3390/nu12041125
Chicago/Turabian StyleNavis, Marit, Vanesa Muncan, Per Torp Sangild, Line Møller Willumsen, Pim J. Koelink, Manon E. Wildenberg, Evan Abrahamse, Thomas Thymann, Ruurd M. van Elburg, and Ingrid B. Renes. 2020. "Beneficial Effect of Mildly Pasteurized Whey Protein on Intestinal Integrity and Innate Defense in Preterm and Near-Term Piglets" Nutrients 12, no. 4: 1125. https://doi.org/10.3390/nu12041125
APA StyleNavis, M., Muncan, V., Sangild, P. T., Møller Willumsen, L., Koelink, P. J., Wildenberg, M. E., Abrahamse, E., Thymann, T., van Elburg, R. M., & Renes, I. B. (2020). Beneficial Effect of Mildly Pasteurized Whey Protein on Intestinal Integrity and Innate Defense in Preterm and Near-Term Piglets. Nutrients, 12(4), 1125. https://doi.org/10.3390/nu12041125





