Feeding Formula Eliminates the Necessity of Bacterial Dysbiosis and Induces Inflammation and Injury in the Paneth Cell Disruption Murine NEC Model in an Osmolality-Dependent Manner
Abstract
:1. Introduction
2. Methods
2.1. Animals and Feeding Protocols
2.2. Formula NEC Models
2.3. Serum and Cytokine Analysis
2.4. Microbiota Analysis
2.5. Injury Scoring
2.6. Statistical Analysis
3. Results
3.1. Formula Feeding in Combination with Dithizone Treatment Results in Inflammation and Removes the Requirement for Bacterial Exposure to Induce NEC-Like Injury in the Immature Murine Intestine
3.2. Paneth Cell Disruption/Formula-Induced NEC is not Dithizone-Dependent
3.3. Immature Murine Generalized Intestinal Injury and Newborn Mortality Induced by Formula Feeding Is Osmolality-Dependent
3.4. Exposure to Mannitol-Increased Osmolality Induced Significant Alterations in the Composition of the Cecal Microbiome in the Immature Intestine
3.5. Osmolality-Induced Effects Are Dependent on the Methodology Used to Increase the Solute Level
3.6. High Osmolality RMS Deceased Survival When Included in Dithizone Paneth Cell Disruption and Formula Feeding NEC Model
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fitzgibbons, S.C.; Ching, Y.; Yu, D.; Carpenter, J.; Kenny, M.; Weldon, C.; Lillehei, C.; Valim, C.; Horbar, J.D.; Jaksic, T. Mortality of necrotizing enterocolitis expressed by birth weight categories. J. Pediatr. Surg. 2009, 44, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Kandefer, S.; Walsh, M.C.; Bell, E.F.; Carlo, W.A.; Laptook, A.R.; Sanchez, P.J.; Shankaran, S.; Van Meurs, K.P.; Ball, M.B.; et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N. Engl. J. Med. 2015, 372, 331–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.W.; Nasr, T.R.; Stoll, B.J. Necrotizing enterocolitis: Recent scientific advances in pathophysiology and prevention. Semin. Perinatol. 2008, 32, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Chung, M.Y.; Chang, J.H.; Lin, H.C. Pathogenesis implication for necrotizing enterocolitis prevention in preterm very-low-birth-weight infants. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet 2012, 13, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Elgin, T.G.; Kern, S.L.; McElroy, S.J. Development of the neonatal intestinal microbiome and its association with necrotizing enterocolitis. Clin. Ther. 2016, 38, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Morrow, A.L.; Lagomarcino, A.J.; Schibler, K.R.; Taft, D.H.; Yu, Z.; Wang, B.; Altaye, M.; Wagner, M.; Gevers, D.; Ward, D.V.; et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome 2013, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Niemarkt, H.J.; de Meij, T.G.; van de Velde, M.E.; van der Schee, M.P.; van Goudoever, J.B.; Kramer, B.W.; Andriessen, P.; de Boer, N.K. Necrotizing enterocolitis: A clinical review on diagnostic biomarkers and the role of the intestinal microbiota. Inflamm. Bowel Dis. 2015, 21, 436–444. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.Y.; Ni, Y.H. Gut microbiota and the development of pediatric diseases. J. Gastroenterol. 2015, 50, 720–726. [Google Scholar] [CrossRef]
- Claud, E.C.; Keegan, K.P.; Brulc, J.M.; Lu, L.; Bartels, D.; Glass, E.; Chang, E.B.; Meyer, F.; Antonopoulos, D.A. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome 2013, 1, 20. [Google Scholar] [CrossRef] [Green Version]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Good, M.; Siggers, R.H.; Sodhi, C.P.; Afrazi, A.; Alkhudari, F.; Egan, C.E.; Neal, M.D.; Yazji, I.; Jia, H.; Lin, J.; et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc. Natl. Acad. Sci. USA 2012, 109, 11330–11335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Good, M.; Sodhi, C.P.; Egan, C.E.; Afrazi, A.; Jia, H.; Yamaguchi, Y.; Lu, P.; Branca, M.F.; Ma, C.; Prindle, T., Jr.; et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 2015, 8, 1166–1179. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.D.; Sodhi, C.P.; Dyer, M.; Craig, B.T.; Good, M.; Jia, H.; Yazji, I.; Afrazi, A.; Richardson, W.M.; Beer-Stolz, D.; et al. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J. Immunol. 2013, 190, 3541–3551. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.J.; Singamsetty, V.K.; Chokshi, N.K.; Boyle, P.; Camerini, V.; Grishin, A.V.; Upperman, J.S.; Ford, H.R.; Prasadarao, N.V. Enterobacter sakazakii enhances epithelial cell injury by inducing apoptosis in a rat model of necrotizing enterocolitis. J. Infect. Dis. 2008, 198, 586–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Managlia, E.; Liu, S.X.L.; Yan, X.; Tan, X.D.; Chou, P.M.; Barrett, T.A.; De Plaen, I.G. Blocking NF-kappaB Activation in Ly6c(+) Monocytes Attenuates Necrotizing Enterocolitis. Am. J. Pathol. 2019, 189, 604–618. [Google Scholar] [CrossRef] [Green Version]
- Lucas, A.; Cole, T.J. Breast milk and neonatal necrotising enterocolitis. Lancet 1990, 336, 1519–1523. [Google Scholar] [CrossRef]
- Liu, Y.; Fatheree, N.Y.; Mangalat, N.; Rhoads, J.M. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NFkappaB signaling in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2011. [Google Scholar] [CrossRef] [Green Version]
- McElroy, S.J.; Weitkamp, J.H. Innate immunity in the small intestine of the preterm infant. NeoReviews 2011, 12, e517–e526. [Google Scholar] [CrossRef] [Green Version]
- Stanford, A.H.; Gong, H.; Noonan, M.; Lewis, A.N.; Gong, Q.; Lanik, W.E.; Hsieh, J.J.; Lueschow, S.R.; Frey, M.R.; Good, M.; et al. A direct comparison of mouse and human intestinal development using epithelial gene expression patterns. Pediatr. Res. 2019. [Google Scholar] [CrossRef]
- Fung, C.M.; White, J.R.; Brown, A.S.; Gong, H.; Weitkamp, J.H.; Frey, M.R.; McElroy, S.J. Intrauterine growth restriction alters mouse intestinal architecture during development. PLoS ONE 2016, 11, e0146542. [Google Scholar] [CrossRef] [PubMed]
- McElroy, S.J.; Castle, S.L.; Bernard, J.K.; Almohazey, D.; Hunter, C.J.; Bell, B.A.; Al Alam, D.; Wang, L.; Ford, H.R.; Frey, M.R. The ErbB4 ligand neuregulin-4 protects against experimental necrotizing enterocolitis. Am. J. Pathol. 2014, 184, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sherman, M.P.; Prince, L.S.; Bader, D.; Weitkamp, J.H.; Slaughter, J.C.; McElroy, S.J. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis. Models Mech. 2012, 5, 522–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, J.R.; Gong, H.; Pope, B.; Schlievert, P.; McElroy, S.J. Paneth-cell-disruption-induced necrotizing enterocolitis in mice requires live bacteria and occurs independently of TLR4 signaling. Dis. Models Mech. 2017, 10, 727–736. [Google Scholar] [CrossRef] [Green Version]
- McElroy, S.J.; Prince, L.S.; Weitkamp, J.H.; Reese, J.; Slaughter, J.C.; Polk, D.B. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: A potential role in neonatal necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G656–G666. [Google Scholar] [CrossRef] [Green Version]
- McElroy, S.J.; Underwood, M.A.; Sherman, M.P. Paneth cells and necrotizing enterocolitis: A novel hypothesis for disease pathogenesis. Neonatology 2013, 103, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, H.B.; da Mota, H.C.; Coutinho, V.B.; Robalinho, T.I.; Furtado, A.F.; Walker, E.; King, G.; Mahida, Y.R.; Sewell, H.F.; Wakelin, D. Absence of lysozyme (muramidase) in the intestinal Paneth cells of newborn infants with necrotising enterocolitis. J. Clin. Pathol. 1998, 51, 512–514. [Google Scholar] [CrossRef] [Green Version]
- Markasz, L.; Wanders, A.; Szekely, L.; Lilja, H.E. Diminished DEFA6 expression in paneth cells is associated with necrotizing enterocolitis. Gastroenterol. Res. Pract. 2018, 2018, 7345426. [Google Scholar] [CrossRef]
- Franklin, A.L.; Said, M.; Cappiello, C.D.; Gordish-Dressman, H.; Tatari-Calderone, Z.; Vukmanovic, S.; Rais-Bahrami, K.; Luban, N.L.; Devaney, J.M.; Sandler, A.D. Are immune modulating single nucleotide polymorphisms associated with necrotizing enterocolitis? Sci. Rep. 2015, 5, 18369. [Google Scholar] [CrossRef] [Green Version]
- Berger, J.N.; Gong, H.; Good, M.; McElroy, S.J. Dithizone-induced Paneth cell disruption significantly decreases intestinal perfusion in the murine small intestine. J. Pediatr. Surg. 2019, 54, 2402–2407. [Google Scholar] [CrossRef]
- Lueschow, S.R.; Stumphy, J.; Gong, H.; Kern, S.L.; Elgin, T.G.; Underwood, M.A.; Kalanetra, K.M.; Mills, D.A.; Wong, M.H.; Meyerholz, D.K.; et al. Loss of murine Paneth cell function alters the immature intestinal microbiome and mimics changes seen in neonatal necrotizing enterocolitis. PLoS ONE 2018, 13, e0204967. [Google Scholar] [CrossRef]
- Garabedian, E.M.; Roberts, L.J.; McNevin, M.S.; Gordon, J.I. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J. Biol. Chem. 1997, 272, 23729–23740. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, D.L.; Gibbins, S.; Kiss, A.; Bando, N.; Brennan-Donnan, J.; Ng, E.; Campbell, D.M.; Vaz, S.; Fusch, C.; Asztalos, E.; et al. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: A randomized clinical trial. JAMA 2016, 316, 1897–1905. [Google Scholar] [CrossRef]
- Sullivan, S.; Schanler, R.J.; Kim, J.H.; Patel, A.L.; Trawoger, R.; Kiechl-Kohlendorfer, U.; Chan, G.M.; Blanco, C.L.; Abrams, S.; Cotten, C.M.; et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J. Pediatr. 2010, 156, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.; Tonkin, E.; Damarell, R.A.; McPhee, A.J.; Suganuma, M.; Suganuma, H.; Middleton, P.F.; Makrides, M.; Collins, C.T. A systematic review and meta-analysis of human milk feeding and morbidity in very low birth weight infants. Nutrients 2018, 10, 707. [Google Scholar] [CrossRef] [Green Version]
- Underwood, M.A. Human milk for the premature infant. Pediatr. Clin. N. Am. 2013, 60, 189–207. [Google Scholar] [CrossRef] [Green Version]
- Breastfeeding, S.O. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef] [Green Version]
- Wojcik, K.Y.; Rechtman, D.J.; Lee, M.L.; Montoya, A.; Medo, E.T. Macronutrient analysis of a nationwide sample of donor breast milk. J. Am. Diet. Assoc. 2009, 109, 137–140. [Google Scholar] [CrossRef]
- Vieira, A.A.; Soares, F.V.; Pimenta, H.P.; Abranches, A.D.; Moreira, M.E. Analysis of the influence of pasteurization, freezing/thawing, and offer processes on human milk’s macronutrient concentrations. Early Hum. Dev. 2011, 87, 577–580. [Google Scholar] [CrossRef]
- Wood, N.S.; Costeloe, K.; Gibson, A.T.; Hennessy, E.M.; Marlow, N.; Wilkinson, A.R.; Group, E.P.S. The EPICure study: Growth and associated problems in children born at 25 weeks of gestational age or less. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F492–F500. [Google Scholar] [CrossRef] [Green Version]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooqi, A.; Hagglof, B.; Sedin, G.; Gothefors, L.; Serenius, F. Growth in 10- to 12-year-old children born at 23 to 25 weeks’ gestation in the 1990s: A Swedish national prospective follow-up study. Pediatrics 2006, 118, e1452–e1465. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, L.; Bokiniec, R.; King, C.; Weaver, G.; Edwards, A.D. Increased osmolality of breast milk with therapeutic additives. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F514–F517. [Google Scholar] [CrossRef] [PubMed]
- Pearson, F.; Johnson, M.J.; Leaf, A.A. Milk osmolality: Does it matter? Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F166–F169. [Google Scholar] [CrossRef] [PubMed]
- Book, L.S.; Herbst, J.J.; Atherton, S.O.; Jung, A.L. Necrotizing enterocolitis in low-birth-weight infants fed an elemental formula. J. Pediatr. 1975, 87, 602–605. [Google Scholar] [CrossRef]
- Santulli, T.V.; Schullinger, J.N.; Heird, W.C.; Gongaware, R.D.; Wigger, J.; Barlow, B.; Blanc, W.A.; Berdon, W.E. Acute necrotizing enterocolitis in infancy: A review of 64 cases. Pediatrics 1975, 55, 376–387. [Google Scholar]
- Commentary on breast-feeding and infant formulas, including proposed standards for formulas. Pediatrics 1976, 57, 278–285.
- Miyake, H.; Chen, Y.; Koike, Y.; Hock, A.; Li, B.; Lee, C.; Zani, A.; Pierro, A. Osmolality of enteral formula and severity of experimental necrotizing enterocolitis. Pediatr. Surg. Int. 2016, 32, 1153–1156. [Google Scholar] [CrossRef]
- Chen, Y.; Koike, Y.; Chi, L.; Ahmed, A.; Miyake, H.; Li, B.; Lee, C.; Delgado-Olguin, P.; Pierro, A. Formula feeding and immature gut microcirculation promote intestinal hypoxia, leading to necrotizing enterocolitis. Dis. Models Mech. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Bry, L.; Falk, P.; Huttner, K.; Ouellette, A.; Midtvedt, T.; Gordon, J.I. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc. Natl. Acad. Sci. USA 1994, 91, 10335–10339. [Google Scholar] [CrossRef] [Green Version]
- Heida, F.H.; Beyduz, G.; Bulthuis, M.L.; Kooi, E.M.; Bos, A.F.; Timmer, A.; Hulscher, J.B. Paneth cells in the developing gut: When do they arise and when are they immune competent? Pediatr. Res. 2016, 80, 306–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yee, W.H.; Soraisham, A.S.; Shah, V.S.; Aziz, K.; Yoon, W.; Lee, S.K. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 2012, 129, e298–e304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvorak, B.; McWilliam, D.L.; Williams, C.S.; Dominguez, J.A.; Machen, N.W.; McCuskey, R.S.; Philipps, A.F. Artificial formula induces precocious maturation of the small intestine of artificially reared suckling rats. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 162–169. [Google Scholar] [CrossRef]
- White, J.R.; Gong, H.; Colaizy, T.T.; Moreland, J.G.; Flaherty, H.; McElroy, S.J. Evaluation of hematologic composition in newborn C57/BL6 mice up to day 35. Vet. Clin. Pathol. 2015. [Google Scholar] [CrossRef]
- Fricke, E.M.; Elgin, T.G.; Gong, H.; Reese, J.; Gibson-Corley, K.N.; Weiss, R.M.; Zimmerman, K.; Bowdler, N.C.; Kalantera, K.M.; Mills, D.A.; et al. Lipopolysaccharide-induced maternal inflammation induces direct placental injury without alteration in placental blood flow and induces a secondary fetal intestinal injury that persists into adulthood. Am. J. Reprod. Immunol. 2018, 79, e12816. [Google Scholar] [CrossRef] [Green Version]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Wynn, J.L.; Wilson, C.S.; Hawiger, J.; Scumpia, P.O.; Marshall, A.F.; Liu, J.H.; Zharkikh, I.; Wong, H.R.; Lahni, P.; Benjamin, J.T.; et al. Targeting IL-17A attenuates neonatal sepsis mortality induced by IL-18. Proc. Natl. Acad. Sci. USA 2016, 113, E2627–E2635. [Google Scholar] [CrossRef] [Green Version]
- Jilling, T.; Simon, D.; Lu, J.; Meng, F.J.; Li, D.; Schy, R.; Thomson, R.B.; Soliman, A.; Arditi, M.; Caplan, M.S. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J. Immunol. 2006, 177, 3273–3282. [Google Scholar] [CrossRef] [Green Version]
- Sangild, P.T.; Siggers, R.H.; Schmidt, M.; Elnif, J.; Bjornvad, C.R.; Thymann, T.; Grondahl, M.L.; Hansen, A.K.; Jensen, S.K.; Boye, M.; et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 2006, 130, 1776–1792. [Google Scholar] [CrossRef]
- Maheshwari, A.; Schelonka, R.L.; Dimmitt, R.A.; Carlo, W.A.; Munoz-Hernandez, B.; Das, A.; McDonald, S.A.; Thorsen, P.; Skogstrand, K.; Hougaard, D.M.; et al. Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr. Res. 2014, 76, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, B.M.; Stanton, B.A. Renal Physiology, 5th ed.; Elsevier Mosby: Philadelphia, PA, USA, 2013; 240p. [Google Scholar]
- Chandran, S.; Chua, M.C.; Lin, W.; Min Wong, J.; Saffari, S.E.; Rajadurai, V.S. Medications that increase osmolality and compromise the safety of enteral feeding in preterm infants. Neonatology 2017, 111, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Warner, B.B.; Deych, E.; Zhou, Y.; Hall-Moore, C.; Weinstock, G.M.; Sodergren, E.; Shaikh, N.; Hoffmann, J.A.; Linneman, L.A.; Hamvas, A.; et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: A prospective case-control study. Lancet 2016, 387, 1928–1936. [Google Scholar] [CrossRef] [Green Version]
- Torrazza, R.M.; Neu, J. The altered gut microbiome and necrotizing enterocolitis. Clin. Perinatol. 2013, 40, 93–108. [Google Scholar] [CrossRef] [PubMed]
- La Scola, B.; Barrassi, L.; Raoult, D. Isolation of new fastidious alpha Proteobacteria and Afipia felis from hospital water supplies by direct plating and amoebal co-culture procedures. FEMS Microbiol. Ecol. 2000, 34, 129–137. [Google Scholar] [PubMed]
- Haahtela, K.; Kari, K.; Sundman, V. Nitrogenase activity (acetylene reduction) of root-associated, cold-climate azospirillum, enterobacter, Klebsiella, and pseudomonas species during growth on various carbon sources and at various partial pressures of oxygen. Appl. Environ. Microbiol. 1983, 45, 563–570. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.K.; Mital, B.K.; Garg, S.K. Characterization of Lactobacillus acidophilus strains for use as dietary adjunct. Int. J. Food Microbiol. 1996, 29, 105–109. [Google Scholar] [CrossRef]
- Genderjahn, S.; Alawi, M.; Mangelsdorf, K.; Horn, F.; Wagner, D. Desiccation- and saline-tolerant bacteria and archaea in kalahari pan sediments. Front. Microbiol. 2018, 9, 2082. [Google Scholar] [CrossRef]
- Oren, A. Microbial life at high salt concentrations: Phylogenetic and metabolic diversity. Saline Syst. 2008, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Alouf, J.E.; Popoff, M.R. The Comprehensive Sourcebook of Bacterial Protein Toxins, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2006; 1047p. [Google Scholar]


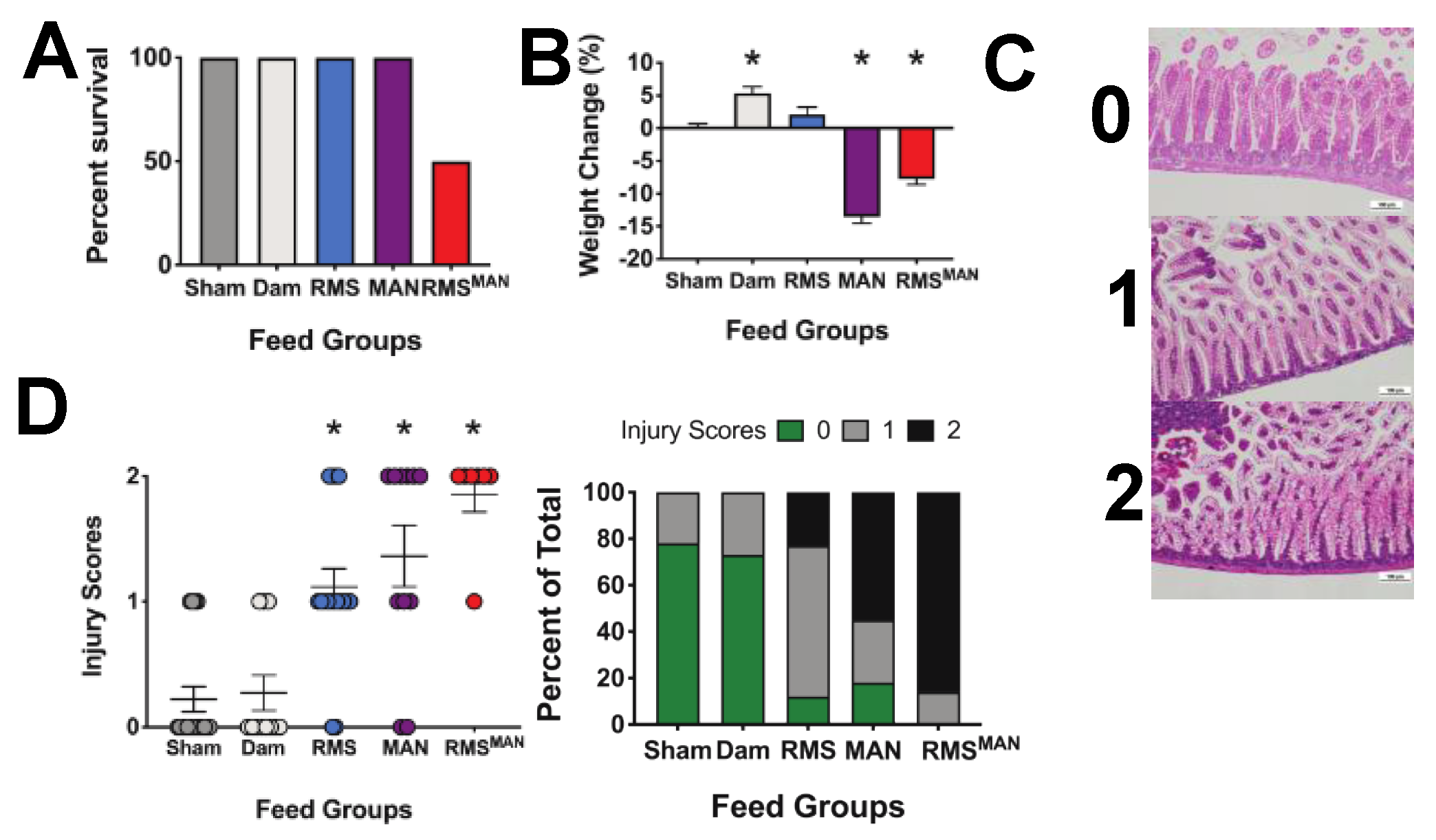
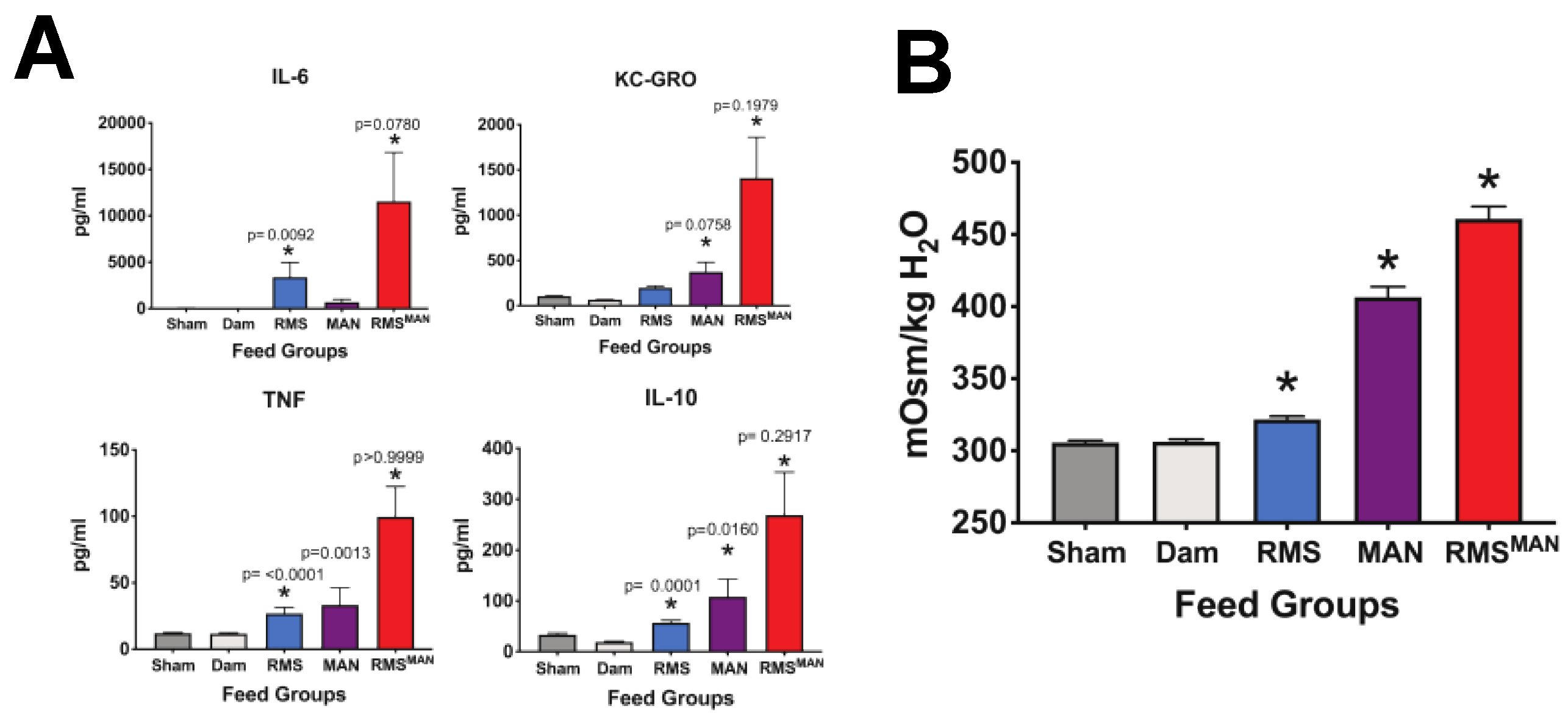

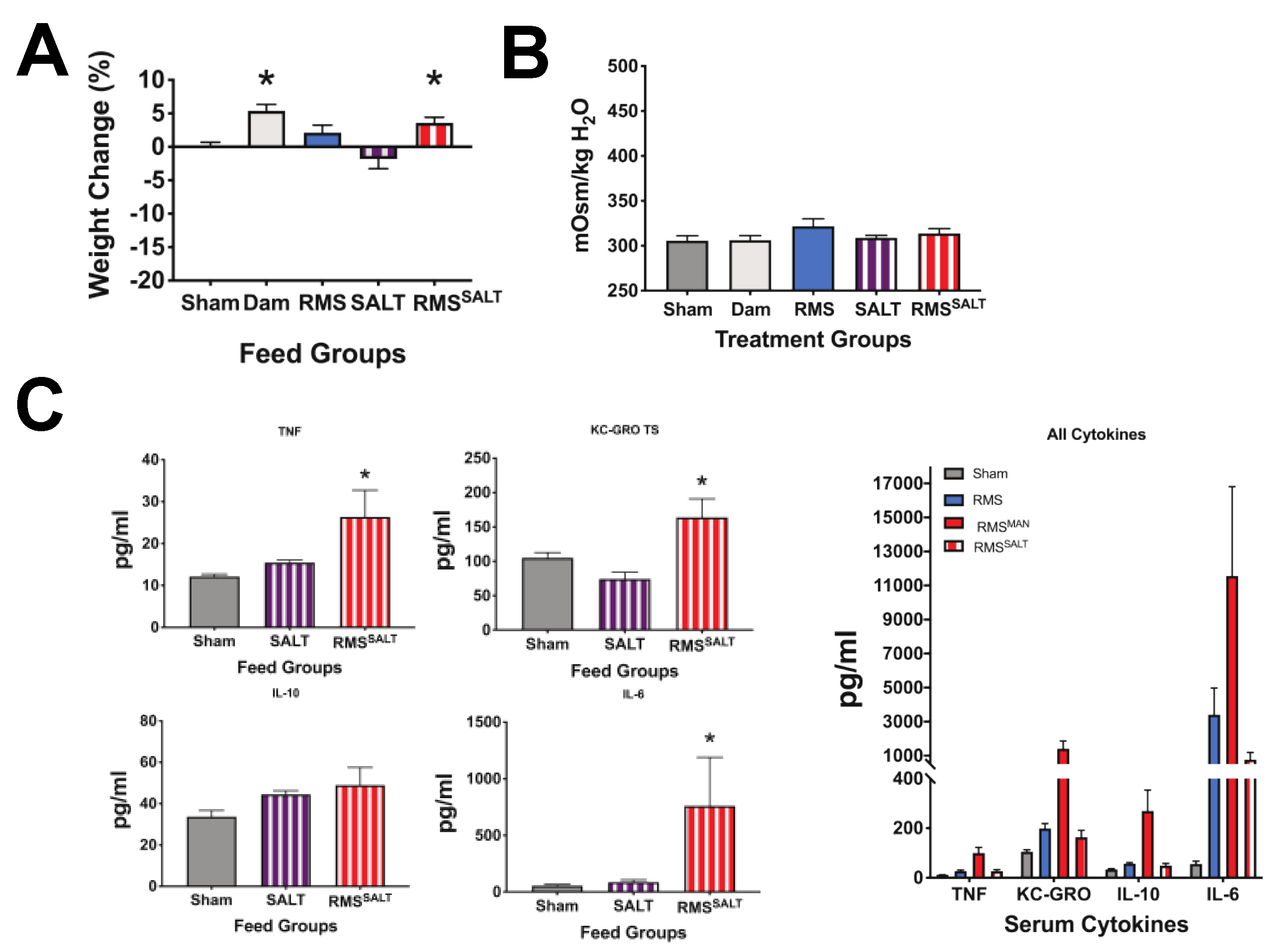


| Feeding Type/Abbreviation | Formulation | Osmolality (mOsm/kg H2O) |
|---|---|---|
| Control | Saline | 250 |
| RMS | Rodent milk substitute | 721 |
| Dam | Ad libitum dam feeding | 300 |
| Saline | Saline | 273 |
| MAN | 10% Mannitol in saline | 873 |
| SALT | Saline + 0.7 g NaCl | 581 |
| RMSMAN | RMS + 10% mannitol | 1491 |
| RMSSALT | RMS + 0.7 g NaCl | 982 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lueschow, S.R.; Kern, S.L.; Gong, H.; Grobe, J.L.; Segar, J.L.; Carlson, S.J.; McElroy, S.J. Feeding Formula Eliminates the Necessity of Bacterial Dysbiosis and Induces Inflammation and Injury in the Paneth Cell Disruption Murine NEC Model in an Osmolality-Dependent Manner. Nutrients 2020, 12, 900. https://doi.org/10.3390/nu12040900
Lueschow SR, Kern SL, Gong H, Grobe JL, Segar JL, Carlson SJ, McElroy SJ. Feeding Formula Eliminates the Necessity of Bacterial Dysbiosis and Induces Inflammation and Injury in the Paneth Cell Disruption Murine NEC Model in an Osmolality-Dependent Manner. Nutrients. 2020; 12(4):900. https://doi.org/10.3390/nu12040900
Chicago/Turabian StyleLueschow, Shiloh R, Stacy L Kern, Huiyu Gong, Justin L Grobe, Jeffrey L Segar, Susan J Carlson, and Steven J McElroy. 2020. "Feeding Formula Eliminates the Necessity of Bacterial Dysbiosis and Induces Inflammation and Injury in the Paneth Cell Disruption Murine NEC Model in an Osmolality-Dependent Manner" Nutrients 12, no. 4: 900. https://doi.org/10.3390/nu12040900
APA StyleLueschow, S. R., Kern, S. L., Gong, H., Grobe, J. L., Segar, J. L., Carlson, S. J., & McElroy, S. J. (2020). Feeding Formula Eliminates the Necessity of Bacterial Dysbiosis and Induces Inflammation and Injury in the Paneth Cell Disruption Murine NEC Model in an Osmolality-Dependent Manner. Nutrients, 12(4), 900. https://doi.org/10.3390/nu12040900





