Spirulina Enhances Bone Modeling in Growing Male Rats by Regulating Growth-Related Hormones
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Diets
2.2. Tissue Collection and Preparation
2.3. Bone Strength and Bone Mineral Content (BMC) Measurement
2.4. Biochemical Analysis
2.5. Antioxidant Enzyme Activities and Lipid Peroxide Measurement
2.6. Statistics
3. Results
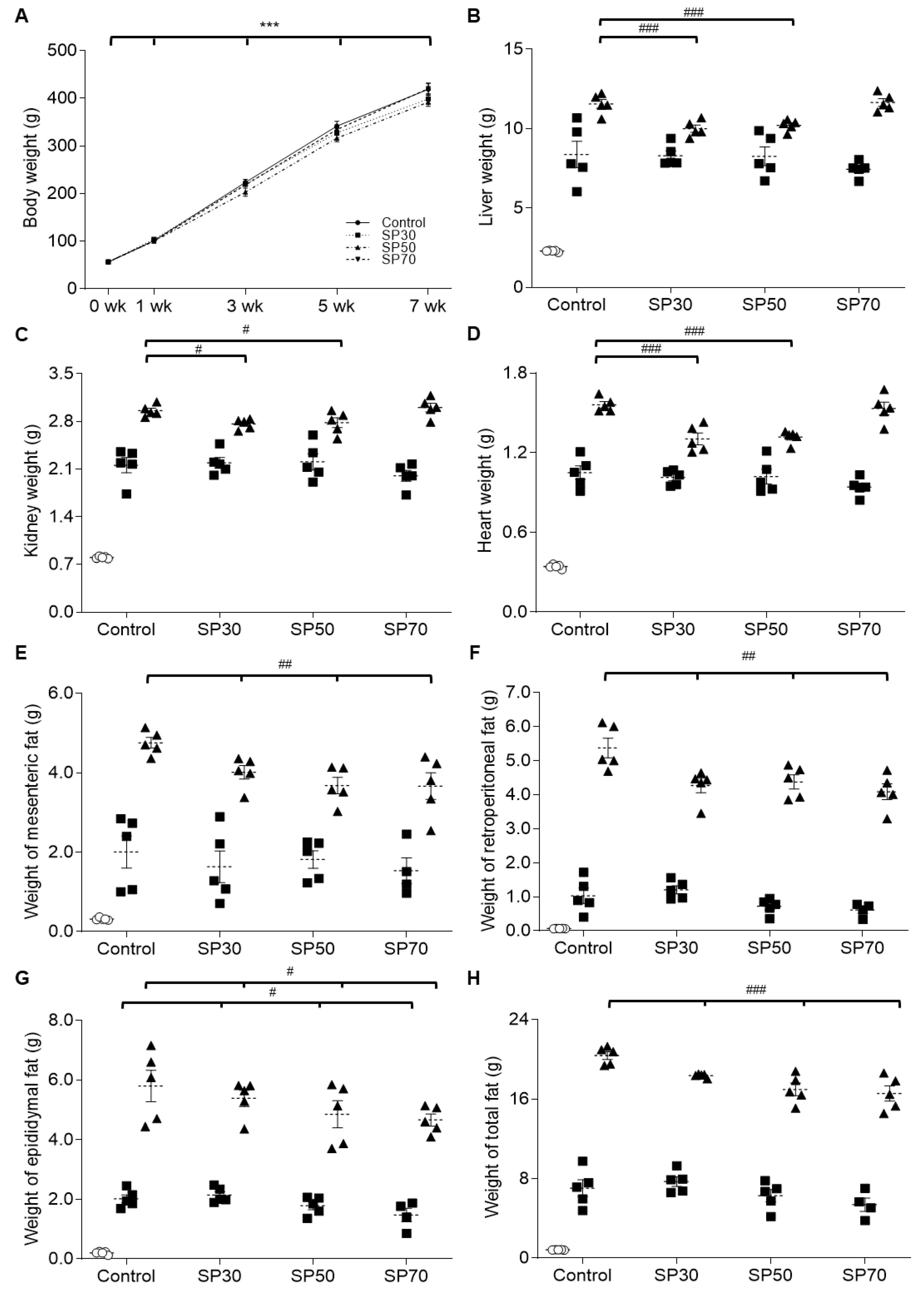
3.1. Body Weight, Food Intake, Tissue Damage, and Blood Lipid Profile
3.2. Change of Organ Growth
3.3. Fat Accumulation during the Growth
3.4. Improvement of Length and Weight of Femur and Lumbar Spine by Spirulina
3.5. Enhanced Bone Strength via Higher Bone Mineral Content by Spirulina
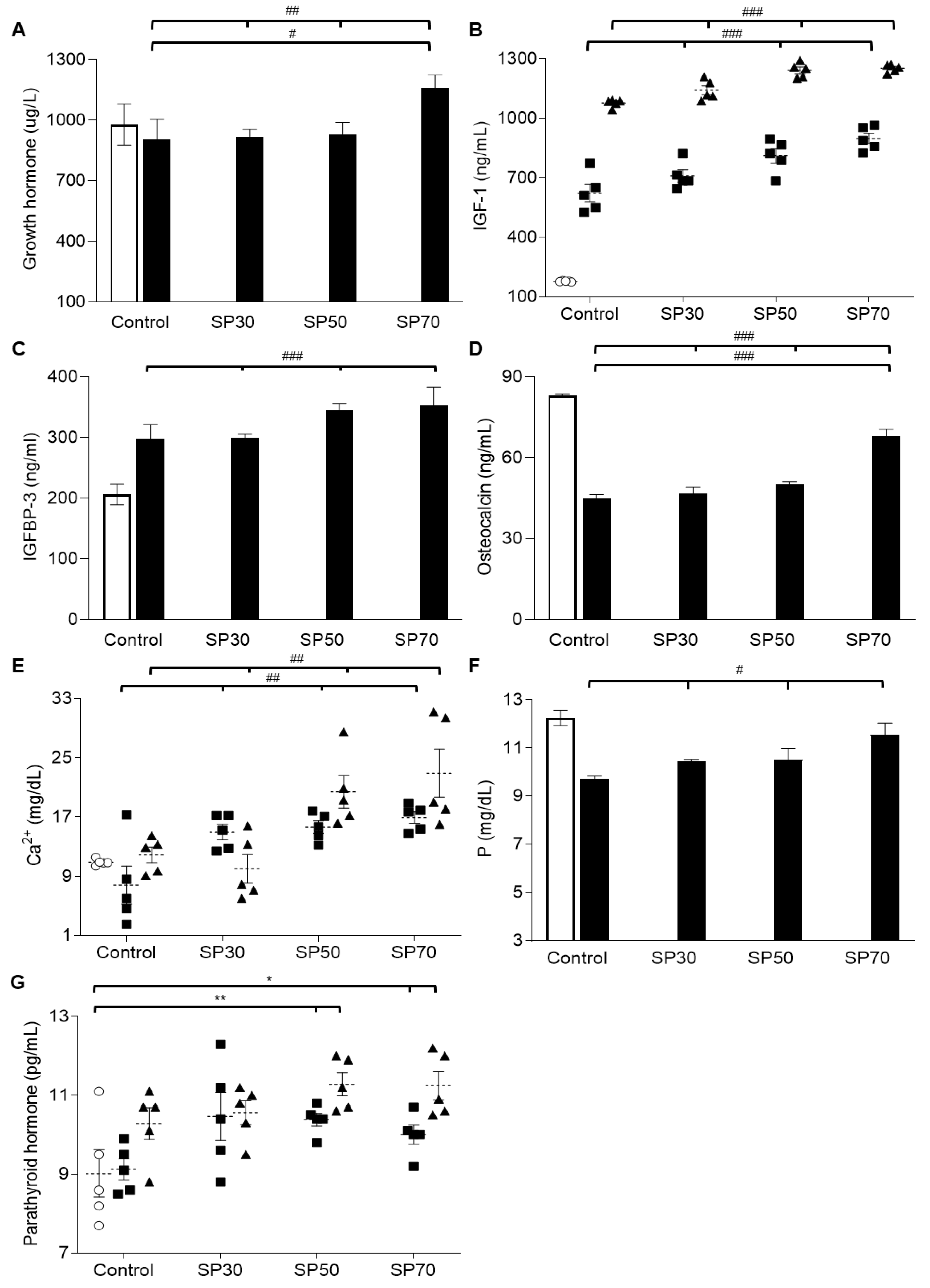
3.6. Increased plasma Growth Hormone, IGF-1, and IGFBP-3 Levels by Spirulina
3.7. Increased Plasma Osteocalcin, Mineral, and Parathyroid Hormone (PTH) Levels Dose-Dependently
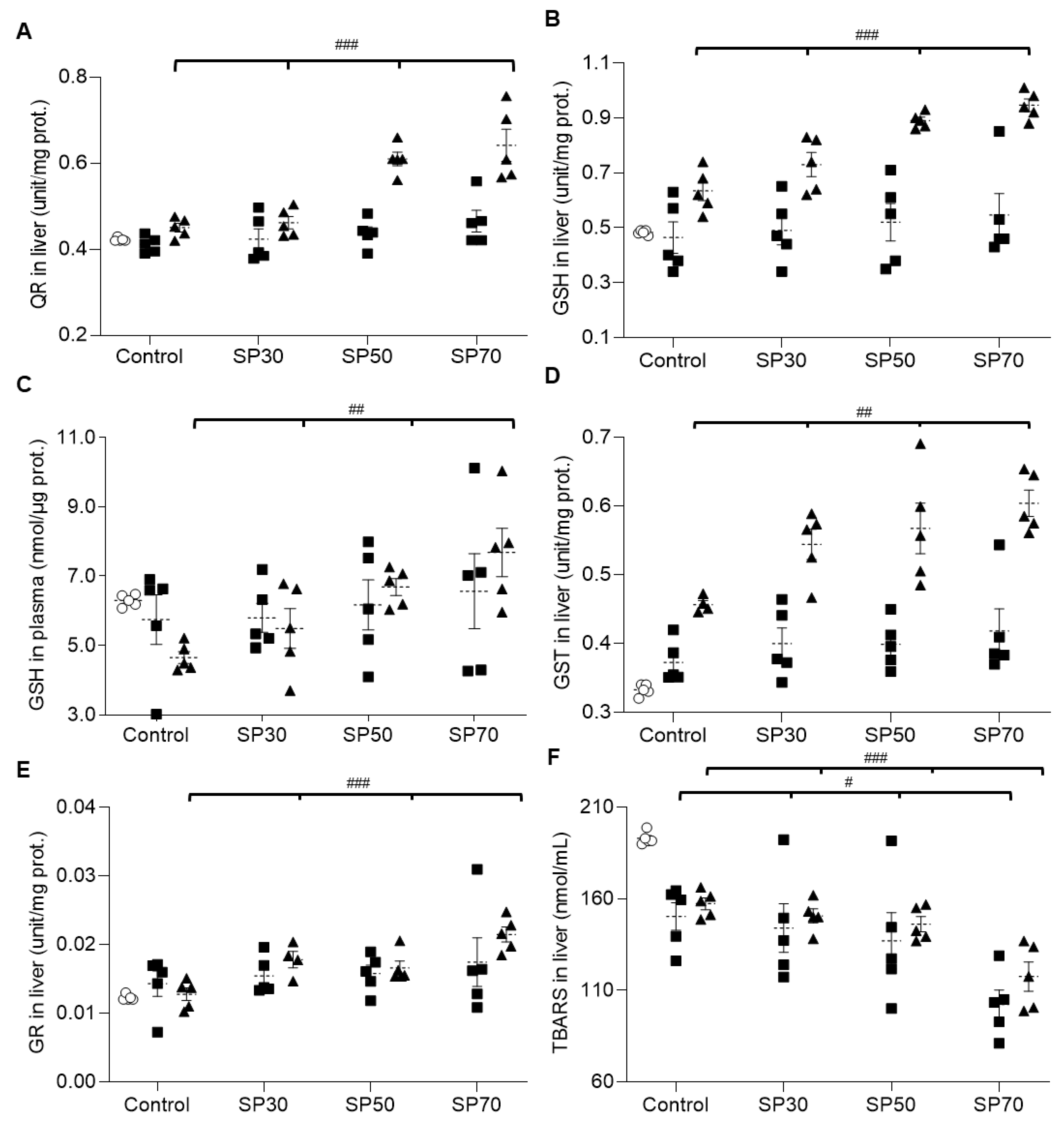
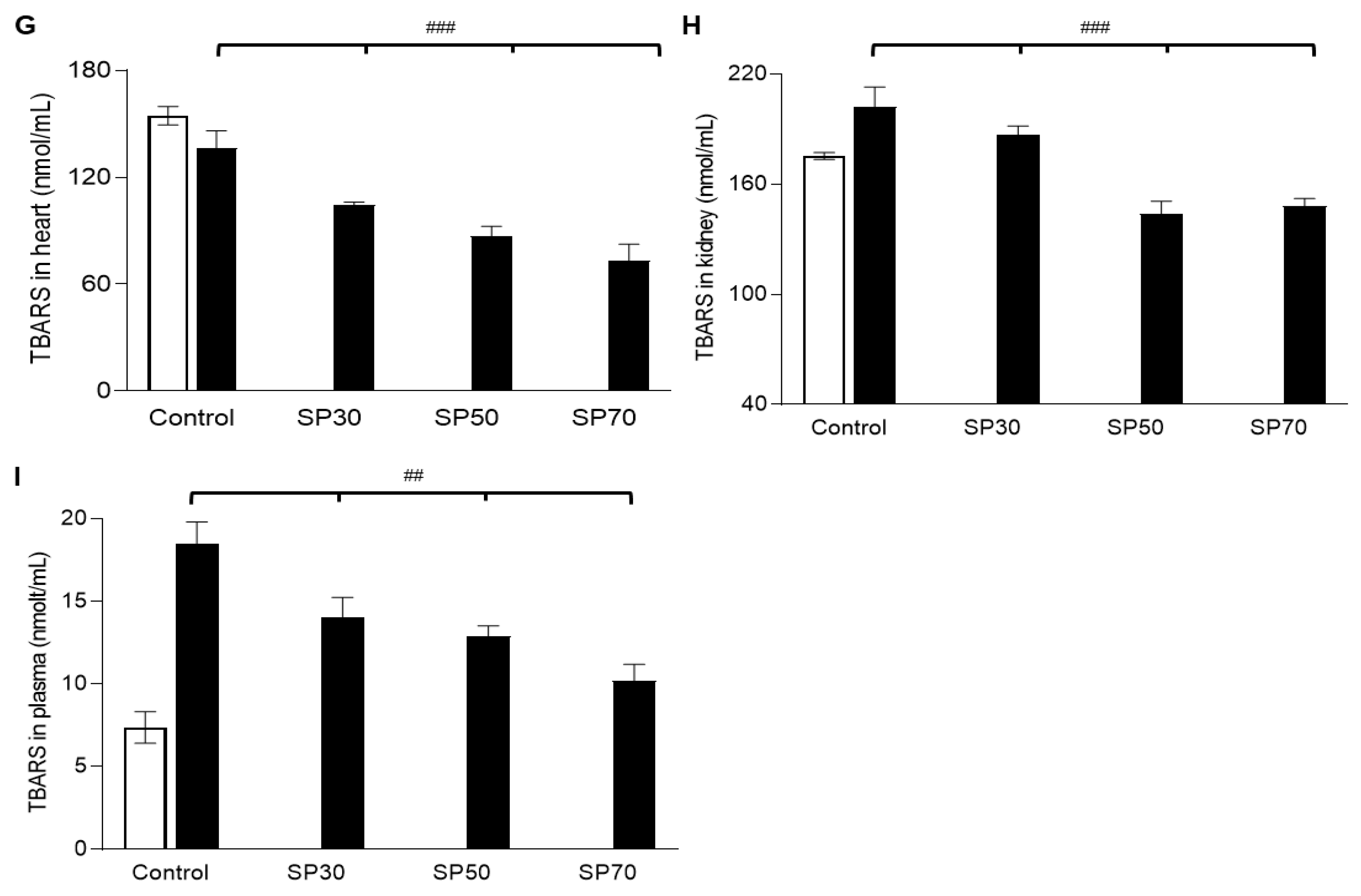
3.8. Increased Antioxidant Enzyme Activities and Reduced Lipid Peroxidation by Spirulina
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barak, M.M. Bone modeling or bone remodeling: That is the question. Am. J. Phys. Anthropol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Boivin, G.; Meunie, P.J. Changes in bone remodeling rate influence the degree of mineralization of bone which is a determinant of bone strength: Therapeutic implications. Adv. Exp. Med. Biol. 2001, 496, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Boivin, G.; Meunier, P.J. Changes in bone remodeling rate influence the degree of mineralization of bone. Connect. Tissue Res. 2002, 43, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Bagi, C.M.; Berryman, E.; Moalli, M.R. Comparative bone anatomy of commonly used laboratory animals: Implications for drug discovery. Comp. Med. 2011, 61, 76–85. [Google Scholar] [PubMed]
- Oberbauer, A.M.; Peng, R. Growth hormone and IGF-I stimulate cell function in distinct zones of the rat epiphyseal growth plate. Connect. Tissue Res. 1995, 31, 189–195. [Google Scholar] [CrossRef]
- Medill, N.J.; Praul, C.A.; Ford, B.C.; Leach, R.M. Parathyroid hormone-related peptide expression in the epiphyseal growth plate of the juvenile chicken: Evidence for the origin of the parathyroid hormone-related peptide found in the epiphyseal growth plate. J. Cell. Biochem. 2001, 80, 504–511. [Google Scholar] [CrossRef]
- Canalis, E. Growth factor control of bone mass. J. Cell. Biochem. 2009, 108, 769–777. [Google Scholar] [CrossRef]
- Yuan, R.; Tsaih, S.W.; Petkova, S.B.; De Evsikova, C.M.; Xing, S.; Marion, M.A.; Bogue, M.A.; Mills, K.D.; Peters, L.L.; Bult, C.J.; et al. Aging in inbred strains of mice: Study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell 2009, 8, 277–287. [Google Scholar] [CrossRef]
- Denley, A.; Cosgrove, L.J.; Booker, G.W.; Wallace, J.C.; Forbes, B.E. Molecular interactions of the IGF system. Cytokine Growth Factor Rev. 2005, 16, 421–439. [Google Scholar] [CrossRef]
- Bennis-Taleb, N.; Remacle, C.; Hoet, J.J.; Reusens, B. A low-protein isocaloric diet during gestation affects brain development and alters permanently cerebral cortex blood vessels in rat offspring. J. Nutr. 1999, 129, 1613–1619. [Google Scholar] [CrossRef]
- Reichling, T.D.; German, R.Z. Bones, muscles and visceral organs of protein-malnourished rats (Rattus norvegicus) grow more slowly but for longer durations to reach normal final size. J. Nutr. 2000, 130, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Fournier, C.; Rizzoli, R.; Ammann, P. Low calcium-phosphate intakes modulate the low-protein diet-related effect on peak bone mass acquisition: A hormonal and bone strength determinants study in female growing rats. Endocrinology 2014, 155, 4305–4315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chevalley, T.; Bonjour, J.P.; Ferrari, S.; Rizzoli, R. High-protein intake enhances the positive impact of physical activity on BMC in prepubertal boys. J. Bone Miner. Res. 2008, 23, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Frenk, S.; Gomez, F.; Ramos-Galvan, R.; Cravioto, J. Fatty liver in children; kwashiorkor. Am. J. Clin. Nutr. 1958, 6, 298–309. [Google Scholar] [CrossRef]
- Omstedt, P.T.; von der Decken, A.; Hedenskog, G.; Mogren, H. Nutritive value of processed Saccharomyces cerevisiae, Scenedesmus obliquus and Spirulina platensis as measured by protein synthesis in vitro in rat skeletal muscle. J. Sci. Food Agric. 1973, 24, 1103–1113. [Google Scholar] [CrossRef]
- Al'bitskaia, O.N.; Zaitseva, G.N.; Rogozhin, S.V.; Pakhomova, M.V.; Oshanina, N.P. [Comparative evaluation of methods for isolating total protein from the biomass of Spirulina platensis]. Prikladnaia Biokhimiia I Mikrobiologiia 1979, 15, 612–617. [Google Scholar]
- Volkmann, H.; Imianovsky, U.; Oliveira, J.L.; Sant’anna, E.S. Cultivation of Arthrospira (spirulina) platensis in desalinator wastewater and salinated synthetic medium: Protein content and amino-acid profile. Braz. J. Microbiol. 2008, 39, 98–101. [Google Scholar] [CrossRef]
- Senachak, J.; Cheevadhanarak, S.; Hongsthong, A. SpirPro: A Spirulina proteome database and web-based tools for the analysis of protein-protein interactions at the metabolic level in Spirulina (Arthrospira) platensis C1. BMC Bioinform. 2015, 16, 233. [Google Scholar] [CrossRef]
- Maranesi, M.; Barzanti, V.; Carenini, G.; Gentili, P. Nutritional studies on Spirulina maxima. Acta Vitaminol. Enzymol. 1984, 6, 295–304. [Google Scholar]
- Padyana, A.K.; Bhat, V.B.; Madyastha, K.M.; Rajashankar, K.R.; Ramakumar, S. Crystal structure of a light-harvesting protein C-phycocyanin from Spirulina platensis. Biochem. Biophys. Res. Commun. 2001, 282, 893–898. [Google Scholar] [CrossRef]
- Sun, J.Y.; Hou, Y.J.; Fu, X.Y.; Fu, X.T.; Ma, J.K.; Yang, M.F.; Sun, B.L.; Fan, C.D.; Oh, J. Selenium-Containing Protein From Selenium-Enriched Spirulina platensis Attenuates Cisplatin-Induced Apoptosis in MC3T3-E1 Mouse Preosteoblast by Inhibiting Mitochondrial Dysfunction and ROS-Mediated Oxidative Damage. Front. Physiol. 2018, 9, 1907. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Cui, Y.; Fan, X.; Qi, P.; Liu, C.; Zhou, X.; Zhang, X. Anti-obesity effects of Spirulina platensis protein hydrolysate by modulating brain-liver axis in high-fat diet fed mice. PLoS ONE 2019, 14, e0218543. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Valan Arasu, M. Quantification of Phytochemicals from Commercial Spirulina Products and Their Antioxidant Activities. Evid. Based Complement. Altern. Med. 2016, 2016, 7631864. [Google Scholar] [CrossRef] [PubMed]
- Pinero Estrada, J.E.; Bermejo Bescos, P.; Villar del Fresno, A.M. Antioxidant activity of different fractions of Spirulina platensis protean extract. Farmaco 2001, 56, 497–500. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, Y.J.; Ryu, H.K.; Kim, M.H.; Chung, H.W.; Kim, W.Y. A randomized double-blind, placebo-controlled study to establish the effects of spirulina in elderly Koreans. Ann. Nutr. Metab. 2008, 52, 322–328. [Google Scholar] [CrossRef]
- Simpore, J.; Kabore, F.; Zongo, F.; Dansou, D.; Bere, A.; Pignatelli, S.; Biondi, D.M.; Ruberto, G.; Musumeci, S. Nutrition rehabilitation of undernourished children utilizing Spiruline and Misola. Nutr. J. 2006, 5, 3. [Google Scholar] [CrossRef]
- Tranquille, N.; Emeis, J.J.; de Chambure, D.; Binot, R.; Tamponnet, C. Spirulina acceptability trials in rats. A study for the “MELISSA” life-support system. Adv. Space Res. 1994, 14, 167–170. [Google Scholar] [CrossRef]
- Salazar, M.; Chamorro, G.A.; Salazar, S.; Steele, C.E. Effect of Spirulina maxima consumption on reproduction and peri- and postnatal development in rats. Food Chem. Toxicol. 1996, 34, 353–359. [Google Scholar] [CrossRef]
- Voltarelli, F.A.; de Mello, M.A. Spirulina enhanced the skeletal muscle protein in growing rats. Eur. J. Nutr. 2008, 47, 393–400. [Google Scholar] [CrossRef]
- Lupatini, A.L.; Colla, L.M.; Canan, C.; Colla, E. Potential application of microalga Spirulina platensis as a protein source. J. Sci. Food Agric. 2017, 97, 724–732. [Google Scholar] [CrossRef]
- Azabji-Kenfack, M.; Dikosso, S.E.; Loni, E.G.; Onana, E.A.; Sobngwi, E.; Gbaguidi, E.; Kana, A.L.; Nguefack-Tsague, G.; Von der Weid, D.; Njoya, O.; et al. Potential of Spirulina Platensis as a Nutritional Supplement in Malnourished HIV-Infected Adults in Sub-Saharan Africa: A Randomised, Single-Blind Study. Nutr. Metab. Insights 2011, 4, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.P.; Puga, G.M.; Beck, W.R.; Teixeira, I.P.; Ghezzi, A.C.; Silva, G.A.; Mello, M.A. Exercise and spirulina control non-alcoholic hepatic steatosis and lipid profile in diabetic Wistar rats. Lipids Health Dis. 2011, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Laib, A.; Barou, O.; Vico, L.; Lafage-Proust, M.H.; Alexandre, C.; Rugsegger, P. 3D micro-computed tomography of trabecular and cortical bone architecture with application to a rat model of immobilisation osteoporosis. Med. Biol. Eng. Comput. 2000, 38, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.D.; Shen, C.; Jiang, L.S.; Dai, L.Y. Differences of bone mass and bone structure in osteopenic rat models caused by spinal cord injury and ovariectomy. Osteoporos. Int. 2007, 18, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Fournier, C.; Rizzoli, R.; Bouzakri, K.; Ammann, P. Selective protein depletion impairs bone growth and causes liver fatty infiltration in female rats: Prevention by Spirulina alga. Osteoporos. Int. 2016, 27, 3365–3376. [Google Scholar] [CrossRef]
- Cheong, S.H.; Kim, M.Y.; Sok, D.E.; Hwang, S.Y.; Kim, J.H.; Kim, H.R.; Lee, J.H.; Kim, Y.B.; Kim, M.R. Spirulina prevents atherosclerosis by reducing hypercholesterolemia in rabbits fed a high-cholesterol diet. J. Nutr. Sci. Vitaminol. (Tokyo) 2010, 56, 34–40. [Google Scholar] [CrossRef]
- Kim, M.Y.; Cheong, S.H.; Lee, J.H.; Kim, M.J.; Sok, D.E.; Kim, M.R. Spirulina improves antioxidant status by reducing oxidative stress in rabbits fed a high-cholesterol diet. J. Med. Food 2010, 13, 420–426. [Google Scholar] [CrossRef]
- Nyberg, F.; Burman, P. Growth hormone and its receptors in the central nervous system--location and functional significance. Horm. Res. Paediatr. 1996, 45, 18–22. [Google Scholar] [CrossRef]
- Forshee, B.A. The aging brain: Is function dependent on growth hormone/insulin-like growth factor-1 signaling? Age 2006, 28, 173–180. [Google Scholar] [CrossRef][Green Version]
- Laron, Z. Childhood-onset growth hormone deficiency, cognitive function and brain N-acetylaspartate. Psychoneuroendocrinology 2006, 31, 680. [Google Scholar] [CrossRef]
- Lazurova, I.; Pura, M.; Wagnerova, H.; Tajtakova, M.; Sedlakova, M.; Tomas, L.; Payer, J.; Hruzikova, P.; Vanuga, P.; Podoba, J.; et al. Effect of growth hormone replacement therapy on plasma brain natriuretic peptide concentration, cardiac morphology and function in adults with growth hormone deficiency. Exp. Clin. Endocrinol. Diabetes 2010, 118, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Nashiro, K.; Guevara-Aguirre, J.; Braskie, M.N.; Hafzalla, G.W.; Velasco, R.; Balasubramanian, P.; Wei, M.; Thompson, P.M.; Mather, M.; Nelson, M.D.; et al. Brain Structure and Function Associated with Younger Adults in Growth Hormone Receptor-Deficient Humans. J. Neurosci. 2017, 37, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- van Dam, P.S.; de Winter, C.F.; de Vries, R.; van der Grond, J.; Drent, M.L.; Lijffijt, M.; Kenemans, J.L.; Aleman, A.; de Haan, E.H.; Koppeschaar, H.P. Childhood-onset growth hormone deficiency, cognitive function and brain N-acetylaspartate. Psychoneuroendocrinology 2005, 30, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Webb, E.A.; O’Reilly, M.A.; Clayden, J.D.; Seunarine, K.K.; Chong, W.K.; Dale, N.; Salt, A.; Clark, C.A.; Dattani, M.T. Effect of growth hormone deficiency on brain structure, motor function and cognition. Brain 2012, 135, 216–227. [Google Scholar] [CrossRef]
- Zhang, H.; Han, M.; Zhang, X.; Sun, X.; Ling, F. The effect and mechanism of growth hormone replacement on cognitive function in rats with traumatic brain injury. PLoS ONE 2014, 9, e108518. [Google Scholar] [CrossRef]
- Baumann, G. Genetic characterization of growth hormone deficiency and resistance: Implications for treatment with recombinant growth hormone. Am. J. Pharmacogenomics 2002, 2, 93–111. [Google Scholar] [CrossRef]
- Juul, A.; Kastrup, K.W.; Pedersen, S.A.; Skakkebaek, N.E. Growth hormone (GH) provocative retesting of 108 young adults with childhood-onset GH deficiency and the diagnostic value of insulin-like growth factor I (IGF-I) and IGF-binding protein-3. J. Clin. Endocrinol. Metab. 1997, 82, 1195–1201. [Google Scholar] [CrossRef][Green Version]
- Coetzee, M.; Kruger, M.C. Osteoprotegerin-receptor activator of nuclear factor-kappaB ligand ratio: A new approach to osteoporosis treatment? South. Med. J. 2004, 97, 506–511. [Google Scholar] [CrossRef]
- Xu, F.; Gao, Z.; Zhang, J.; Rivera, C.A.; Yin, J.; Weng, J.; Ye, J. Lack of SIRT1 (Mammalian Sirtuin 1) activity leads to liver steatosis in the SIRT1+/- mice: A role of lipid mobilization and inflammation. Endocrinology 2010, 151, 2504–2514. [Google Scholar] [CrossRef]
- Barclay, J.L.; Nelson, C.N.; Ishikawa, M.; Murray, L.A.; Kerr, L.M.; McPhee, T.R.; Powell, E.E.; Waters, M.J. GH-dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology 2011, 152, 181–192. [Google Scholar] [CrossRef]
- Bao, Y.; Ma, X.; Yang, R.; Wang, F.; Hao, Y.; Dou, J.; He, H.; Jia, W. Inverse relationship between serum osteocalcin levels and visceral fat area in Chinese men. J. Clin. Endocrinol. Metab. 2013, 98, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Addai, D.; Zarkos, J.; Tolekova, A. The bone hormones and their potential effects on glucose and energy metabolism. Endocr. Regul. 2019, 53, 268–273. [Google Scholar] [CrossRef]
- Bae, S.J.; Choe, J.W.; Chung, Y.E.; Kim, B.J.; Lee, S.H.; Kim, H.Y.; Koh, J.M.; Kim, H.K.; Kim, G.S. The association between serum osteocalcin levels and metabolic syndrome in Koreans. Osteoporos. Int. 2011, 22, 2837–2846. [Google Scholar] [CrossRef] [PubMed]
- Buday, B.; Kulcsar, E.; Literati Nagy, B.; Horvath, T.; Vitai, M.; Vecsei, I.; Bezzegh, K.; Kiss, J.; Peterfai, E.; Koltay, L.; et al. [The role of osteocalcin in the connection of bone and glucose metabolism in humans]. Orv. Hetil. 2008, 149, 2453–2461. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Q.; Yang, Z.; Ye, Z.; Huang, Y.; He, M.; Wen, J.; Wang, X.; Lu, B.; Hu, J.; et al. Osteocalcin, glucose metabolism, lipid profile and chronic low-grade inflammation in middle-aged and elderly Chinese. Diabet. Med. 2013, 30, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Guedes, J.A.C.; Esteves, J.V.; Morais, M.R.; Zorn, T.M.; Furuya, D.T. Osteocalcin improves insulin resistance and inflammation in obese mice: Participation of white adipose tissue and bone. Bone 2018, 115, 68–82. [Google Scholar] [CrossRef]
- Wu, L.C.; Ho, J.A.; Shieh, M.C.; Lu, I.W. Antioxidant and antiproliferative activities of Spirulina and Chlorella water extracts. J. Agric. Food Chem. 2005, 53, 4207–4212. [Google Scholar] [CrossRef]
- Miranda, M.S.; Cintra, R.G.; Barros, S.B.; Mancini Filho, J. Antioxidant activity of the microalga Spirulina maxima. Braz. J. Med. Biol. Res. 1998, 31, 1075–1079. [Google Scholar] [CrossRef]
- Bhat, V.B.; Madyastha, K.M. Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: Protection against oxidative damage to DNA. Biochem. Biophys. Res. Commun. 2001, 285, 262–266. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Triratana, P.; Loha, V.; Tia, S.; Bunnag, B. Polysaccharide extraction from Spirulina sp. and its antioxidant capacity. Int. J. Biol. Macromol. 2013, 58, 73–78. [Google Scholar] [CrossRef]
- Koh, E.J.; Kim, K.J.; Song, J.H.; Choi, J.; Lee, H.Y.; Kang, D.H.; Heo, H.J.; Lee, B.Y. Spirulina maxima Extract Ameliorates Learning and Memory Impairments via Inhibiting GSK-3beta Phosphorylation Induced by Intracerebroventricular Injection of Amyloid-beta 1-42 in Mice. Int. J. Mol. Sci. 2017, 18, 2401. [Google Scholar] [CrossRef]
- Khan, M.; Shobha, J.C.; Mohan, I.K.; Naidu, M.U.; Sundaram, C.; Singh, S.; Kuppusamy, P.; Kutala, V.K. Protective effect of Spirulina against doxorubicin-induced cardiotoxicity. Phytother. Res. 2005, 19, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Shobha, J.C.; Mohan, I.K.; Rao Naidu, M.U.; Prayag, A.; Kutala, V.K. Spirulina attenuates cyclosporine-induced nephrotoxicity in rats. J. Appl. Toxicol. 2006, 26, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Colla, L.M.; Oliveira Reinehr, C.; Reichert, C.; Costa, J.A. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour. Technol. 2007, 98, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.R.; Prassad, V.S.; Das, U.N. Intratumoural injection of gamma leinolenic acid in malignant gliomas. J. Clin. Neurosci. 1998, 5, 36–39. [Google Scholar] [CrossRef]
- Jenkins, A.P.; Thompson, R.P. Trophic effect of Efamol on the rat small-intestinal mucosa. Clin. Sci. (Lond.) 1989, 77, 555–559. [Google Scholar] [CrossRef]
- Uccella, R.; Contini, A.; Sartorio, M. [Action of evening primrose oil on cardiovascular risk factors in insulin-dependent diabetics]. La Clinica Terapeutica 1989, 129, 381–388. [Google Scholar]
- Ishikawa, T.; Fujiyama, Y.; Igarashi, O.; Morino, M.; Tada, N.; Kagami, A.; Sakamoto, T.; Nagano, M.; Nakamura, H. Effects of gammalinolenic acid on plasma lipoproteins and apolipoproteins. Atherosclerosis 1989, 75, 95–104. [Google Scholar] [CrossRef]
- Maggioli, C.; Stagi, S. Bone modeling, remodeling, and skeletal health in children and adolescents: Mineral accrual, assessment and treatment. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 1–5. [Google Scholar] [CrossRef]
- Buck, D.W., 2nd; Dumanian, G.A. Bone biology and physiology: Part II. Clinical correlates. Plast. Reconstr. Surg. 2012, 129, 950e–956e. [Google Scholar] [CrossRef]
- Buck, D.W., 2nd; Dumanian, G.A. Bone biology and physiology: Part I. The fundamentals. Plast. Reconstr. Surg. 2012, 129, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Spangler, J.G. Bone biology and physiology: Implications for novel osteoblastic osteosarcoma treatments? Med. Hypotheses 2008, 70, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Iwaniec, U.T.; Yuan, D.; Power, R.A.; Wronski, T.J. Strain-dependent variations in the response of cancellous bone to ovariectomy in mice. J. Bone Miner. Res. 2006, 21, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H. Bone strength: Current concepts. Ann. N. Y. Acad. Sci. 2006, 1068, 429–446. [Google Scholar] [CrossRef]
- Thompson, D.D.; Simmons, H.A.; Pirie, C.M.; Ke, H.Z. FDA Guidelines and animal models for osteoporosis. Bone 1995, 17, 125S–133S. [Google Scholar] [CrossRef]
- Bagi, C.M.; Wilkie, D.; Georgelos, K.; Williams, D.; Bertolini, D. Morphological and structural characteristics of the proximal femur in human and rat. Bone 1997, 21, 261–267. [Google Scholar] [CrossRef]
- Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Cerrudo, V.; Audebert, M.; Dumont, F.; Mancano, G.; Khodorova, N.; Andriamihaja, M.; et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: A randomized, parallel, double-blind trial in overweight humans. Am. J. Clin. Nutr. 2017, 106, 1005–1019. [Google Scholar] [CrossRef]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef]
- Pi, Y.; Gao, K.; Peng, Y.; Mu, C.L.; Zhu, W.Y. Antibiotic-induced alterations of the gut microbiota and microbial fermentation in protein parallel the changes in host nitrogen metabolism of growing pigs. Animal 2019, 13, 262–272. [Google Scholar] [CrossRef]
- Mafra, D.; Barros, A.F.; Fouque, D. Dietary protein metabolism by gut microbiota and its consequences for chronic kidney disease patients. Future Microbiol. 2013, 8, 1317–1323. [Google Scholar] [CrossRef]
- Nakatani, A.; Li, X.; Miyamoto, J.; Igarashi, M.; Watanabe, H.; Sutou, A.; Watanabe, K.; Motoyama, T.; Tachibana, N.; Kohno, M.; et al. Dietary mung bean protein reduces high-fat diet-induced weight gain by modulating host bile acid metabolism in a gut microbiota-dependent manner. Biochem. Biophys. Res. Commun. 2018, 501, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Chi, L.; Tu, P.; Xue, J.; Ru, H.; Lu, K. Quantitative proteomics reveals systematic dysregulations of liver protein metabolism in sucralose-treated mice. J. Proteom. 2019, 196, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hua, P.; Xiong, Y.; Yu, Z.; Liu, B.; Zhao, L. Effect of Chlorella Pyrenoidosa Protein Hydrolysate-Calcium Chelate on Calcium Absorption Metabolism and Gut Microbiota Composition in Low-Calcium Diet-Fed Rats. Mar. Drugs 2019, 17, 348. [Google Scholar] [CrossRef] [PubMed]

| Experimental Group | Diet | Number of Mice |
|---|---|---|
| Control | AIN93G | 17 |
| SP30 | 30% of AIN93G replaced by spirulina | 17 |
| SP50 | 50% of AIN93G replaced by spirulina | 17 |
| SP70 | 70% of AIN93G replaced by spirulina | 17 |
| Total | 68 |
| Composition | |||
|---|---|---|---|
| Macronutrients (g/100 g) | Phytonutrients (mg/100 g) | ||
| Calories (kcal/100 g) | 360.70 | Phycocyanin | 8000.00 |
| Moisture (%/100 g) | 8.70 | Chlorophyll a | 1300.00 |
| Carbohydrate | 17.50 | ||
| Fat | 4.30 | ||
| Protein | 63.00 | ||
| Dietary fiber | 6.50 | ||
| Vitamins (mg/100 g) | Minerals (mg/100 g) | ||
| Vitamin A | 2.95 | Calcium | 98.80 |
| β-Carotene | 177.00 | Iron | 40.90 |
| Vitamin B1 | 3.08 | Phosphorus | 859.00 |
| Vitamin B2 | 3.74 | Magnesium | 319.00 |
| Vitamin B6 | 0.83 | Zinc | 1.28 |
| Vitamin B12 | 0.18 | Copper | 0.32 |
| Vitamin E | 12.70 | Manganese | 3.77 |
| α-Tocopherol | 12.50 | Chromium | 0.06 |
| β-Tocopherol | 0.50 | Potassium | 1560.00 |
| Vitamin K1 | 1.59 | ||
| Vitamin K2 | 0.08 | ||
| Folic acid | 0.08 | ||
| Niacin | 23.50 | ||
| Components (g/kg Diet) | Group | |||
|---|---|---|---|---|
| Control | SP30 | SP50 | SP70 | |
| Casein | 200.00 | 140.00 | 100.00 | 60.00 |
| Corn starch | 397.49 | 377.28 | 363.81 | 350.34 |
| Dyetrose | 132.00 | 132.00 | 132.00 | 132.00 |
| Sucrose | 100.00 | 100.00 | 100.00 | 100.00 |
| Cellulose | 50.00 | 43.81 | 39.68 | 35.56 |
| Soybean oil | 70.00 | 65.91 | 63.18 | 60.45 |
| t-Butylhydroquinone | 0.01 | 0.01 | 0.01 | 0.01 |
| Salt mix | 35.00 | 31.06 | 28.43 | 25.80 |
| Vitamin mix | 10.00 | 9.20 | 8.66 | 8.13 |
| L-cystine | 3.00 | 3.00 | 3.00 | 3.00 |
| Choline bitartrate | 2.50 | 2.50 | 2.50 | 2.50 |
| Spirulina | 0.00 | 95.24 | 158.73 | 222.22 |
| TOTAL | 1000.00 | 1000.00 | 1000.00 | 1000.00 |
| Total energy (kcal) | 3948.07 | 3945.63 | 3944.00 | 3942.29 |
| Nutrition from spirulina | ||||
| Energy (kcal) | 0 | 355.24 | 592.06 | 828.81 |
| Protein | 0 | 60.00 | 100.00 | 139.99 |
| Fat | 0 | 4.10 | 6.83 | 9.56 |
| Carbohydrate | 0 | 16.67 | 27.78 | 38.89 |
| Fiber | 0 | 6.19 | 10.32 | 14.44 |
| Total vitamin | 0 | 0.80 | 1.34 | 1.87 |
| Total mineral | 0 | 3.95 | 6.57 | 9.20 |
| Control | SP30 | SP50 | SP70 | ||
|---|---|---|---|---|---|
| 3 weeks | Weight (g) | 179.1 ± 11.0 NS | 188.7 ± 2.2 | 180.4 ± 5.3 | 176.9 ± 6.1 |
| Food intake (g/d) | 24.6 ± 0.6 NS | 24.6 ± 0.4 | 24.4 ± 0.4 | 24.6 ± 0.4 | |
| FER (%) | 13.9 ± 0.6 NS | 13.0 ± 0.3 | 13.6 ± 0.2 | 13.9 ± 0.3 | |
| 7 weeks | Weight (g) | 378.9 ± 12.2 NS | 356.6 ± 11.8 | 341.8 ± 2.5 | 369.4 ± 13.4 |
| Food intake (g/d) | 30.0 ± 1.0 NS | 28.3 ± 0.8 | 27.5 ± 0.6 | 26.7 ± 1.7 | |
| FER (%) | 8.0 ± 0.3 NS | 7.9 ± 0.2 | 8.1 ± 0.2 | 8.2 ± 0.3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.A.; Baek, S.Y.; Cheong, S.H.; Kim, M.R. Spirulina Enhances Bone Modeling in Growing Male Rats by Regulating Growth-Related Hormones. Nutrients 2020, 12, 1187. https://doi.org/10.3390/nu12041187
Cho JA, Baek SY, Cheong SH, Kim MR. Spirulina Enhances Bone Modeling in Growing Male Rats by Regulating Growth-Related Hormones. Nutrients. 2020; 12(4):1187. https://doi.org/10.3390/nu12041187
Chicago/Turabian StyleCho, Jin Ah, Seong Yeon Baek, Sun Hee Cheong, and Mee Ree Kim. 2020. "Spirulina Enhances Bone Modeling in Growing Male Rats by Regulating Growth-Related Hormones" Nutrients 12, no. 4: 1187. https://doi.org/10.3390/nu12041187
APA StyleCho, J. A., Baek, S. Y., Cheong, S. H., & Kim, M. R. (2020). Spirulina Enhances Bone Modeling in Growing Male Rats by Regulating Growth-Related Hormones. Nutrients, 12(4), 1187. https://doi.org/10.3390/nu12041187







