Facial EMG Correlates of Subjective Hedonic Responses During Food Consumption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli
2.3. Procedure
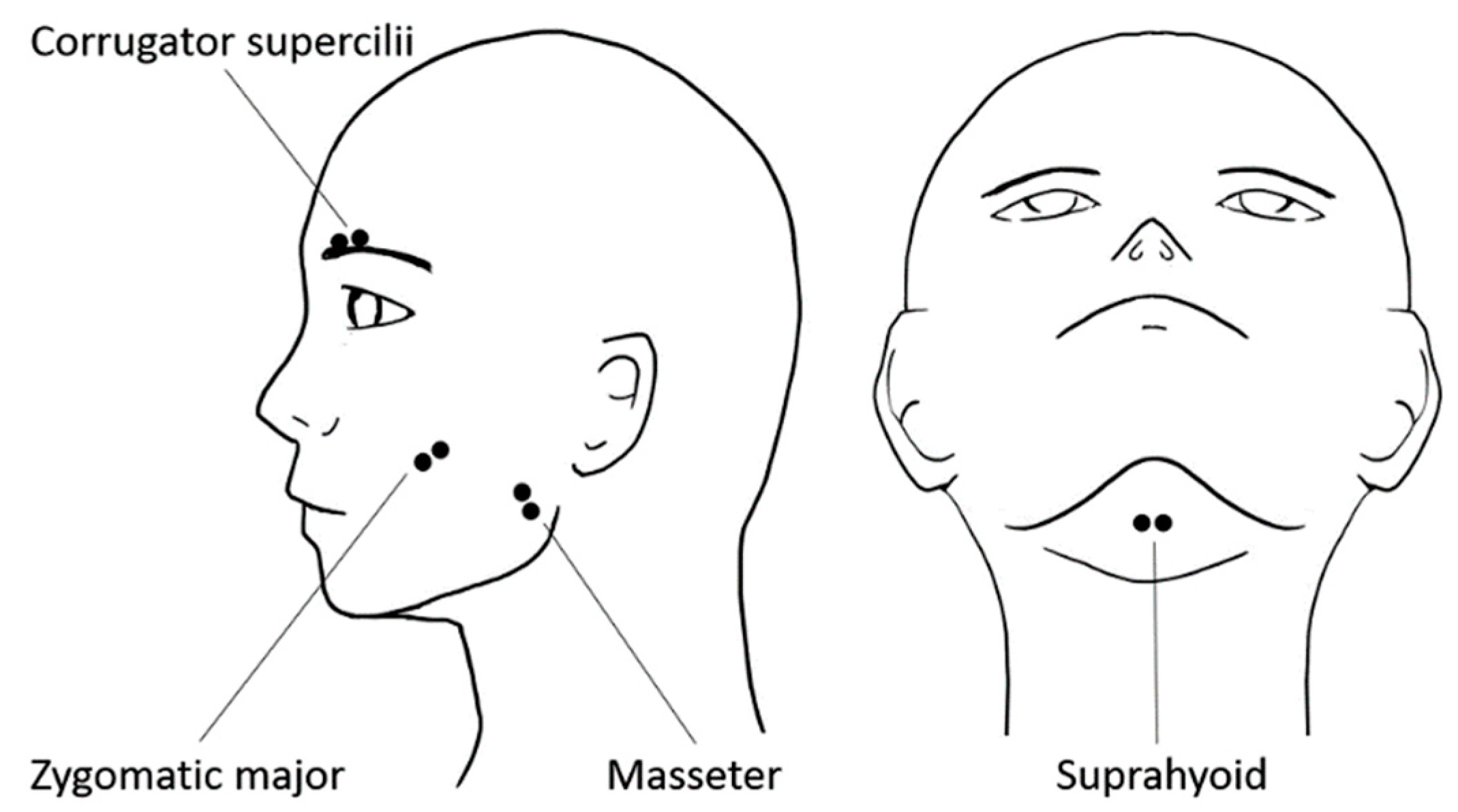
2.4. Physiological Data Recording
2.5. Data Analysis
2.5.1. Preprocessing
2.5.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orne, M.T. On the social psychology of the psychological experiment: With particular reference to demand characteristics and their implications. Am. Psychol. 1962, 17, 776–783. [Google Scholar] [CrossRef]
- James, W. What is an emotion? Mind 1884, 9, 188–205. [Google Scholar] [CrossRef]
- Friedman, B.H. Feelings and the body: The Jamesian perspective on autonomic specificity of emotion. Biol. Psychol. 2010, 84, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J. The varieties of emotional experience: A meditation on James-Lange theory. Psychol. Rev. 1994, 101, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, J.T.; Berntson, G.G.; Klein, D.J. What is an emotion? The role of somatovisceral afference, with special emphasis on somatovisceral “illusions”. In Emotion and Social Behavior. ix.; Clark, M.S., Ed.; Sage: Thousand Oaks, CA, USA, 1992; pp. 63–98. [Google Scholar]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biol. Psychiatry 1998, 44, 1248–1263. [Google Scholar] [CrossRef]
- Dimberg, U. Facial electromyography and emotional reactions. Psychophysiology 1990, 27, 481–494. [Google Scholar] [CrossRef]
- Greenwald, M.K.; Cook, E.W.; Lang, P.J. Affective judgment and psychophysiological response: Dimensional covariation in the evaluation of pictorial stimuli. J. Psychophysiol. 1989, 3, 51–64. [Google Scholar]
- Horio, T. EMG activities of facial and chewing muscles of human adults in response to taste stimuli. Percept. Mot. Skills 2003, 97, 289–298. [Google Scholar] [CrossRef]
- Armstrong, J.E.; Hutchinson, I.; Laing, D.G.; Jinks, A.L. Facial electromyography: Responses of children to odor and taste stimuli. Chem. Senses 2007, 32, 611–621. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, J.E.; Laing, D.G.; Jinks, A.L. Taste-elicited activity in facial muscle regions in 5–8-week-old infants. Chem. Senses 2017, 42, 443–453. [Google Scholar] [CrossRef]
- Hu, S.; Player, K.A.; Mcchesney, K.A.; Dalistan, M.D.; Tyner, C.A.; Scozzafava, J.E. Facial EMG as an indicator of palatability in humans. Physiol. Behav. 1999, 68, 31–35. [Google Scholar] [CrossRef]
- Danner, L.; Haindl, S.; Joechl, M.; Duerrschmid, K. Facial expressions and autonomous nervous system responses elicited by tasting different juices. Food Res. Int. 2014, 64, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Greimel, E.; Macht, M.; Krumhuber, E.; Ellgring, H. Facial and affective reactions to tastes and their modulation by sadness and joy. Physiol. Behav. 2006, 89, 261–269. [Google Scholar] [CrossRef] [PubMed]
- de Wijk, R.A.; Kooijman, V.; Verhoeven, R.H.G.; Holthuysen, N.T.E.; de Graaf, C. Autonomic nervous system responses on and facial expressions to the sight, smell, and taste of liked and disliked foods. Food Qual. Prefer. 2012, 26, 196–203. [Google Scholar] [CrossRef]
- Weiland, R.; Ellgring, H.; Macht, M. Gustofacial and olfactofacial responses in human adults. Chem. Senses 2010, 35, 841–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendin, K.; Allesen–Holm, B.H.; Bredie, W.L.P. Do facial reactions add new dimensions to measuring sensory responses to basic tastes? Food Qual. Prefer. 2011, 22, 346–354. [Google Scholar] [CrossRef]
- Steiner, J.E.; Glaser, D.; Hawilo, M.E.; Berridge, K.C. Comparative expression of hedonic impact: Affective reactions to taste by human infants and other primates. Neurosci. Biobehav. Rev. 2001, 25, 53–74. [Google Scholar] [CrossRef]
- Berridge, K.C.; Kringelbach, M.L. Pleasure systems in the brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef] [Green Version]
- Coles, N.A.; Larsen, J.T.; Lench, H.C. A meta-analysis of the facial feedback literature: Effects of facial feedback on emotional experience are small and variable. Psychol. Bull. 2019, 145, 610–651. [Google Scholar] [CrossRef]
- Kostyra, E.; Rambuszek, M.; Waszkiewicz–Robak, B.; Laskowski, W.; Blicharski, T.; Poławska, E. Consumer facial expression in relation to smoked ham with the use of face reading technology. The methodological aspects and informative value of research results. Meat Sci. 2016, 119, 22–31. [Google Scholar] [CrossRef]
- Curran, P.J.; Bauer, D.J. The disaggregation of within–person and between–person effects in longitudinal models of change. Annu. Rev. Psychol. 2011, 62, 583–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, S. The stability of behavior: II. Implications for psychological research. Am. Psychol. 1980, 35, 790–806. [Google Scholar] [CrossRef]
- Ostrof, C. Comparing Correlations Based on Individual–Level and Aggregated Data. J. Appl. Psychol. 1993, 78, 569–582. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Wang, L.P. Aggregating and testing intra–individual correlations: Methods and comparisons. Multivar. Behav. Res. 2014, 49, 130–148. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A. Taste preferences and food intake. Annu. Rev. Nutr. 1997, 17, 237–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, I. Sensory perception is related to the rate of change of volatile concentration in-nose during eating of model gels. Chem. Senses 1999, 24, 155–160. [Google Scholar] [CrossRef]
- Carr, J.; Baloga, D.; Guinard, J.-X.; Lawter, L.; Marty, C.; Squire, C. The effect of gelling agent type and concentration on flavor release in model systems. In Flavor-Food Interactions; McGorrin, R.J., Leland, J.V., Eds.; American Chemical Society: Washington, DC, USA, 1996; pp. 98–108. [Google Scholar]
- Clark, R. Influence of hydrocolloids on flavour release and sensory-instrumental correlations. In Gums and Stabilisers for the Food Industry 11; Williams, P.A., Phillips, G.O., Eds.; Royal Society of Chemistry: Cambridge, UK, 2007; pp. 217–225. [Google Scholar]
- Weel, K.G.C.; Boelrijk, A.E.M.; Alting, A.C.; van Mil, P.J.J.M.; Burger, J.J.; Gruppen, H.; Voragen, A.G.J.; Smit, G. Flavor release and perception of flavored whey protein gels: Perception is determined by texture rather than by release. J. Agric. Food Chem. 2002, 50, 5149–5155. [Google Scholar] [CrossRef]
- Finlayson, G.; King, N.; Blundell, J.E. Liking vs. wanting food: Importance for human appetite control and weight regulation. Neurosci. Biobehav. Rev. 2007, 31, 987–1002. [Google Scholar] [CrossRef] [Green Version]
- Pool, E.; Sennwald, V.; Delplanque, S.; Brosch, T.; Sander, D. Measuring wanting and liking from animals to humans: A systematic review. Neurosci. Biobehav. Rev. 2016, 63, 124–142. [Google Scholar] [CrossRef] [Green Version]
- Attuquayefio, T.; Stevenson, R.J.; Boakes, R.A.; Oaten, M.J.; Yeomans, M.R.; Mahmut, M.; Francis, H.M. A high-fat high-sugar diet predicts poorer hippocampal-related memory and a reduced ability to suppress wanting under satiety. J. Exp. Psychol. Anim. Learn. Cogn. 2016, 42, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Finlayson, G.; King, N.; Blundell, J.E. Is it possible to dissociate ‘liking’ and ‘wanting’ for foods in humans? A novel experimental procedure. Physiol. Behav. 2007, 90, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.J.; Francis, H.M.; Attuquayefio, T.; Ockert, C. Explicit wanting and liking for palatable snacks are differentially affected by change in physiological state, and differentially related to salivation and hunger. Physiol. Behav. 2017, 182, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Nakauma, M.; Hunami, T.; Tanaka, T.; Nishinari, K.; Kohyama, K. Electromyography during oral processing in relation to mechanical and sensory properties of soft gels. J. Texture Stud. 2011, 42, 254–267. [Google Scholar] [CrossRef]
- Kohyama, K.; Gao, Z.; Ishihara, S.; Funami, T.; Nishinari, K. Electromyography analysis of natural mastication behavior using varying mouthful quantities of two types of gels. Physiol. Behav. 2016, 161, 174–182. [Google Scholar] [CrossRef]
- Boucsein, W. Electrodermal Activity; Plenum: New York, NY, USA, 1992. [Google Scholar]
- Rousmans, S.; Robin, O.; Dittmar, A.; Vernet–Maury, E. Autonomic nervous system responses associated with primary tastes. Chem. Senses 2000, 25, 709–718. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Drewnowski, A. Individual differences in sensory preferences for fat in model sweet dairy products. Acta. Psychol. 1993, 84, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Bradley, M.M.; Lang, P.J. Measuring emotion: The Self–Assessment Manikin and the Semantic Differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef]
- Fridlund, A.J.; Cacioppo, J.T. Guidelines for human electromyographic research. Psychophysiology 1986, 23, 567–589. [Google Scholar] [CrossRef]
- Schumann, N.P.; Bongers, K.; Guntinas-Lichius, O.; Scholle, H.C. Facial muscle activation patterns in healthy male humans: A multi–channel surface EMG study. J. Neurosci. Methods 2010, 187, 120–128. [Google Scholar] [CrossRef]
- Van Boxtel, A. Optimal signal bandwidth for the recording of surface EMG activity of facial, jaw, oral, and neck muscles. Psychophysiology 2001, 38, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Christie, M.J.; Venables, P.H. Change in palmar skin potential level during relaxation after stress. J. Psychosom. Res. 1974, 18, 301–306. [Google Scholar] [CrossRef]
- Boucsein, W.; Fowles, D.C.; Grimnes, S.; Ben-Shakhar, G.; Roth, W.T.; Dawson, M.E.; Filion, D.L. Publication recommendations for electrodermal measurements. Psychophysiology 2012, 49, 1017–1034. [Google Scholar] [PubMed]
- Jennings, J.R.; Bberg, W.K.; Hutcheson, J.S.; Obrist, P.; Porges, S.; Turpin, G. Publication guidelines for heart rate studies in man. Psychophysiology 1981, 18, 226–231. [Google Scholar] [CrossRef]
- Van der Zwaag, M.D.; Westerink, J.; Van den Broek, E.L. Emotional and psychophysiological responses to tempo, mode, and percussiveness. Music. Sci. 2011, 15, 250–269. [Google Scholar] [CrossRef]
- Holmes, A.P.; Friston, K.J. Generalisability, random effects and population inference. Neuroimage 1998, 7, S754. [Google Scholar] [CrossRef]
- Sørensen, L.B.; Møller, P.; Flint, A.; Martens, M.; Raben, A. Effect of sensory perception of foods on appetite and food intake: A review of studies on humans. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1152–1166. [Google Scholar] [CrossRef] [Green Version]
- Bornemann, B.; Winkielman, P.; van der Meer, E. Can you feel what you do not see? Using internal feedback to detect briefly presented emotional stimuli. Int. J. Psychophysiol. 2012, 85, 116–124. [Google Scholar] [CrossRef]
- Kaiser, J.; Davey, G.C.; Parkhouse, T.; Meeres, J.; Scott, R.B. Emotional facial activation induced by unconsciously perceived dynamic facial expressions. Int. J. Psychophysiol. 2016, 110, 207–211. [Google Scholar] [CrossRef]
- Sato, W.; Kochiyama, T.; Minemoto, K.; Sawada, R.; Fushiki, T. Amygdala activation during unconscious visual processing of food. Sci. Rep. 2019, 9, 7277. [Google Scholar] [CrossRef] [Green Version]
- Sato, W.; Rymarczyk, K.; Minemoto, K.; Wojciechowski, J.; Hyniewska, S. Cultural moderation of unconscious hedonic responses to food. Nutrients 2019, 11, 2832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, W.; Sawada, R.; Kubota, T.; Toichi, M.; Fushiki, T. Unconscious affective responses to food. PLoS ONE 2016, 11, e0160956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, W.; Sawada, R.; Kubota, T.; Toichi, M.; Fushiki, T. Homeostatic modulation on unconscious hedonic responses to food. BMC Res. Notes 2017, 10, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekman, P. Methods for measuring facial action. In Handbook of Methods in Nonverbal Behavior Research; Scherer, K.R., Ekman, P., Eds.; Cambridge University Press: Cambridge, UK, 1982; pp. 45–90. [Google Scholar]
- Cattaneo, L.; Pavesi, G. The facial motor system. Neurosci. Biobehav. Rev. 2014, 38, 135–159. [Google Scholar] [CrossRef]
- Critchley, H.D.; Garfinkel, S.N. Interoception and emotion. Curr. Opin. Psychol. 2017, 17, 7–14. [Google Scholar] [CrossRef]
- Holstege, G. The emotional motor system in relation to the supraspinal control of micturition and mating behavior. Behav. Brain Res. 1998, 92, 103–109. [Google Scholar] [CrossRef]
- Hemphill, J.F. Interpreting the magnitude of correlation coefficients. Am. Psychol. 2003, 58, 78–79. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Hu, X. Interference removal from electromyography based on independent component analysis. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 887–894. [Google Scholar] [CrossRef]
- Tan, J.-W.; Andrade, A.O.; Li, H.; Walter, S.; Hrabal, D.; Rukavina, S.; Limbrecht-Ecklundt, K.; Hoffmann, H.; Traue, H.C. Recognition of intensive valence and arousal affective states via facial electromyographic activity in young and senior adults. PLoS ONE 2016, 11, e0146691. [Google Scholar] [CrossRef] [Green Version]




| Subjective | Statistic | Physiological | |||||
|---|---|---|---|---|---|---|---|
| Corrugator | Zygomatic | Masseter | Suprahyoid | SPL | HR | ||
| Liking | t | 4.31 | 0.53 | 0.14 | 1.29 | 1.09 | 0.60 |
| p | 0.000 | 0.602 | 0.893 | 0.211 | 0.289 | 0.552 | |
| Wanting | t | 3.98 | 0.08 | 0.42 | 0.72 | 1.22 | 0.29 |
| p | 0.001 | 0.936 | 0.680 | 0.481 | 0.234 | 0.775 | |
| Valence | t | 3.56 | 0.91 | 0.31 | 0.33 | 0.70 | 0.88 |
| p | 0.002 | 0.373 | 0.760 | 0.744 | 0.492 | 0.389 | |
| Arousal | t | 0.27 | 1.52 | 0.47 | 0.11 | 1.23 | 1.22 |
| p | 0.788 | 0.143 | 0.646 | 0.916 | 0.231 | 0.235 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, W.; Minemoto, K.; Ikegami, A.; Nakauma, M.; Funami, T.; Fushiki, T. Facial EMG Correlates of Subjective Hedonic Responses During Food Consumption. Nutrients 2020, 12, 1174. https://doi.org/10.3390/nu12041174
Sato W, Minemoto K, Ikegami A, Nakauma M, Funami T, Fushiki T. Facial EMG Correlates of Subjective Hedonic Responses During Food Consumption. Nutrients. 2020; 12(4):1174. https://doi.org/10.3390/nu12041174
Chicago/Turabian StyleSato, Wataru, Kazusa Minemoto, Akira Ikegami, Makoto Nakauma, Takahiro Funami, and Tohru Fushiki. 2020. "Facial EMG Correlates of Subjective Hedonic Responses During Food Consumption" Nutrients 12, no. 4: 1174. https://doi.org/10.3390/nu12041174
APA StyleSato, W., Minemoto, K., Ikegami, A., Nakauma, M., Funami, T., & Fushiki, T. (2020). Facial EMG Correlates of Subjective Hedonic Responses During Food Consumption. Nutrients, 12(4), 1174. https://doi.org/10.3390/nu12041174





