1. Introduction
Data from the 2009–2012 National Health and Nutrition Examination Survey (NHANES) indicate that the majority of adults ages 51–70 years in the U.S. do not meet hydration criteria [
1]. An estimated 72.1% (68.4%–75.5%) of men and 65.9% (61.0%–70.6%) of women who are free of acute illness have a serum sodium below 135 mmol/L, a serum sodium of 145 mmol/L or higher, a spot urine volume less than 50 mL, or a urine osmolality of 500 mmol/kg or higher. Individuals who do not meet these hydration criteria have hyponatremia and/or a condition described as “underhydration” by Kavouras [
2], which is characterized by elevated vasopressin and renal conservation of water despite normal plasma osmolality. The 2009–2012 NHANES analysis [
1] has implications for national water intake recommendations, if hyponatremia and/or underhydration signal risk for poor health outcomes. Given controversy over hydration criteria [
3] and no population-representative estimates of health benefit associated with euhydration as defined by Stookey [
1], this analysis extends work on the same 2009–2012 NHANES study population to describe the prevalence of chronic health conditions and risk of mortality associated with not meeting the same hydration criteria.
1.1. Hydration Classification
The public health significance of not meeting the hydration criteria specified by Stookey [
1] is uncertain, because it is possible to define hydration relative to various standards and index hydration using multiple different measures, either alone or in combination. Hydration may be defined relative to requirements for various aspects of metabolism, such as sodium balance, protein turnover, and hormone release and action, requirements for various aspects of physiological homeostasis, such as maintenance of blood volume, blood pressure, and thermoregulation; requirements for various aspects of physical function or performance, such as urine concentration, endurance, and cognition; and/or myriad other requirements for health. Hydration measures, such as serum sodium, blood urea nitrogen creatinine ratio, anti-diuretic hormone, hematocrit, heart rate, urine concentration, sweat rate, skin turgor, and others, have been developed to reflect one or more of the above aspects of metabolism, physiology, function, or health [
4,
5,
6]. No one gold standard of hydration has been identified [
7,
8]. Available hydration indices vary in sensitivity and specificity to a range of mild to severe, acute to chronic, fluid disorders, including hyper-, iso-, and hypovolemic dehydration. The hydration indices vary in terms of prevalence under conditions of daily life [
9]. Assessment of one or more hydration measures may be appropriate to address any given public health question.
1.2. Cross-Sectional Association between Hydration Classification and Chronic Health Conditions
The 2009–2012 NHANES data suggest that the high prevalence of not meeting the hydration criteria is associated with risk of chronic disease. Compared with the prevalence estimated for the total population of non-acutely ill men and women ages 51–70 years (noted above), the prevalence estimated for the sub-group without chronic disease risk factors is over 15 percentage points lower (52.4% (40.1%–64.4%) for males, 49.0% (34.3%–63.9%) for females) [
1]. The chronic disease risk factors considered in the analysis are underweight, overweight or obesity, hyperglycemia, insulin resistance, hypertriglyceridemia, low high-density lipoprotein (HDL), and chronic kidney disease.
Individuals with chronic disease are more likely to have hyponatremia than individuals without chronic disease. NHANES data from 1999–2004 indicate that, in U.S. adults, hyponatremia is significantly more common among those with hypertension, diabetes, coronary artery disease, stroke, chronic obstructive pulmonary disease, cancer, and psychiatric disorders, and less common in those with no comorbidities. Controlling for demographics, smoking, comorbidities, and insurance, hyponatremia more than doubles the risk of death within 3 to 6 years [
10].
Individuals with chronic disease are also at increased risk of underhydration. At a given point in time, conditions of the metabolic syndrome predispose to higher serum sodium, low urine volume, and/or concentrated urine. In cross-sectional data, obese individuals are significantly more likely to have a serum sodium above 140 mmol/L and an increased extracellular relative to intracellular (ECF/ICF) fluid ratio than normal weight individuals [
11]. Insulin resistance causes extracellular glucose to become osmotically effective, favoring osmotic cell shrinkage, vasopressin release, and vasopressin action on the kidney to reduce urine volume and concentrate urine [
12,
13,
14]. Impaired insulin-stimulated glucose metabolism and salt-sensitive hypertension occur with renal sodium retention [
15,
16,
17]. Plasma copeptin, a biomarker of osmotic shrinkage of cells and vasopressin release [
18], is cross-sectionally associated with diabetes and insulin resistance [
19]. Higher systolic blood pressure is cross-sectionally associated with higher serum sodium [
20,
21,
22,
23,
24]. Across studies, individuals with metabolic syndrome have significantly higher levels of sodium compared to healthy controls [
25].
Cross sectional associations are not informative about the temporality of association. Although chronic health conditions may increase risk of underhydration, the reverse is also possible. Underhydration status may determine chronic health conditions. Prospectively, hypernatremia, low urine volume, and/or concentrated urine predict the risk of new onset or worsening metabolic dysregulation. In controlled experiments, increasing the serum sodium over 145 mmol/L of healthy adults via fluid restriction or hypertonic saline infusion decreases insulin sensitivity, increases hyperglycemia, and alters lipid metabolism [
26,
27,
28]. At the cell level, experiments show that extracellular hypertonicity causes cell shrinkage, which upregulates proteolysis and glycogenolysis [
29]. The free amino acids and glucose end-products, in turn, trigger insulin release. Extracellular hypertonicity, furthermore, causes insulin resistance by counteracting cell swelling, which is required for the effect of insulin on metabolism, such as proteolysis [
30]. Sustained over time, extracellular hypertonicity causes cells to alter gene transcription to support cellular accumulation of organic osmolytes [
31]. Over the longer term, higher serum sodium predicts many age-related degenerative diseases [
32].
1.3. Longitudinal Association between Hydration Classification and Mortality
Longitudinal, population-representative data are consistent with a negative feedback system of not meeting hydration criteria and metabolic dysregulation that magnifies risk of incident disease. As the number of metabolic syndrome components increases, the body sodium level increases [
25]. In people with hyperglycemia, independent of the level of plasma glucose, a unit increase in serum sodium increases the relative odds of incident diabetes by 26% [
33]. In people with diabetes, independent of fasting glucose and hemoglobin A1c, plasma copeptin significantly interacts with diabetes to predict 33% greater risk of coronary artery disease, 62% greater risk of heart failure, and 32% greater risk of death from diabetes [
34]. Longitudinal data indicate significantly greater mortality when serum sodium is outside the normal range or plasma copeptin or urine osmolality is elevated [
10,
19,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45].
The ultimate goal of this analysis was to facilitate interpretation of recent results from the 2009–2012 NHANES [
1]. To align with that analysis, the specific aims of this analysis were to determine if “not meeting hydration criteria”, as defined in that analysis [
1], was (1) cross-sectionally associated with chronic health conditions in 2009–2012 and (2) longitudinally associated with death within 3–6 years. The cross-sectional analysis checked for potential intercorrelation or effect mediation between hydration classification and chronic health conditions in 2009–2012. The survival analysis specified the joint effect of baseline hydration and chronic health condition as main exposure.
Given the working hypothesis that hyponatremia and/or underhydration exacerbate metabolic dysregulation characteristics of metabolic syndrome, this analysis also reports cross-sectional associations between underhydration and chronic health conditions. Cross-sectional associations between hyponatremia and chronic health conditions have already been described for the NHANES population [
10].
3. Results
3.1. Sample Characteristics
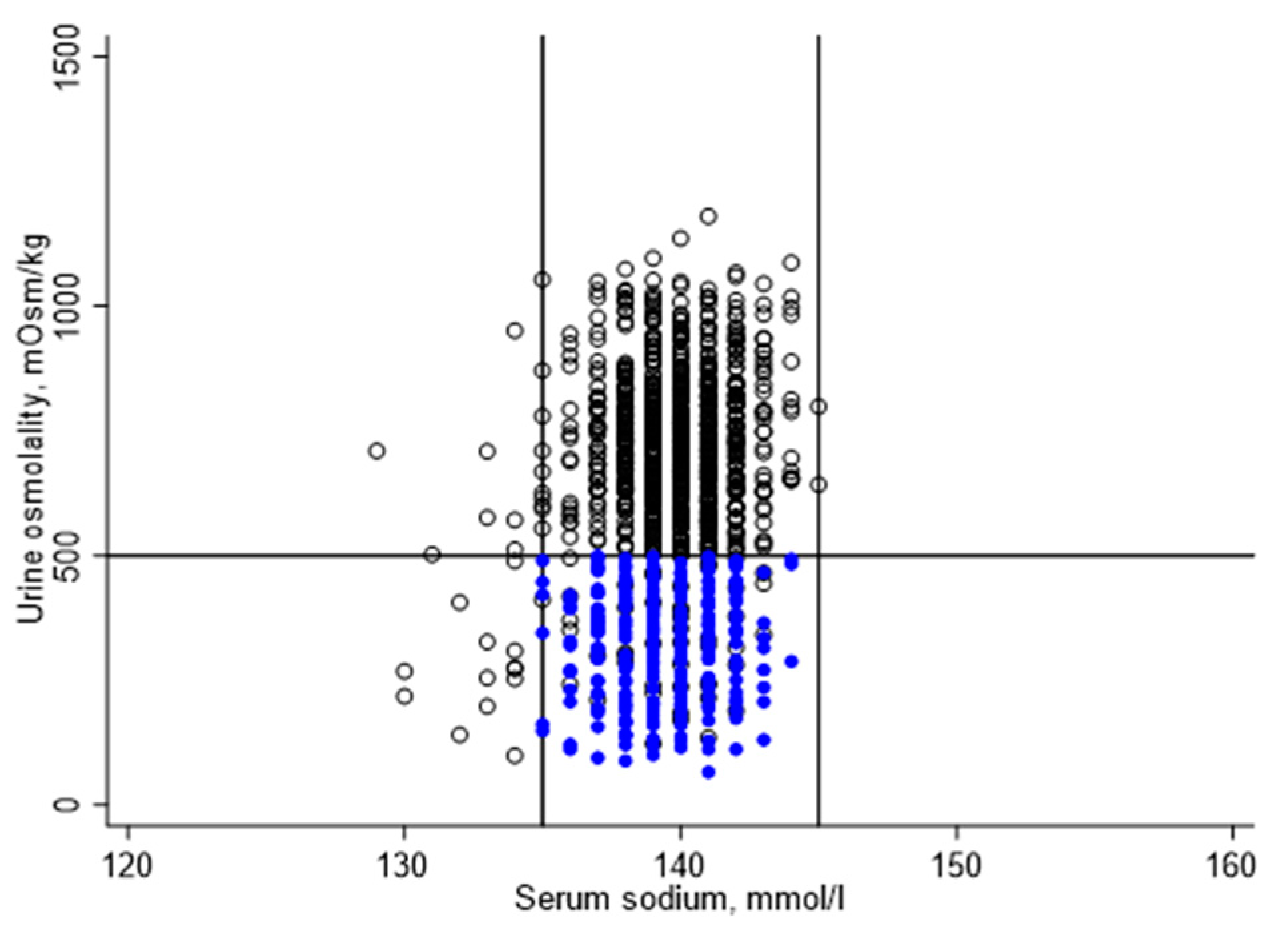
Figure 2 illustrates the distribution of serum sodium and urine osmolality for the study sample. Serum sodium ranged from 129 to 145 mmol/L, with a mean (standard error (SE)) of 139 (0.1) mmol/l. Urine osmolality ranged from 66 to 1180 mmol/kg, with a mean (SE) of 537 (11) mmol/kg. Urine volume ranged from 1 to 466 mL, with a mean (SE) of 77 (3) mL.
Of 1200 study participants, 881 did not meet one or more hydration criteria. A total of 22 participants were hyponatremic, 10 of which had a urine osmolality below 500 mmol/kg. A total of 859 participants were classified as underhydrated, including 322 participants with zero urine or a urine volume below 50 mL. Urine osmolality was 500 mmol/kg or higher for 796 participants, including 537 participants with urine volume of 50 mL or more.
Applying sample weights, an estimated 71.4% (67.0%–75.4%) of fasting U.S. adults aged 51–70 years in 2009–2012 did not meet one or more hydration criteria. Among those who did not meet the hydration criteria, 2.7% (1.3%–5.5%) had hyponatremia and 97.3% (94.5%–98.7%) were underhydrated. An estimated 69.4% (65.5%–73.1%) of the study population was underhydrated.
Table 1 describes the hydration classification by risk factors for chronic health conditions and/or mortality. Compared to euhydration, the prevalence of not meeting the hydration criteria varied significantly by race/ethnicity, cigarette smoking, total daily dietary solute load, total energy, and carbohydrate intake.
3.2. Prevalence of Not Meeting Hydration Criteria by Chronic Health Condition
Table 2A describes the number of NHANES participants and weighted proportion of the study population who did not meet one or more hydration criteria, stratified by chronic condition. The table aligns with groups defined in the prior NHANES analysis [
1]. Independent of the covariates controlled in Model 2, the prevalence of not meeting hydration criteria was significantly higher among individuals with obesity, high waist circumference, insulin resistance, low HDL, and metabolic syndrome than among individuals without these conditions. Except for the relationship between obesity and not meeting hydration criteria, the observed associations were explained away by control for other chronic conditions (Model 3).
Whereas
Table 2A describes the prevalence of not meeting one or more hydration criteria,
Table 2B focuses on the prevalence of underhydration, more specifically, excluding hyponatremia. The same pattern of results was observed for underhydration. Independent of the covariates controlled in Model 2, the prevalence of underhydration was significantly higher among individuals with obesity, high waist circumference, insulin resistance, low HDL, and metabolic syndrome than among individuals without these conditions.
3.3. Prevalence of Chronic Health Condition by Hydration Classification
Table 3A,B describes the number of NHANES participants and weighted prevalence of each chronic health condition, stratified by hydration classification. As above,
Table 3A describes associations for not meeting one or more of the hydration criteria, whereas
Table 3B focuses on underhydration specifically. In unadjusted weighted Poisson models, obesity, high waist circumference, insulin resistance, diabetes, low HDL, hypertension, and metabolic syndrome were significantly more prevalent among individuals who did not meet hydration criteria. The proportion of people without any chronic condition was two times greater for people who were euhydrated. The relationships remained statistically significant after adjustment for all covariates in Model 2. The multivariable-adjusted prevalence ratio for each condition (Model 2) was reduced by more than 10% after further adjustment for all other chronic conditions (Model 3).
3.4. Mortality Associated with Hyponatremia or Underhydration vs. Euhydration
3.4.1. All-Cause Mortality
Table 4 summarizes the number of deaths that occurred within 3 to 6 years of the 2009–2012 MEC examination. A total of 52 deaths in the 2009–2012 NHANES study sample represented 1,724,051 deaths to persons ages 51–70 years, nationally. The observed deaths were concentrated among persons who were underhydrated in 2009–2012.
Figure 3 (a) illustrates the difference in survival for people who were hyponatremic or underhydrated vs. euhydrated. The unadjusted all-cause death rate was over two times higher for people who did not meet the hydration criteria in 2009–2012 (weighted hazard ratio (HR): 2.37, 95% confidence interval (CI): 1.02–5.50,
p = 0.044), either because of hyponatremia (weighted HR: 2.32, 95% CI: 0.32–16.89,
p = 0.396) or underhydration (weighted HR: 2.37, 95% CI: 1.03–5.49,
p = 0.044). The estimated increase in all-cause mortality associated with not meeting hydration criteria remained over two times higher after adjustment for covariates (adjusted weighted HR: 2.32, 95% CI: 0.96–5.63,
p = 0.062). The estimated increase in all-cause mortality associated with underhydration, more specifically, also remained over two times higher (adjusted weighted HR: 2.33, 95% CI: 0.97–5.60,
p = 0.059).
3.4.2. Chronic Disease Mortality
An estimated 1,084,144 deaths in the study population were attributed to or associated with heart disease, malignant neoplasms, hypertension, and/or diabetes. The unadjusted risk of chronic disease-associated death was about four times higher for underhydrated vs. euhydrated people in 2009–2012 (unadjusted weighted HR: 4.44, 95% CI: 1.57–12.54,
p = 0.006). After adjustment for covariates, underhydration remained significantly associated with chronic disease-associated death (adjusted weighted HR: 4.21, 95% CI: 1.29–13.78,
p = 0.019).
Figure 3 (b) illustrates the difference in survival.
3.4.3. Sensitivity Analyses
For adults aged 51–80 years, a U-shaped relationship was observed between serum osmolality and all-cause as well as chronic disease mortality. For the 51–70-year-old age group, a U-shaped relationship was observed between serum osmolality and all-cause, but not chronic disease mortality (see
Table A1). The tails of the serum osmolality distribution, below 285 mmol/kg and above 300 mmol/kg, were associated with the increased risk of chronic disease mortality (see
Table A2).
The effect of underhydration on chronic disease associated mortality was significantly modified by age group (adjusted weighted HR (SE) for the interaction term: 0.23 (0.17), p-value = 0.048).
For adults aged 51–70 years with a serum osmolality between 285–295 mmol/kg, underhydration was associated with significantly increased risk of chronic disease mortality (unadjusted weighted HR: 3.52, 95% CI: 1.12–11.10, p = 0.032; adjusted weighted HR: 3.45, 95% CI: 0.94–12.64, p = 0.061).
Focusing on underhydrated individuals with urine osmolality below 500 mmol/kg, the unadjusted and multivariable-adjusted relative hazards for chronic disease mortality associated with low urine volume vs. euhydration were as follows: unadjusted weighted HR: 7.86, 95% CI: 1.14–54.38, p = 0.037; adjusted weighted HR: 13.32, 95% CI: 1.01–176.25, p = 0.049.
3.5. Mortality Associated with the Joint Effect of Hydration Classification and Chronic Health Condition
Across the different chronic health conditions in 2009–2012, deaths were concentrated among those who were underhydrated. Health condition-specific data were not reported because of small numbers. There were fewer than 10 chronic disease-associated deaths in the euhydrated group.
Table 4 summarizes the number of deaths that occurred by presence or absence of chronic health condition in 2009–2012.
Figure 4 describes survival by the joint exposure of hydration classification and chronic health condition in 2009–2012.
Among those with one or more chronic health conditions in 2009–2012, compared to euhydration, not meeting hydration criteria was associated with significantly greater risk of all-cause mortality within 3 to 6 years (unadjusted weighted HR: 2.51, 95% CI: 1.14–5.51, p = 0.024; adjusted weighted HR: 2.50, 95% CI: 1.07–5.85, p = 0.036). Compared to euhydration, underhydration was associated with significantly greater all-cause mortality (unadjusted weighted HR: 2.51, 95% CI: 1.13–5.54, p = 0.025; adjusted weighted HR: 2.47, 95% CI: 1.06–5.71, p = 0.036).
Among those with one or more chronic health conditions in 2009–2012, the risk of death attributed to chronic disease was over three times greater for people who were underhydrated vs. euhydrated (unadjusted weighted HR: 3.51, 95% CI: 1.17–10.53, p = 0.027; adjusted weighted HR: 3.08, 95% CI: 0.92–10.32, p = 0.067).
It was not possible to estimate the effect of underhydration on mortality for participants with none of the chronic conditions at baseline, due to the small number of deaths in this group. No chronic disease-associated deaths were observed during the follow-up period for people who did not have any of the chronic conditions and were, also, euhydrated in 2009–2012. Compared with people who did have one or more chronic condition, but were classified as euhydrated, in 2009–2012, people who were not identified as having any chronic condition at baseline but were underhydrated were significantly more likely to die (unadjusted weighted HR: 5.41, 95% CI: 1.32–22.15, p = 0.020; adjusted weighted HR: 8.43, 95% CI: 1.15–61.75, p = 0.037).
4. Discussion
The results of this study suggest that over 95% of U.S. adults aged 51–70 years who do not meet hydration criteria are underhydrated. The results further suggest that underhydration signals a statistically significant health risk. Underhydration, that is, serum sodium above the 135–144 mmol/L range, spot urine volume less than 50 mL, or urine osmolality of 500 mmol/kg or higher, was cross-sectionally associated with increased prevalence of multiple chronic health conditions. Underhydration was longitudinally associated with increased risk of all-cause and chronic disease associated mortality within 3–6 years.
4.1. Hydration Classification
Researchers debate which biomarker to use for epidemiologic analysis, dietary recommendations, and public health policy. Various hydration biomarkers have been developed to index a variety of body fluid disorders [
7,
83,
84]. Any one biomarker may not be optimally sensitive to all types of body fluid disorders [
9]. This analysis explored the public health significance of not meeting hydration criteria specified in a previous NHANES analysis [
1], testing for health effects of hyponatremia and/or underhydration, conditions that would be flagged by the hydration criteria.
Researchers agree that serum osmolality and its main component, serum sodium, are sensitive biomarkers of total body water deficit. With every 2% body weight loss via sweat during exercise, plasma osmolality increases about 5 mmol/kg [
85]. Researchers further agree that serum osmolality and sodium have limited variability under conditions of daily life due to tight homeostatic regulation [
55,
86]. Researchers disagree, however, about relying on a biomarker that has limited variability under conditions of daily life to index hydration in people who are not hospitalized or not performing extreme exercise [
86]. By itself, a normal serum osmolality or sodium can mask variability in metabolic and/or physiologic compensation. It does not discriminate individuals who have normal serum osmolality and/or normal serum sodium, thanks to urine concentration, from individuals who have normal values with no compensation required. Beyond the potential for unmeasured heterogeneity due to homeostatic compensation, use of serum osmolality or serum sodium to index hydration is complicated by the narrow 10 mmol/L normal range, variation in the set-point for homeostasis, and U-shaped relationship with health outcomes. A one-unit difference in serum osmolality or sodium may be clinically insignificant or only reflect measurement error. Cutoffs for defining the optimal normal range for these biomarkers may differ within and between individuals because the homeostatic set-point for serum osmolality or sodium can vary [
61,
87]. As hypo- and hypernatremia are both associated with adverse health outcomes, including death [
10,
88], use of continuous serum osmolality and serum sodium in epidemiologic analysis inappropriately masks U-shaped effects. Indeed, continuous serum osmolality is not linearly associated with mortality in NHANES data [
89].
Researchers agree that spot urine osmolality is sensitive to acute and mild changes in fluid intake in the short-term [
55,
86], but disagree about using this biomarker to index ‘usual’ status in epidemiologic hydration studies. Despite disagreement, spot urine osmolality at a given time of day may be relatively stable among free-living individuals, as free-living individuals have stable within-person 24 h urine volume and osmolality, as well as intransigent fluid intake patterns [
90]. Community-dwelling individuals who report lower fluid intake have elevated antidiuretic hormone (arginine vasopressin, AVP) and higher urine osmolality [
57]. In non-acutely ill adults, following a sustained increase in total daily water intake (i.e., a change in the ‘usual’ level of water intake), spot urine osmolality decreases for all urine collections across the day, including the morning fasting collection [
90,
91]. The hydration classification used in this analysis was selected to match that defined for a previous NHANES analysis [
1]. The hydration classification represents a compromise between only using serum osmolality or serum sodium and only using urine osmolality. It also represents an alignment of epidemiological research on free-living non-acutely ill populations with clinical differential diagnosis of fluid disorders in hospitalized patients [
92].
4.2. Cross-Sectional Association between Hydration Classification and Chronic Health Conditions
The relationship between hydration classification and chronic health condition was described from two points of view, with hydration classification treated as either the dependent or independent variable. One or more chronic health condition was cross-sectionally associated with not meeting the hydration criteria and underhydration, more specifically. Conversely, not meeting hydration criteria and underhydration were respectively associated with chronic health conditions. Although a statistically significant association in both directions is anticipated when a statistically significant association is observed in one direction, the study provides weighted estimates of the proportion exposed and magnitudes of association for each direction of effect for U.S. adults aged 51–70 years. Population-representative estimates of each effect may be useful for future meta-analysis, causal inference do-calculus, program planning, or policy making.
Independent of covariates, obesity, high waist circumference, and insulin resistance each respectively increased the prevalence of not meeting the hydration criteria by 20%. Low HDL cholesterol increased the prevalence of not meeting the hydration criteria by 15%. The prevalence ratio for not meeting the hydration criteria was 15% higher for individuals with metabolic syndrome compared to individuals without metabolic syndrome. The results suggest need or opportunity for clinicians to systematically assess hydration for individuals with conditions of the metabolic syndrome, and chronic disease researchers to consider hydration as a potential confounder and/or effect modifier for chronic disease morbidity and mortality. The National Academy of Medicine report recognizes that illness increases water requirements [
71]. Further research is needed to determine what level of water intake reduces the risk of underhydration for individuals with metabolic syndrome.
With hydration classification treated as the independent variable, the results suggest that not meeting the hydration criteria and underhydration, more specifically, have public health significance. Not meeting the hydration criteria and underhydration were associated with significantly increased prevalence of obesity, insulin resistance, diabetes, dyslipidemia, hypertension, and metabolic syndrome. Independent of age, sex, race/ethnicity, height, education, poverty level, physical activity, dietary solute load, total energy intake, carbohydrate intake, past or current cigarette smoking, prescription medication use, season, and length of fast, the proportion of people without any of the aforementioned conditions was 39% lower for individuals who did not meet hydration criteria compared to individuals who did meet the hydration criteria.
4.3. Potential for Endogenous Effects of Hydration and Chronic Disease
The results of this study were consistent with a potential negative feedback mechanism. Excepting the association between obesity and hydration classification, control for all other chronic health conditions explained away each observed association, signaling that the relationship between each health condition and hydration could conceivably be mediated by metabolic dysregulation due to other chronic conditions. Alternatively, each association might be confounded by one or more other health condition(s). If metabolic dysregulation mediates the relationship between each health condition and hydration, then metabolic dysregulation and the health conditions that are upstream to the metabolic dysregulation should not be controlled in multivariable analysis [
93], but rather treated as a joint exposure. If, on the other hand, one or more health conditions confounds the association, explaining the relationship by a pathway that does not involve the index health condition, then stratified analyses would be needed to test for each association among those with no other condition. Longitudinal data with carefully selected study populations are needed to determine if each chronic health condition prospectively predicts underhydration.
The finding that obesity remained cross-sectionally associated with hydration classification after control for all other health conditions might suggest that this relationship preceded associations between underhydration and the other health conditions assessed in this analysis. Obesity might be a cause or consequence of underhydration. Large body size increases water intake requirements and likelihood of inadequate water intake. On the other hand, lower absolute and relative intake of drinking water have been observed to reduce energy expenditure, reduce fat oxidation, and induce insulin resistance, which contribute to positive fat balance [
94]. Obesity reflects a sustained period of positive fat balance, due primarily to reduced oxidation of fat consumed [
95,
96]. The residual association that remained after control for other health conditions might also reflect measurement or misclassification error related to the method and cutoffs used to assess each health condition. Individuals with insulin resistance detectable by a HOMA-IR value of 2.5 may be more likely to already be obese than individuals with a milder HOMA-IR of 1.5. Longitudinal data and carefully defined health conditions would be required to determine the order of associations.
4.4. Potential Causal Mechanisms Linking Hydration with Metabolic Syndrome
Underhydration, that is, having a serum sodium above the normal range, low urine volume and/or concentrated urine, implies osmotic stress on cells. Osmotic stress on cells is a plausible causal factor for the metabolic syndrome, which is characterized by multiple simultaneous health conditions, as osmotic cell shrinkage acts as a metabolic switch that simultaneously alters multiple biochemical pathways, cellular processes, and physiological systems [
29].
Longitudinal data suggest parallel relationships between various correlates of osmotic stress on cells and risk of the metabolic syndrome.
Behavioral determinants of hyperosmotic stress on cells, such as salt intake, lower absolute water intake, and intake of hypertonic beverages such as sugar-sweetened beverages instead of drinking water [
97,
98,
99,
100,
101];
blood biomarker measures of hyperosmotic stress on cells, such as serum hypernatremia, hypertonicity, or hyperosmolality [
26,
32,
33,
38,
88,
102]; intracellular measures of biochemical response to hyperosmotic cell shrinkage such as upregulation of the serum- and glucocorticoid-inducible kinase 1 (SGK1) [
103,
104]; and
physiologic measures of response to hyperosmotic cell shrinkage, such as increases in plasma copeptin [
18,
19,
105,
106,
107,
108], are independently associated with incident metabolic dysregulation and/or chronic disease risk.
The parallel results suggest that osmotic stress on cells or some correlate(s) of osmotic stress on cells may be causally related to metabolic dysregulation and chronic disease risk. Any one or more measure might conceivably be the necessary and sufficient etiologic factor(s) for metabolic syndrome. Given that osmotic cell shrinkage simultaneously impacts multiple biochemical pathways, multiple cellular mechanisms may mediate effects of suboptimal fluid intake on pathophysiology and life span. Additional experimental effort is needed to unravel the molecular pathophysiology of the relationship between not meeting hydration criteria, underhydration, and chronic health conditions. The success of disease prevention and treatment efforts depends on identification of the necessary and sufficient causal factor(s).
4.5. Longitudinal Association between Hydration Classification and Mortality
The above paragraphs consider the potential for endogenous, interactive effects of hydration and health conditions on disease progression, as well as the potential for health conditions to simply confound effects of hydration on disease progression. If the underlying mechanism(s) involve(s) feedback of endogenous effects, then an individual’s stage in the feedback cycle at baseline would be an appropriate exposure for longitudinal mortality analysis. The effect to be estimated would be the joint effect of the hydration classification and chronic condition on mortality risk. If, on the other hand, baseline chronic health condition confounds the effect of hydration on disease progression, then the longitudinal effect to estimate would be the effect of hydration among individuals without chronic health conditions at baseline.
In the present analysis, the combination of one or more chronic health conditions in 2009–2012 and underhydration in 2009–2012 was associated with significantly greater all-cause and chronic disease mortality compared to the combination of one or more chronic conditions and euhydration in 2009–2012. The data are consistent with sub-optimal hydration exacerbating metabolic dysregulation and accelerating disease progression. It was not possible to estimate the effect of underhydration on mortality among those without a chronic health condition at baseline, as only five deaths occurred in this group. Zero chronic disease associated deaths were observed for people who did not have any of the chronic conditions and, also, met the hydration criteria in 2009–2012.
The results of the present NHANES analysis are consistent with the results of Mohan et al. [
10] and inconsistent with those reported by Kant and Graubard [
89]. Mohan et al. [
10] report significantly greater mortality associated with hypernatremia vs. normonatremia in NHANES participants followed from 1999–2004 through December 31, 2006. They also report significantly greater mortality associated with hyponatremia vs. normonatremia. The U-shaped relationship between serum sodium and mortality may explain why Kant and Graubard [
89] observed no linear association between continuous serum osmolality and all-cause mortality with up to 13 years of follow-up. The present sensitivity analyses confirmed a significant U-shaped relationship between serum osmolality and mortality in the 2009–2012 NHANES data (see
Appendix). Null effects of individual hydration parameters on mortality may also reflect the fact that no single hydration measure isolates optimal hydration for non-acutely ill individuals. Normal serum osmolality may mask heterogeneity due to homeostatic compensation. Low urine osmolality may mask hyponatremia. The present sensitivity analyses confirmed significant variability in mortality associated with underhydration (low urine volume or urine osmolality above 500 mmol/kg) for individuals with a serum osmolality in the normal range, between 285–295 mmol/kg.
4.6. Confounding
Effects of hydration on the metabolic syndrome and chronic disease risk may be confounded by determinants of water requirements, determinants of water intake, and determinants of water handling in the body. Time invariant and time-varying environmental, socio-economic, behavioral, psychological, and biological factors may determine an individual’s water requirements and/or water intake at a given point in time. Environmental factors may include ambient temperature; altitude; the availability, accessibility, and quality or palatability of water; and place-based conditions, such as school or workplace policy (e.g., vending machine rules, recess or toilet breaks). Socio-economic factors may include cultural norms or ethnicity, education, and poverty. Behavioral factors include physical activity, solute intake, sleep, smoking, alcohol intake, and medication use. Psychological factors include stress and pain. Biological factors include age, sex, body size, race, metabolic adaptation (e.g., to habitual caffeine intake or heat stress), renal concentrating/diluting capacity, disease related factors (e.g., insult accumulation/wear and tear on organ systems, glycosuria), and genetic factors.
The present cross-sectional analysis was able to control for some environmental, socio-economic, behavioral, and genetic factors, but data were not available to also control for psychological factors. Stress and pain may confound the observed associations because stress and pain can trigger vasopressin and cortisol release [
109,
110], which stimulate urine concentration. At the same time, stress and pain induce metabolic shifts that favor the flight or fight response, a profile of metabolic dysregulation that is characteristic of the metabolic syndrome. In addition to better control for environmental and psychological factors, future prospective studies of hydration effects on chronic disease risk should address time-varying factors.
Variables which might confound the observed cross-sectional associations between hydration and chronic health conditions may also confound the observed longitudinal relationships between hydration and mortality. In the present study, longitudinal models predicting death within 3–6 years controlled for the same covariates addressed in the cross-sectional analysis. On the basis of the hypothesized causal mechanism illustrated in
Figure 1, the analysis did not control for all possible confounding variables.
The present study observed that spot urine volume below 50 mL was associated with significantly increased mortality risk. This finding, considered with the association between serum hypernatremia and increased mortality reported by Mohan et al. [
10], suggests that the observed association between underhydration and increased mortality is not solely attributable to urine osmolality of 500 mmol/kg or more due to uncontrolled diabetes-related glycosuria. Urine glucose data were not available to rule out a confounding by glycosuria. Future work to confirm and characterize an independent effect of underhydration on mortality should consider potential confounding by glycosuria.
Acknowledging that observational data might be informative regarding causal effects, even when confounding variables are unmeasured [
93], the present analysis reported weighted probabilities of having health conditions and estimated number of deaths, in addition to relative hazard ratios, in case readers wish to apply front door or do-calculus techniques to estimate the probability of death caused by underhydration. Front door adjustment may not be applicable to the present estimation problem, however, if the unmeasured confounders (e.g., environmental factors, pain, psychological stress) have a strong effect on the mediating metabolic dysregulation. Independence of the intermediate from the unobserved confounder is a prerequisite for using front door adjustment [
93]. The lack of data regarding metabolic dysregulation from an intermediate time point limits the approach. Information or assumptions about a weak, negligible effect of environmental and psychological factors on metabolic dysregulation, which remains consistent over time, in the population of interest might enable front door adjustment.
4.7. Analysis Limitations
The present analysis was limited by the non-randomized, observational design, lack of serial measures of hydration biomarkers, chronic health conditions, relatively short 3 to 6 year follow-up period and small number of deaths. Although chronic health condition was determined from laboratory tests and multivariable models were used to control for confounding, the results are vulnerable to measurement or misclassification error and residual confounding. Interpretation of the results is limited to descriptive purposes and hypothesis generation about underlying causal mechanisms.
The analysis defined hydration in terms of biomarkers, which are sensitive to hypertonic dehydration but may be relatively insensitive to isotonic dehydration and/or hypohydration for persons with deficits in urine concentrating ability. The hydration criteria may have limited interpretation for individuals with advanced chronic disease. Serum sodium and urine osmolality are influenced by chronic health conditions. Hyperglycemia can distort serum sodium downwards. Urine glucose may distort urine osmolality upwards. Medications prescribed for chronic disease, such as diuretics, may disassociate serum sodium and urine osmolality from dietary intake. Renal urine concentration and/or urine dilution capacity may influence urine osmolality. The present sensitivity analyses confirmed that older age significantly modifies the effect of underhydration on chronic disease mortality.
4.8. Future Directions
In addition to the question of whether or not underhydration truly causes an increased risk of chronic disease and mortality, the present results raise questions about what type and amount of water intake could reduce said risk. In the preceding NHANES analysis, the prevalence of not meeting hydration criteria was significantly higher for non-acutely ill men and women aged 51–70 years with daily total water intake (TWI) below 45 mL/kg or plain drinking water intake (PWI) below 20 mL/kg [
1]. The discussion of that paper calls for careful sensitivity and specificity analyses to identify the optimal ml/kg TWI and PWI for each population sub-group and condition. Group- and condition-specific randomized trials are needed to test effects of different levels of water intake, defined relative to optimal ml/kg TWI or PWI cutoffs, on chronic disease incidence and progression.