Calorie Restriction Improves Physical Performance and Modulates the Antioxidant and Inflammatory Responses to Acute Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Calorie Restriction Prescription
2.3. Exercise Energy Efficiency Stress Test
2.4. Experimental Procedure
2.5. NEFA Determinations
2.6. Enzymatic Determinations
2.7. Malonyldialdehyde Determination
2.8. Assay of Nitrotyrosine and Protein Carbonyls
2.9. Nitrite and Nitrate Determination
2.10. PBMC RNA Extraction and Real-Time PCR Assay
2.11. Statistical Analysis
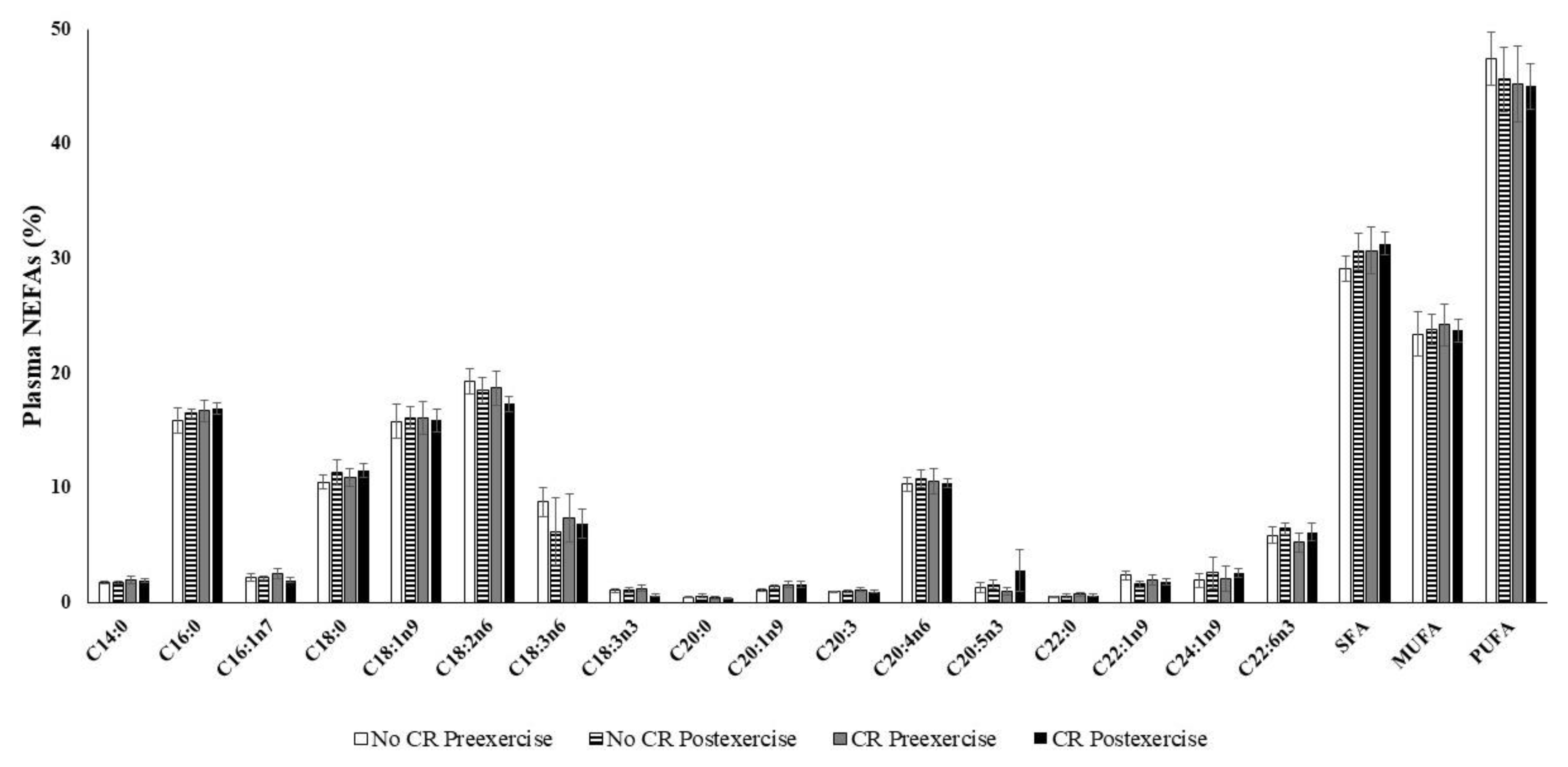
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CAT | catalase |
| NEFA | non-esterified fatty acid |
| SOD | superoxide dismutase |
References
- Piper, M.D.; Bartke, A. Diet and aging. Cell Metab. 2008, 8, 99–104. [Google Scholar] [CrossRef] [PubMed]
- McCay, C.M.; Crowell, M.F.; Maynard, L.A. Nutrition metabolism classic—The effect of retarded growth upon the length of life-span and upon the ultimate body size (reprinted from journal of nutrition, voL 10, pg 63–79, 1935). Nutrition 1989, 5, 155–171. [Google Scholar] [PubMed]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending Healthy Life Span-From Yeast to Humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric Restriction Delays Disease Onset and Mortality in Rhesus Monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef]
- Colman, R.J.; Beasley, T.M.; Kemnitz, J.W.; Johnson, S.C.; Weindruch, R.; Anderson, R.M. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Ketonen, J.; Pilvi, T.; Mervaala, E. Caloric restriction reverses high-fat diet-induced endothelial dysfunction and vascular superoxide production in C57Bl/6 mice. Heart Vessels 2010, 25, 254–262. [Google Scholar] [CrossRef]
- Minamiyama, Y.; Bito, Y.; Takemura, S.; Takahashi, Y.; Kodai, S.; Mizuguchi, S.; Nishikawa, Y.; Suehiro, S.; Okada, S. Calorie restriction improves cardiovascular risk factors via reduction of mitochondrial reactive oxygen species in type II diabetic rats. J. Pharmacol. Exp. Ther. 2007, 320, 535–543. [Google Scholar] [CrossRef]
- Castello, L.; Froio, T.; Cavallini, G.; Biasi, F.; Sapino, A.; Leonarduzzi, G.; Bergamini, E.; Poli, G.; Chiarpotto, E. Calorie restriction protects against age-related rat aorta sclerosis. FASEB J. 2005, 19, 1863–1865. [Google Scholar] [CrossRef]
- Mattison, J.A.; Roth, G.S.; Beasley, T.M.; Tilmont, E.M.; Handy, A.M.; Herbert, R.L.; Longo, D.L.; Allison, D.B.; Young, J.E.; Bryant, M.; et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 2012, 489, 318–321. [Google Scholar] [CrossRef]
- Cava, E.; Fontana, L. Will calorie restriction work in humans? Aging (Albany N. Y.) 2013, 5, 507–514. [Google Scholar] [CrossRef]
- Locher, J.L.; Goldsby, T.U.; Goss, A.M.; Kilgore, M.L.; Gower, B.; Ard, J.D. Calorie restriction in overweight older adults: Do benefits exceed potential risks? Exp. Gerontol. 2016, 86, 4–13. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Liu, D. Mechanistic perspectives of calorie restriction on vascular homeostasis. Sci. China Life Sci. 2014, 57, 742–754. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnson, J.B.; Summer, W.; Cutler, R.G.; Martin, B.; Hyun, D.H.; Dixit, V.D.; Pearson, M.; Nassar, M.; Telljohann, R.; Tellejohan, R.; et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 2007, 42, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Vaziri, N.D.; Norris, K.; An, W.S.; Quiroz, Y.; Rodriguez-Iturbe, B. High-calorie diet with moderate protein restriction prevents cachexia and ameliorates oxidative stress, inflammation and proteinuria in experimental chronic kidney disease. Clin. Exp. Nephrol. 2010, 14, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Feng, F.; Xiong, X.; Li, R.; Chen, N. Exercise coupled with dietary restriction reduces oxidative stress in male adolescents with obesity. J. Sports Sci. 2017, 35, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Civitarese, A.E.; Carling, S.; Heilbronn, L.K.; Hulver, M.H.; Ukropcova, B.; Deutsch, W.A.; Smith, S.R.; Ravussin, E.; Team, C.P. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007, 4, e76. [Google Scholar] [CrossRef]
- Das, S.K.; Balasubramanian, P.; Weerasekara, Y.K. Nutrition modulation of human aging: The calorie restriction paradigm. Mol. Cell. Endocrinol. 2017, 455, 148–157. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Redman, L.; Heilbronn, L.K.; Martin, C.K.; Ravussin, E. Caloric restriction with or without exercise: The fitness versus fatness debate. Med. Sci. Sports Exerc. 2010, 42, 152–159. [Google Scholar] [CrossRef]
- Friedlander, A.L.; Braun, B.; Pollack, M.; MacDonald, J.R.; Fulco, C.S.; Muza, S.R.; Rock, P.B.; Henderson, G.C.; Horning, M.A.; Brooks, G.A.; et al. Three weeks of caloric restriction alters protein metabolism in normal-weight, young men. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E446–E455. [Google Scholar] [CrossRef]
- Vessby, B.; Gustafsson, I.B.; Boberg, J.; Karlström, B.; Lithell, H.; Werner, I. Substituting polyunsaturated for saturated fat as a single change in a Swedish diet: Effects on serum lipoprotein metabolism and glucose tolerance in patients with hyperlipoproteinaemia. Eur. J. Clin. Investig. 1980, 10, 193–202. [Google Scholar] [CrossRef]
- Zock, P.L.; Mensink, R.P.; Harryvan, J.; de Vries, J.H.; Katan, M.B. Fatty acids in serum cholesteryl esters as quantitative biomarkers of dietary intake in humans. Am. J. Epidemiol. 1997, 145, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Glatz, J.F.; Soffers, A.E.; Katan, M.B. Fatty acid composition of serum cholesteryl esters and erythrocyte membranes as indicators of linoleic acid intake in man. Am. J. Clin. Nutr. 1989, 49, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Martorell, M.; Capo, X.; Sureda, A.; Batle, J.M.; Llompart, I.; Argelich, E.; Tur, J.A.; Pons, A. Effect of DHA on plasma fatty acid availability and oxidative stress during training season and football exercise. Food Funct. 2014, 5, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Fock, K.M.; Khoo, J. Diet and exercise in management of obesity and overweight. J. Gastroenterol. Hepatol. 2013, 28, 59–63. [Google Scholar] [CrossRef]
- Miller, W.C.; Koceja, D.M.; Hamilton, E.J. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int. J. Obes. 1997, 21, 941–947. [Google Scholar] [CrossRef]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef]
- Farrán, A.; Zamora, R.; Cervera, P. Tablas de Composición de Alimentos Del CESNID (Food Composition Tables of CESNID). 2004. Available online: http://www.sennutricion.org/en/2013/05/13/tablas-de-composicin-de-alimentos-del-cesnid (accessed on 26 March 2020).
- Buchfuhrer, M.J.; Hansen, J.E.; Robinson, T.E.; Sue, D.Y.; Wasserman, K.; Whipp, B.J. Optimizing the exercise protocol for cardiopulmonary assessment. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 1558–1564. [Google Scholar] [CrossRef]
- Wasserman, K.; Beaver, W.L.; Whipp, B.J. Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation 1990, 81, II14-30. [Google Scholar]
- Peronnet, F.; Massicotte, D. Table of nonprotein respiratory quotient—An update. Can. J. Sport Sci. Rev. Can. Sci. Sport 1991, 16, 23–29. [Google Scholar]
- Martorell, M.; Capó, X.; Bibiloni, M.M.; Sureda, A.; Mestre-Alfaro, A.; Batle, J.M.; Llompart, I.; Tur, J.A.; Pons, A. Docosahexaenoic acid supplementation promotes erythrocyte antioxidant defense and reduces protein nitrosative damage in male athletes. Lipids 2015, 50, 131–148. [Google Scholar] [CrossRef]
- Martorell, M.; Capo, X.; Sureda, A.; Tur, J.A.; Pons, A. Chromatographic and Enzymatic Method to Quantify Individual Plasma Free and Triacylglycerol Fatty Acids. Chromatographia 2015, 78, 259–266. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kiningham, R.B.; Gorenflo, D.W. Weight loss methods of high school wrestlers. Med. Sci. Sports Exerc. 2001, 33, 810–813. [Google Scholar] [CrossRef] [PubMed]
- King, N.A.; Caudwell, P.P.; Hopkins, M.; Stubbs, J.R.; Naslund, E.; Blundell, J.E. Dual-process action of exercise on appetite control: Increase in orexigenic drive but improvement in meal-induced satiety. Am. J. Clin. Nutr. 2009, 90, 921–927. [Google Scholar] [CrossRef]
- Villeneuve, P.J.; Morrison, H.I.; Craig, C.L.; Schaubel, D.E. Physical activity, physical fitness, and risk of dying. Epidemiology 1998, 9, 626–631. [Google Scholar] [CrossRef]
- Willcockson, M.A.; Wall-Scheffler, C.M. Reconsidering the Effects of Respiratory Constraints on the Optimal Running Speed. Med. Sci. Sports Exerc. 2012, 44, 1344–1350. [Google Scholar] [CrossRef]
- Egan, B.; D’Agostino, D.P. Fueling Performance: Ketones Enter the Mix. Cell Metab. 2016, 24, 373–375. [Google Scholar] [CrossRef]
- Fontan-Lozano, A.; Lopez-Lluch, G.; Maria Delgado-Garcia, J.; Navas, P.; Manuel Carrion, A. Molecular Bases of Caloric Restriction Regulation of Neuronal Synaptic Plasticity. Mol. Neurobiol. 2008, 38, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Hancock, C.R.; Han, D.H.; Higashida, K.; Kim, S.H.; Holloszy, J.O. Does calorie restriction induce mitochondrial biogenesis? A reevaluation. FASEB J. 2011, 25, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.J.; Wang, B.; Yardley, J.; Edwards, J.; Merry, B.J. The effect of aging and caloric restriction on mitochondrial protein density and oxygen consumption. Exp. Gerontol. 2004, 39, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Nisoli, E.; Tonetto, C.; Cardile, A.; Cozzi, V.; Bracale, R.; Tedesco, L.; Falcone, S.; Valerio, A.; Cantoni, O.; Clementi, E.; et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 2005, 310, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lluch, G.; Hunt, N.; Jones, B.; Zhu, M.; Jamieson, H.; Hilmer, S.; Cascajo, M.V.; Allard, J.; Ingram, D.K.; Navas, P.; et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. USA 2006, 103, 1768–1773. [Google Scholar] [CrossRef]
- Ramsey, J.J.; Harper, M.E.; Weindruch, R. Restriction of energy intake, energy expenditure, and aging. Free Radic. Biol. Med. 2000, 29, 946–968. [Google Scholar] [CrossRef]
- Nicholls, D.G. Mitochondrial membrane potential and aging. Aging Cell 2004, 3, 35–40. [Google Scholar] [CrossRef]
- Sohal, R.S.; Agarwal, S.; Candas, M.; Forster, M.J.; Lal, H. Effect of age and caloric restriction on dna oxidative damage in different tissues of c57bl/6 mice. Mech. Ageing Dev. 1994, 76, 215–224. [Google Scholar] [CrossRef]
- Busquets-Cortes, C.; Capo, X.; Argelich, E.; Ferrer, M.D.; Mateos, D.; Bouzas, C.; Abbate, M.; Tur, J.A.; Sureda, A.; Pons, A. Effects of Millimolar Steady-State Hydrogen Peroxide Exposure on Inflammatory and Redox Gene Expression in Immune Cells from Humans with Metabolic Syndrome. Nutrients 2018, 10, 1920. [Google Scholar] [CrossRef]
- Capo, X.; Martorell, M.; Sureda, A.; Tur, J.A.; Pons, A. Effects of docosahexaenoic supplementation and in vitro vitamin C on the oxidative and inflammatory neutrophil response to activation. Oxidative Med. Cell. Longev. 2015, 2015, 187849. [Google Scholar] [CrossRef]
- Vince, R.V.; Kirk, R.J.; Aye, M.M.; Atkin, S.L.; Madden, L.A. Impaired heat shock protein 72 expression in women with polycystic ovary syndrome following a supervised exercise programme. Cell Stress Chaperones 2020, 25, 73–80. [Google Scholar] [CrossRef]
- Cases, N.; Sureda, A.; Maestre, I.; Tauler, P.; Aguilo, A.; Cordova, A.; Roche, E.; Tur, J.A.; Pons, A. Response of antioxidant defences to oxidative stress induced by prolonged exercise: Antioxidant enzyme gene expression in lymphocytes. Eur. J. Appl. Physiol. 2006, 98, 263–269. [Google Scholar] [CrossRef]
- Hooper, P.L. Insulin Signaling, GSK-3, Heat Shock Proteins and the Natural History of Type 2 Diabetes Mellitus: A Hypothesis. Metab. Syndr. Relat. Disord. 2007, 5, 220–230. [Google Scholar] [CrossRef]
- Soti, C.; Nagy, E.; Giricz, Z.; Vigh, L.; Csermely, P.; Ferdinandy, P. Heat shock proteins as emerging therapeutic targets. Br. J. Pharmacol. 2005, 146, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G. Vitamin E regulatory mechanisms. Annu. Rev. Nutr. 2007, 27, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Stocker, A. Molecular mechanisms of vitamin E transport. Ann. N. Y. Acad. Sci. 2004, 1031, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Morley, S.; Wilson, K.; Nava, P.; Atkinson, J.; Manor, D. Intracellular trafficking of vitamin E in hepatocytes: The role of tocopherol transfer protein. J. Lipid Res. 2005, 46, 2072–2082. [Google Scholar] [CrossRef]
- Marcos, P.; Gonzalez-Fuentes, J.; Castro-Vazquez, L.; Lozano, M.V.; Santander-Ortega, M.J.; Rodriguez-Robledo, V.; Villaseca-Gonzalez, N.; Arroyo-Jimenez, M.M. Vitamin transporters in mice brain with aging. J. Anat. 2018, 232, 699–715. [Google Scholar] [CrossRef]
- Wenz, T. Regulation of mitochondrial biogenesis and PGC-1alpha under cellular stress. Mitochondrion 2013, 13, 134–142. [Google Scholar] [CrossRef]
- Busquets-Cortes, C.; Capo, X.; Bibiloni, M.D.M.; Martorell, M.; Ferrer, M.D.; Argelich, E.; Bouzas, C.; Carreres, S.; Tur, J.A.; Pons, A.; et al. Peripheral Blood Mononuclear Cells Antioxidant Adaptations to Regular Physical Activity in Elderly People. Nutrients 2018, 10, 1555. [Google Scholar] [CrossRef]
- Piccolo, B.D.; Keim, N.L.; Fiehn, O.; Adams, S.H.; Van Loan, M.D.; Newman, J.W. Habitual Physical Activity and Plasma Metabolomic Patterns Distinguish Individuals with Low vs. High Weight Loss during Controlled Energy Restriction. J. Nutr. 2015, 145, 681–690. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Mougios, V. Effects of exercise on the fatty-acid composition of blood and tissue lipids. Sports Med. 2004, 34, 1051–1076. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N. Fat as a fuel: Emerging understanding of the adipose tissue-skeletal muscle axis. Acta Physiol. (Oxf.) 2010, 199, 509–518. [Google Scholar] [CrossRef] [PubMed]


| Control | Calorie Restriction | ||
|---|---|---|---|
| Initial | Final | ||
| Energy (Kcal) | 2534 ± 639 | 2292 ± 137 | 1537 ± 84 * |
| Carbohydrate (% Energy) | 38.8 ± 6.7 | 41.7 ± 2.9 | 41.4 ± 2.3 |
| Protein (% Energy) | 22.3 ± 7.4 | 18.7 ± 0.9 | 20.5 ± 1.0 |
| Lipids (% Energy) | 38.9 ± 15.4 | 39.7 ± 2.7 | 38.1 ± 1.7 |
| Gene | Primer | Annealing Temperature |
|---|---|---|
| 18S | Fw: 5′-ATGTGAAGTCACTGTGCCAG-3′ | 60 °C |
| Rv:5′-GTGTAATCCGTCTCCACAGA-3′ | ||
| Catalase | Fw: 5′-TTT GGC TAC TTT GAG GTC AC-3′ | 60 °C |
| Rv: 5′-TCC CCA TTT GCA TTA ACC AG-3′ | ||
| Mn-SOD | Fw: 5′CGTGCTCCCACAC ATCAATC-3′ | 60 °C |
| Rev Rv: 5′-TGAACGTCACCG AGGAGAAG-3′ | ||
| Cu/Zn-SOD | Fw: 5′-TCA GGA GAC CAT TGC ATC ATT-3′ | 63 °C |
| Rv: 5′-CGC TTT CCT GTC TTT GTA CTT TCT TC-3′ | ||
| UCP-3 | Fw: 5′-CGT GGT GAT GTT CAT AAC CTA TG-3′ | 60 °C |
| Rv: 5′-CGG TGA TTC CCG TAA CAT CTG-3′ | ||
| NFκB | Fw:5′-AAACACTGTGAGGATGGGATCTG-3′ | 60 °C |
| Rv:5′-CGAAGCCGACCACCATGT-3′ | ||
| PGC-1α | Fw 5′-CAC TTA CAA GCC AAA CCA ACA ACT -3 | 62 °C |
| Rv 5′-CAA TAG TCT TGT TCT CAA ATG GGG A-3 | ||
| iNOS | Fw: 5′--TCTGCAGACACGTGCGTTACT-3′ | 62 °C |
| Rv: 5′-ATGCACAGCTGAGCATTCCA-3′ | ||
| HSP72 | Fw: 5′-CCGGCAAGGCCAACAAGATC-3′ | 59 °C |
| Rv: 5′-CCTCCACGGCGCTCTTCATG-3′ | ||
| IL-6 | Fw: 5′-TACATCCTCGACGGCATCTC-3′ | 63 °C |
| Rv: 5′-ACTCATCTGCACAGCTCTGG-3′ | ||
| GRd | Fw: 5′-TCA CGC AGT TAC CAA AAG GAA A-3′ | 64 °C |
| Rev: 5′-CAC ACC CAA GTC CCC TGC ATA T-3′ | ||
| HO-1 | Fw: 5′-CCA GCG GGC CAG CAA CAA AGT GC-3′ | 60 °C |
| Rev: 5′-AAG CCT TCA GTG CCC ACG GTA AGG-3′ | ||
| SIRT-3 | Fw: 5′-GAG CTT CTG GGC TGG ACA GA-3′ | 65 °C |
| Rev: 5′-TGG GAT GTG GAT GTC TCC TAT G-3′ | ||
| TFAM | Fw: 5′-TCGCTCTTCCTCTGCCTAAC-3′ | 60 °C |
| Rev: 5′-CAAGAGATGAAAACCACCTC-3′ | ||
| PPARγ | Fw: 5′ CCATTCTGGCCCACCAAC-3′ | 64 °C |
| Rev: 5′-AATGCGAGTGGTCTTCCATCA-5′ | ||
| p53 | Fw: 5′ AAGTCTGTACTTGCACG-3′ | 62 °C |
| Rev: 5′CTGGAGTCTTCCAGTGTG-3′ | ||
| α-TTP | Fw: 5′-CACCCCGAAATAACACCTTC-3′ | 62 °C |
| Rev: 5′TCGCTCTTCCTCTGCCTAAC-3′ |
| Resting Condition | Control | Calorie Restriction | ||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| Weight (kg) | 85.5 ± 14.3 | 85.8 ± 14.7 | 81.0 ± 1.9 | 76.2 ± 1.9 * |
| VO2 (mL/min) | 412.1 ± 124.1 | 489.9 ± 152.2 | 408 ± 18 | 405 ± 20 |
| VO2 (mL/min/kg) | 4.9 ±1.6 | 5.8 ± 1.8 | 5.0 ± 0.2 | 5.3 ± 0.2 |
| Expiratory volume (L/min) | 15.1 ± 3.1 | 16.5 ± 4.3 | 12.6 ± 0.5 | 12.0 ± 0.5 |
| Maximal exercise test | ||||
| Speed (km/h) | 12.3 ± 1.4 | 12.5 ± 1.3 | 14.6 ± 0.4 | - |
| VO2 (mL/min) | 3896.8 ± 402.8 | 3846.8 ± 420.4 | 3832 ± 141 | - |
| VO2 (mL/min/kg) | 46.1 ± 4.8 | 45.5 ± 5.1 | 47.5 ± 1.7 | - |
| 50% maximal speed | ||||
| Speed (km/h) | 6.1 ± 0.7 | 6.3 ± 0.7 | 7.7 ± 0.3 | 7.7 ± 0.3 |
| VO2 (mL/kg.min) | 27.8 ± 3.2 | 26.4 ± 3.7 | 29.5 ± 1.4 | 28.0 ± 1.4 |
| Expiratory volume (L/min) | 65.9 ± 8.6 | 63.5 ± 5.9 | 53.0 ± 2.2 | 46.0 ± 2.0 * |
| VO2 (mL/min) | 2351.4 ± 239.9 | 2227.3 ± 189.8 | 2366 ± 93 | 2115 ± 96 * |
| VCO2 (mL/min) | 2268.8 ± 265.7 | 2185.9 ± 164.8 | 2126 ± 99 | 1797 ± 103 * |
| Respiratory quotient | 0.96 ± 0.04 | 0.98 ± 0.05 | 0.900 ± 0.081 | 0.850 ± 0.110 * |
| 60% maximal speed | ||||
| Speed (km/h) | 7.4 ± 0.9 | 7.5 ± 0.8 | 9.1 ± 0.3 | 9.1 ± 0.3 |
| VO2 (mL/kg.min) | 32.2 ± 4.1 | 31.9 ± 3.4 | 34.3± 1.4 | 32.9 ± 1.3 |
| Expiratory volume (L/min) | 77.4 ± 9.3 | 81.4 ± 11.3 | 65.5 ± 2.7 | 57.7 ± 2.1 * |
| VO2 (mL/min) | 2726.7 ± 349.5 | 2701.5 ± 256.2 | 2755 ± 95 | 2484 ± 81 * |
| VCO2 (mL/min) | 2652.1 ± 321.4 | 2708.3 ± 244.1 | 2616 ± 105 | 2273 ± 106* |
| Respiratory quotient | 0.97 ± 0.04 | 1.003 ± 0.04 | 0.950 ± 0.071 | 0.915 ± 0.104 |
| 70% maximal speed | ||||
| Speed (km/h) | 8.6 ± 1 | 8.8 ± 0.9 | 10.4 ± 0.3 | 10.4 ± 0.3 |
| VO2 (mL/kg.min) | 37.2 ± 4 | 36.5 ± 4.0 | 38.8 ± 1.6 | 37.1 ± 1.6 * |
| Expiratory volume (L/min) | 97.3 ± 13.1 | 98.7 ± 18.3 | 80.0 ± 4.0 | 67.0 ± 2.2 * |
| VO2 (mL/min) | 3154.8 ± 346.8 | 3083.3 ± 314.3 | 3117 ± 109 | 2804 ± 101 * |
| VCO2 (mL/min) | 3201.2 ± 364.5 | 3185.2 ± 326.3 | 3067 ± 134 | 2617 ± 116 * |
| Respiratory quotient | 1.02 ± 0.04 | 1.03 ± 0.04 | 0.984 ± 0.080 | 0.933 ± 0.241 * |
| Threshold | ||||
| Speed (km/h) | 10.1 ± 1.3 | 10.5 ± 1.5 | 11.7 ± 0.3 | 11.7 ± 0.3 |
| VO2 (mL/kg.min) | 41.6 ± 4.5 | 41.2 ± 4.8 | 42.6 ± 1.6 | 40.8 ± 1.1 |
| Expiratory volume (L/min) | 123.5 ± 19.1 | 126.7 ± 20.4 | 92.6 ± 3.2 | 81.6 ± 3.0 * |
| VO2 (mL/min) | 3530.6 ± 418.5 | 3471.9 ± 366.2 | 3428 ± 100 | 3112 ± 74 * |
| VCO2 (mL/min) | 3830.3 ± 498.5 | 3838.5 ± 532.1 | 3427 ± 110 | 2959 ± 77 * |
| Respiratory quotient | 1.09 ± 1.4 | 1.1 ± 0.07 | 1.000 ± 0.080 | 0.951 ± 0.081 * |
| ANOVA | ||||||
|---|---|---|---|---|---|---|
| No CR | CR | E | CR | E × CR | ||
| Catalase (K/L) | Pre-exercise | 374 ± 186 | 389 ± 174 | 0.933 | 0.438 | 0.480 |
| Post-exercise | 207 ± 65.6 | 520 ± 312 | ||||
| SOD (pkatal/L) | Pre-exercise | 740 ± 51 | 717 ± 96 | 0.259 | 0.372 | 0.573 |
| Post-exercise | 700 ± 56 | 728 ± 63 | ||||
| MDA (mM) | Pre-exercise | 8.27 ± 1.29 | 9.98 ± 2.99 | 0.202 | 0.256 | 0.112 |
| Post-exercise | 8.91 ± 4.99 | 8.79 ± 1.65 | ||||
| Carbonyl (%) | Pre-exercise | 100 ± 7 | 101 ± 9 | 0.295 | 0.983 | 0.920 |
| Post-exercise | 111 ± 11 | 110 ± 7 | ||||
| N-Tyr (%) | Pre-exercise | 100 ± 7 | 109 ± 5 | 0.096 | 0.121 | 0.943 |
| Post-exercise | 90.5 ± 3.1 | 101 ± 8 | ||||
| Nitrite (nM) | Pre-exercise | 167 ± 10 | 183 ± 12 | 0.325 | 0.206 | 0.449 |
| Post-exercise | 180 ± 13 | 186 ± 13 | ||||
| Nitrate (μM) | Pre-exercise | 33.5 ± 3.4 | 35.4 ± 3.2 | 0.561 | 0.957 | 0.574 |
| Post-exercise | 33.4 ± 2.8 | 31.8 ± 1.8 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capó, X.; Martorell, M.; Ferrer, M.D.; Sureda, A.; Pons, V.; Domingo, J.C.; Drobnic, F.; Martínez-Rodríguez, A.; Leyva-Vela, B.; Sarabia, J.M.; et al. Calorie Restriction Improves Physical Performance and Modulates the Antioxidant and Inflammatory Responses to Acute Exercise. Nutrients 2020, 12, 930. https://doi.org/10.3390/nu12040930
Capó X, Martorell M, Ferrer MD, Sureda A, Pons V, Domingo JC, Drobnic F, Martínez-Rodríguez A, Leyva-Vela B, Sarabia JM, et al. Calorie Restriction Improves Physical Performance and Modulates the Antioxidant and Inflammatory Responses to Acute Exercise. Nutrients. 2020; 12(4):930. https://doi.org/10.3390/nu12040930
Chicago/Turabian StyleCapó, Xavier, Miquel Martorell, Miguel D. Ferrer, Antoni Sureda, Victoria Pons, Juan C. Domingo, Franchek Drobnic, Alejandro Martínez-Rodríguez, Belén Leyva-Vela, José M. Sarabia, and et al. 2020. "Calorie Restriction Improves Physical Performance and Modulates the Antioxidant and Inflammatory Responses to Acute Exercise" Nutrients 12, no. 4: 930. https://doi.org/10.3390/nu12040930
APA StyleCapó, X., Martorell, M., Ferrer, M. D., Sureda, A., Pons, V., Domingo, J. C., Drobnic, F., Martínez-Rodríguez, A., Leyva-Vela, B., Sarabia, J. M., Herranz-López, M., Roche, E., Tur, J. A., & Pons, A. (2020). Calorie Restriction Improves Physical Performance and Modulates the Antioxidant and Inflammatory Responses to Acute Exercise. Nutrients, 12(4), 930. https://doi.org/10.3390/nu12040930














