Does ENaC Work as Sodium Taste Receptor in Humans?
Abstract
:1. Introduction
2. Psychophysics
3. Electrophysiology
4. Molecular Biology and Immunohistochemistry
5. Genetics
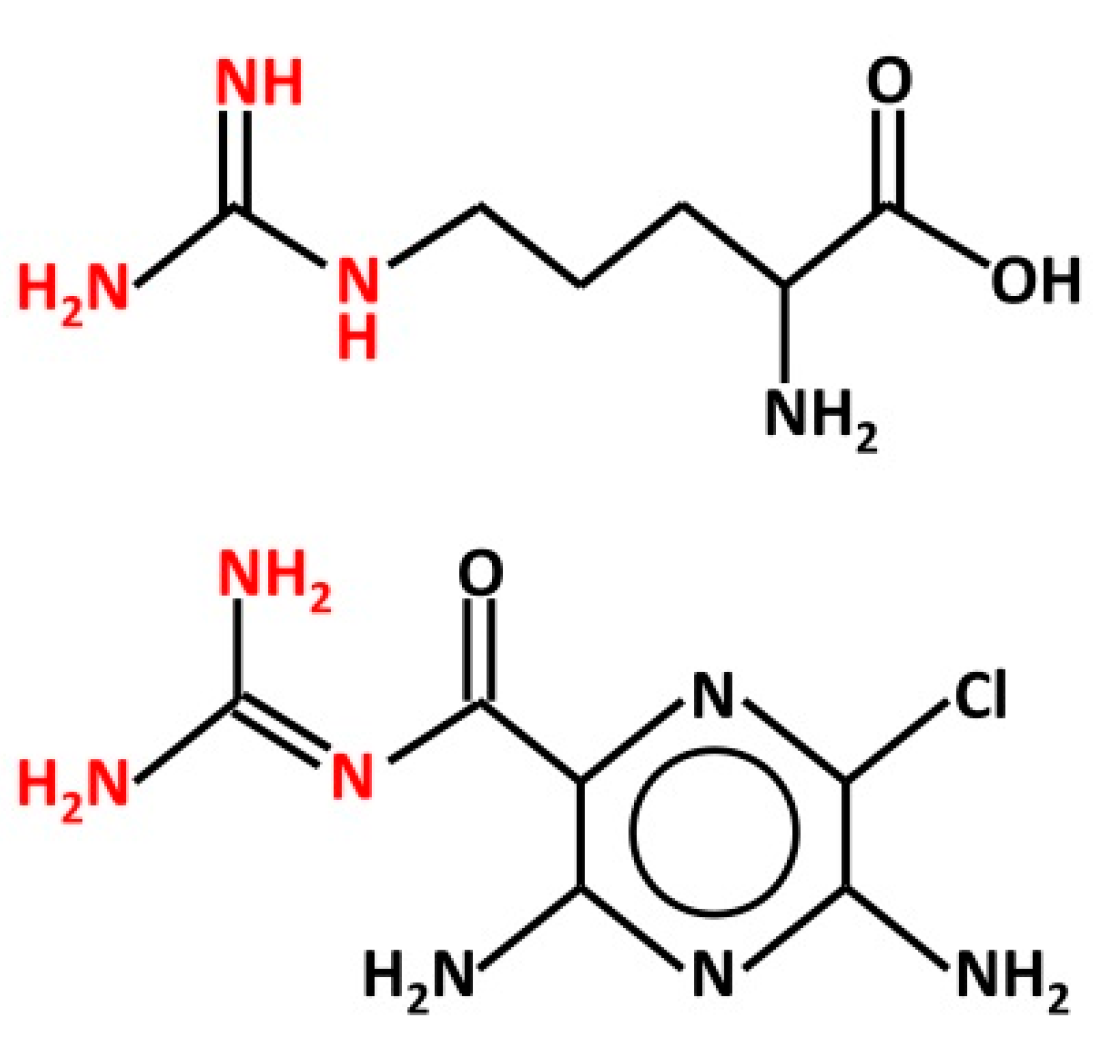
6. Salt Taste Enhancers
7. Salivary Proteins
8. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Schiffman, S.S.; Erickson, R.P. A psychophysical model for gustatory quality. Physiol. Behav. 1971, 7, 617–633. [Google Scholar] [CrossRef]
- Mattes, R.D. The taste for salt in humans. Am. J. Clin. Nutr. 1997, 65, 692S–697S. [Google Scholar] [CrossRef] [Green Version]
- Lindemann, B. Taste reception. Physiol. Rev. 1996, 76, 718–766. [Google Scholar] [CrossRef]
- McCaughey, S.A.; Scott, T.R. The taste of sodium. Neurosci. Biobehav. Rev. 1998, 22, 663–676. [Google Scholar] [CrossRef]
- Bigiani, A. Salt taste. In The Senses. Olfaction and Taste, 2nd ed.; Meyerhof, W., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume III, in press. [Google Scholar] [CrossRef]
- Breslin, P.A.; Huang, L. Human taste: Peripheral anatomy, taste transduction, and coding. Adv. Otorhinolaryngol. 2006, 63, 152–190. [Google Scholar] [CrossRef]
- Witt, M. Anatomy and development of the human taste system. In Handbook of clinical neurology. Smell and taste; Doty, R.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 164, pp. 147–171. [Google Scholar]
- Bigiani, A. Electrophysiology of sodium receptors in taste cells. J. Biomed. Sci. Eng. 2016, 9, 367–383. [Google Scholar] [CrossRef] [Green Version]
- Heck, G.L.; Mierson, S.; DeSimone, J.A. Salt taste transduction occurs through an amiloride-sensitivesodium transport pathway. Science 1984, 223, 403–405. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Kuhn, C.; Oka, Y.; Yarmolinsky, D.A.; Hummler, E.; Ryba, N.J.P.; Zuker, C.S. The cells and peripheral representation of sodium taste in mice. Nature 2010, 464, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Schiffman, S.S.; Lockhead, E.; Maes, F.W. Amiloride redcuces the taste intensity of Na+ and Li+ salts and sweeteners. Proc. Natl. Acad. Sci. USA 1983, 80, 6136–6140. [Google Scholar] [CrossRef] [Green Version]
- Tennissen, A.M. Amiloride reduces intensity responses of human fungiform papillae. Physiol. Behav. 1992, 51, 1061–1068. [Google Scholar] [CrossRef]
- McCutcheon, N.B. Human psychophysical studies of saltiness suppression by amiloride. Physiol. Behav. 1992, 51, 1069–1074. [Google Scholar] [CrossRef]
- Tennissen, A.M.; McCutcheon, N.B. Anterior tongue stimulation with amiloride suppress NaCl saltiness, but not citric acid sourness in humans. Chem. Senses 1996, 21, 113–120. [Google Scholar] [CrossRef]
- Anand, K.K.; Zuniga, J.R. Effect of amiloride on suprathreshold NaCl, LiCl, and KCl salt taste in humans. Physiol. Behav. 1997, 62, 925–929. [Google Scholar] [CrossRef]
- Desor, J.A.; Finn, J. Effects of amiloride on salt taste in humans. Chem. Senses 1989, 14, 793–803. [Google Scholar] [CrossRef]
- Ossebaard, C.A.; Smith, D.V. Effect of amiloride on the taste of NaCl, Na-gluconate and KCl in humans: Implications for Na+ receptor mechanisms. Chem. Senses 1995, 20, 37–46. [Google Scholar] [CrossRef]
- Smith, D.V.; Ossebaard, C.A. Amiloride suppression of the taste intensity of sodium chloride: Evidence from direct magnitude scaling. Physiol. Behav. 1995, 4, 773–777. [Google Scholar] [CrossRef]
- Ossebaard, C.A.; Smith, D.V. Amiloride suppresses the sourness of NaCl and LiCl. Physiol. Behav. 1996, 60, 1317–1322. [Google Scholar] [CrossRef]
- Ossebaard, C.A.; Polet, I.A.; Smith, D.V. Amiloride effects on taste quality: Comparison of single and multiple response category procedures. Chem. Senses 1997, 22, 267–275. [Google Scholar] [CrossRef]
- Halpern, B.P.; Darlington, R.B. Effects of amiloride on gustatory quality descriptions and temporal patterns produced by NaCl. Chem. Senses 1998, 23, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Simon, S.A.; de Araujo, I.E.; Stapleton, J.R.; Nicolelis, M.A. Multisensory processing of gustatory stimuli. Chemosens. Percept. 2008, 1, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Kleyman, T.R.; Cragoe, E.J., Jr. Amiloride and its analogs as tools in the study of ion transport. J. Membr. Biol. 1988, 105, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-M.; Presser, F.; Morad, M. Amiloride selectively blocks the low threshold (T) calcium channel. Science 1988, 240, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Béhé, P.; DeSimone, J.A.; Avenet, P.; Lindemann, B. Membrane currents in taste cells of the rat fungiform papilla. J. Gen. Physiol. 1990, 96, 1061–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigiani, A.; Cuoghi, V. Localization of amiloride-sensitive sodium current and voltage-gated calcium currents in rat fungiform taste cells. J. Neurophysiol. 2007, 98, 2483–2487. [Google Scholar] [CrossRef]
- Kinsella, J.L.; Aronson, P.S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am. J. Physiol. 1981, 241, F374–F379. [Google Scholar] [CrossRef]
- Lundy, R.F., Jr.; Pittman, D.W.; Contreras, R.J. Role for epithelial Na+ channels and putative Na+/H+ exchangers in salt taste transduction in rats. Am. J. Physiol. 1997, 273, R1923–R1931. [Google Scholar] [CrossRef]
- Vinnikova, A.K.; Alam, R.I.; Malik, S.A.; Ereso, G.L.; Feldman, G.M.; McCarty, J.M.; Knepper, M.A.; Heck, G.L.; DeSimone, J.A.; Lyall, V. Na+-H+ exchange activity in taste receptor cells. J. Neurophysiol. 2004, 91, 1297–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyall, V.; Alam, R.I.; Malik, S.A.; Phan, T.H.; Vinnikova, A.K.; Heck, G.L.; DeSimone, J.A. Basolateral Na+-H+ exchanger-1 in rat taste receptor cells is involved in neural adaptation to acidic stimuli. J. Physiol. 2004, 556, 159–173. [Google Scholar] [CrossRef]
- Kuijpers, G.A.; De Pont, J.J.; Van Nooy, I.G.; Fleuren-Jakobs, A.M.; Bonting, S.L.; Rodrigues de Miranda, J.F. Amiloride is a cholinergic antagonist in the rabbit pancreas. Biochim. Biophys. Acta 1984, 804, 237–244. [Google Scholar] [CrossRef]
- Ogura, T. Acetylcholine increases intracellular Ca2+ in taste cells via activation of muscarinic receptors. J. Neurophysiol. 2002, 87, 2643–2649. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, K.; Ohtubo, Y.; Yoshii, K. Functional expression of M3, a muscarinic acetylcholine receptor subtype, in taste bud cells of mouse fungiform papillae. Chem. Senses 2008, 33, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSimone, J.A.; Heck, G.L.; DeSimone, S.K. Active ion transport in dog tissue: A possible role in taste. Science 1981, 214, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, J.A.; Heck, G.L.; Mierson, S.; DeSimone, S.K. The active ion transport properties of canine lingual epithelia in vitro. Implications forgustatory transduction. J. Gen. Physiol. 1984, 83, 633–656. [Google Scholar] [CrossRef] [Green Version]
- Feldman, G.M.; Mogyorósi, A.; Heck, G.L.; DeSimone, J.A.; Santos, C.R.; Clary, R.A.; Lyall, V. Salt-evoked lingual surface potential in humans. J. Neurophysiol. 2003, 90, 2060–2064. [Google Scholar] [CrossRef] [PubMed]
- Feldman, G.M.; Heck, G.L.; Smith, N.L. Human salt taste and the lingual surface potential correlate. Chem. Senses 2009, 34, 373–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benos, D.J.; Stanton, B.A. Functional domains within the degenerin/epithelial sodium channel (Deg/ENaC) superfamily of ion channels. J. Physiol. 1999, 520, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Kellenberger, S.; Schild, L. International Union of Basic and Clinical Pharmacology. XCI. structure, function, and pharmacology of acid-sensing ion channels and the epithelial Na+ channel. Pharmacol. Rev. 2015, 67, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Canessa, C.M.; Schild, L.; Buell, G.; Thorens, B.; Gautschi, I.; Horisberger, J.D.; Rossier, B.C. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 1994, 367, 463–467. [Google Scholar] [CrossRef]
- Lossow, K.; Hermans-Borgmeyer, I.; Meyerhof, W.; Behrens, M. Segregated expression of ENaC subunits in taste cells. Chem. Senses 2020, in press. [Google Scholar] [CrossRef]
- Simon, S.A.; Holland, V.F.; Benos, D.J.; Zampighi, G.A. Transcellular and paracellular pathways in lingual epithelia and their influence in taste transduction. Microsc. Res. Tech. 1993, 26, 196–208. [Google Scholar] [CrossRef]
- Kretz, O.; Barbry, P.; Bock, R.; Lindemann, B. Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J. Histochem. Cytochem. 1999, 47, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Finger, T.E.; Rossier, B.C.; Kinnamon, S.C. Epithelial Na+ channel subunits in rat taste cells: Localization and regulation by aldosterone. J. Comp. Neurol. 1999, 405, 406–420. [Google Scholar] [CrossRef]
- Shigemura, N.; Islam, A.A.; Sadamitsu, C.; Yoshida, R.; Yasumatsu, K.; Ninomiya, Y. Expression of amiloride-sensitive epithelial sodium channels in mouse taste cells after chorda tympani nerve crush. Chem. Senses 2005, 30, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, R.; Horio, N.; Murata, Y.; Yasumatsu, K.; Shigemura, N.; Ninomiya, Y. NaCl responsive taste cells in the mouse fungiform taste buds. Neuroscience 2009, 159, 795–803. [Google Scholar] [CrossRef]
- Rossier, O.; Cao, J.; Huque, T.; Spielman, A.I.; Feldman, R.S.; Medrano, J.F.; Brand, J.G.; le Coutre, J. Analysis of a human fungiform papillae cDNA library and identification of taste-related genes. Chem. Senses 2004, 29, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Stähler, F.; Riedel, K.; Demgensky, S.; Neumann, K.; Dunkel, A.; Täubert, A.; Raab, B.; Behrens, M.; Raguse, J.-D.; Hofmann, T.; et al. A role of the epithelial sodium channel in human salt taste transduction? Chemosens. Percept. 2008, 1, 78–90. [Google Scholar] [CrossRef]
- Huque, T.; Cowart, B.J.; Dankulich-Nagrudny, L.; Pribitkin, E.A.; Bayley, D.L.; Spielman, A.I.; Feldman, R.S.; Mackler, S.A.; Brand, J.G. Sour ageusia in two individuals implicates ion channels of the ASIC and PKD families in human sour taste perception at the anterior tongue. PLoS ONE 2009, 4, e7347. [Google Scholar] [CrossRef]
- Waldmann, R.; Champigny, G.; Bassilana, F.; Voilley, N.; Lazdunski, M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. J. Biol. Chem. 1995, 270, 27411–27414. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.-L.; LaToya, R.; Bishop, L.R.; Anderson, S.J.; Catherine, M.; Fuller, C.M.; Benos, D.J. The role of Pre-H2 domains of α- and δ-epithelial Na+ channels in ion permeation, conductance, and amiloride sensitivity. J. Biol. Chem. 2004, 279, 8428–8440. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.-L.; Su, X.-F.; Kedar, S.; Li, J.; Barbry, P.; Smith, P.R.; Matalon, S.; Benos, D.J. δ-subunit confers novel biophysical features to αβγ-human epithelial sodium channel (ENaC) via a physical interaction. J. Biol. Chem. 2006, 281, 8233–8241. [Google Scholar] [CrossRef] [Green Version]
- Bachmanov, A.A.; Bosak, N.P.; Lin, C.; Matsumoto, I.; Ohmoto, M.; Reed, D.R.; Nelson, T.M. Genetics of taste receptors. Curr. Pharmaceut. Des. 2014, 20, 2669–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrik, D.; Myoga, M.H.; Grade, S.; Gerkau, N.J.; Pusch, M.; Rose, C.R.; Grothe, B.; Götz, M. Epithelial sodium channel regulates adult neural stem cell proliferation in a flow-dependent manner. Cell Stem Cell 2018, 22, 865–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, P.; Huang, L.; Wang, H. Taste bud homeostasis in health, disease, and aging. Chem. Senses 2014, 39, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barlow, L.A. Progress and renewal in gustation: New insights into taste bud development. Development 2015, 142, 3620–3629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, A.G.; Rousseau, D.; Duizer, L.; Cockburn, M.; Chiu, W.; Nielsen, D.; El-Sohemy, A. Genetic variation in putative salt taste receptors and salt taste perception in humans. Chem. Senses 2013, 38, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Firsov, D.; Schild, L.; Gautschi, I.; Mérillat, A.M.; Schneeberger, E.; Rossier, B.C. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: A quantitative approach. Proc. Natl. Acad. Sci. USA 1996, 93, 15370–15375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstas, A.A.; Korbmacher, C. The γ-subunit of ENaC is more important for channel surface expression than the β-subunit. Am. J. Physiol. Cell Physiol. 2003, 284, C447–C456. [Google Scholar] [CrossRef] [Green Version]
- Contreras, A.C. Salt taste and disease. Am. J. Clin. Nutr. 1978, 31, 1088–1097. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, G.K. The human preference for excess salt. Am. Sci. 1987, 75, 27–33. [Google Scholar]
- Duncan, C.J. Salt preference of birds and mammals. Physiol. Zool. 1962, 35, 120–132. [Google Scholar] [CrossRef]
- Oka, Y.; Butnaru, M.; von Buchholtz, L.; Ryba, N.J.; Zuker, C.S. High salt recruits aversive taste pathways. Nature 2013, 494, 472–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dötsch, M.; Busch, J.; Batenburg, M.; Liem, G.; Tareilus, E.; Mueller, R.; Meijer, G. Strategies to reduce sodium consumption: A food industry perspective. Crit. Rev. Food Sci. Nutr. 2009, 49, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Burnier, M.; Wuerzner, G.; Bochud, M. Salt, blood pressure and cardiovascular risk: What is the most adequate preventive strategy? A Swiss perspective. Front. Physiol. 2015, 6, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J. Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Global sodium consumption and death from cardiovascular causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.J.; Elkaddi, N.; Garcia-Blanco, A.; Spielman, A.I.; Bachmanov, A.A.; Chung, H.Y.; Ozdener, M.H. Arginyl dipeptides increase the frequency of NaCl-elicited responses via epithelial sodium channel alpha and delta subunits in cultured human fungiform taste papillae cells. Sci. Rep. 2017, 7, 7483. [Google Scholar] [CrossRef]
- Schindler, A.; Dunkel, A.; Stähler, F.; Backes, M.; Ley, J.; Meyerhof, W.; Hofmann, T. Discovery of salt taste enhancing arginyl dipeptides in protein digests and fermented fish sauces by means of a sensomics approach. J. Agric. Food Chem. 2011, 59, 12578–12588. [Google Scholar] [CrossRef]
- Kellenberger, S.; Schild, L. Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiol. Rev. 2002, 82, 735–767. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, T.; Nakamura, T.; Tsuji, E.; Miyanaga, Y.; Nakagawa, H.; Hirabayashi, H.; Uchida, T. The combination effect of L-arginine and NaCl on bitterness suppression of amino acid solutions. Chem. Pharm. Bull. 2004, 52, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Stolle, T.; Grondinger, F.; Dunkel, A.; Meng, C.; Médard, G.; Kuster, B.; Hofmann, T. Salivary proteome patterns affecting human salt taste sensitivity. J. Agric. Food Chem. 2017, 65, 9275–9286. [Google Scholar] [CrossRef]
- Hughey, R.P.; Carattino, M.D.; Kleyman, T.R. Role of proteolysis in the activation of epithelial sodium channels. Curr. Opin. Nephrol. Hypertens. 2007, 16, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kleyman, T.R.; Carattino, M.D.; Hughey, R.P. ENaC at the cutting edge: Regulation of epithelial sodium channels by proteases. J. Biol. Chem. 2009, 284, 20447–20451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolle, T.; Grondinger, F.; Dunkel, A.; Hofmann, T. Quantitative proteomics and SWATH-MS to elucidate peri-receptor mechanisms in human salt taste sensitivity. Food Chem. 2018, 254, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-J.; Blackshaw, S.; Snyder, S.H. Expression and localization of amiloride-sensitive sodium channel indicate a role for non-taste cells in taste perception. Proc. Natl. Acad. Sci. USA 1994, 91, 1814–1818. [Google Scholar] [CrossRef] [Green Version]
- Weisz, O.A.; Johnson, J.P. Noncoordinate regulation of ENaC: Paradigm lost? Am. J. Physiol. Ren. Physiol. 2002, 285, F833–F842. [Google Scholar] [CrossRef] [Green Version]
| Molecular Target | Ki (µM) | Cell/Tissue | Occurrence in Rodent Taste Cells |
|---|---|---|---|
| T-type calcium channel | 30 | Mouse neuroblastoma and chick DRG 1 neurons [24] | [25,26] |
| Na+/H+ exchanger | 30 | Rabbit renal microvillous membrane [27] | [28,29,30] |
| Muscarinic receptors | 40–80 | Rabbit pancreatic acini [31] | [32,33] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bigiani, A. Does ENaC Work as Sodium Taste Receptor in Humans? Nutrients 2020, 12, 1195. https://doi.org/10.3390/nu12041195
Bigiani A. Does ENaC Work as Sodium Taste Receptor in Humans? Nutrients. 2020; 12(4):1195. https://doi.org/10.3390/nu12041195
Chicago/Turabian StyleBigiani, Albertino. 2020. "Does ENaC Work as Sodium Taste Receptor in Humans?" Nutrients 12, no. 4: 1195. https://doi.org/10.3390/nu12041195
APA StyleBigiani, A. (2020). Does ENaC Work as Sodium Taste Receptor in Humans? Nutrients, 12(4), 1195. https://doi.org/10.3390/nu12041195





