Erythropoiesis and Red Cell Indices Undergo Adjustments during Pregnancy in Response to Maternal Body Size but not Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Blood Sampling and Metabolite Analysis
2.4. Statistical Analyse
- (1)
- To identify the relationship between EPO and independent factors such as iron intake, pgBMI, gestational week and the presence of inflammation, as well as the relationship of EPO with hepcidin and iron nutrition status indicators. Pearson bivariate correlations were carried out using log-transformed data for variables without normal distribution.
- (2)
- Differences in EPO along pregnancy between the AW and Ob groups were analyzed using two generalized linear models (GLM):
- (3)
- Differences in red cell indices between study groups were also analyzed using GLM. These models included study group (Ob and AW), gestational age, presence of an underlying health condition (yes/no) and presence of pregnancy complications (yes/no) as fixed factors, and vitamin B12 and EPO as covariates. As with EPO, models for red cell indices were performed with and without adding IL–6 to control for inflammation.
3. Results
3.1. Relationship between Erythropoietin and Other Factors
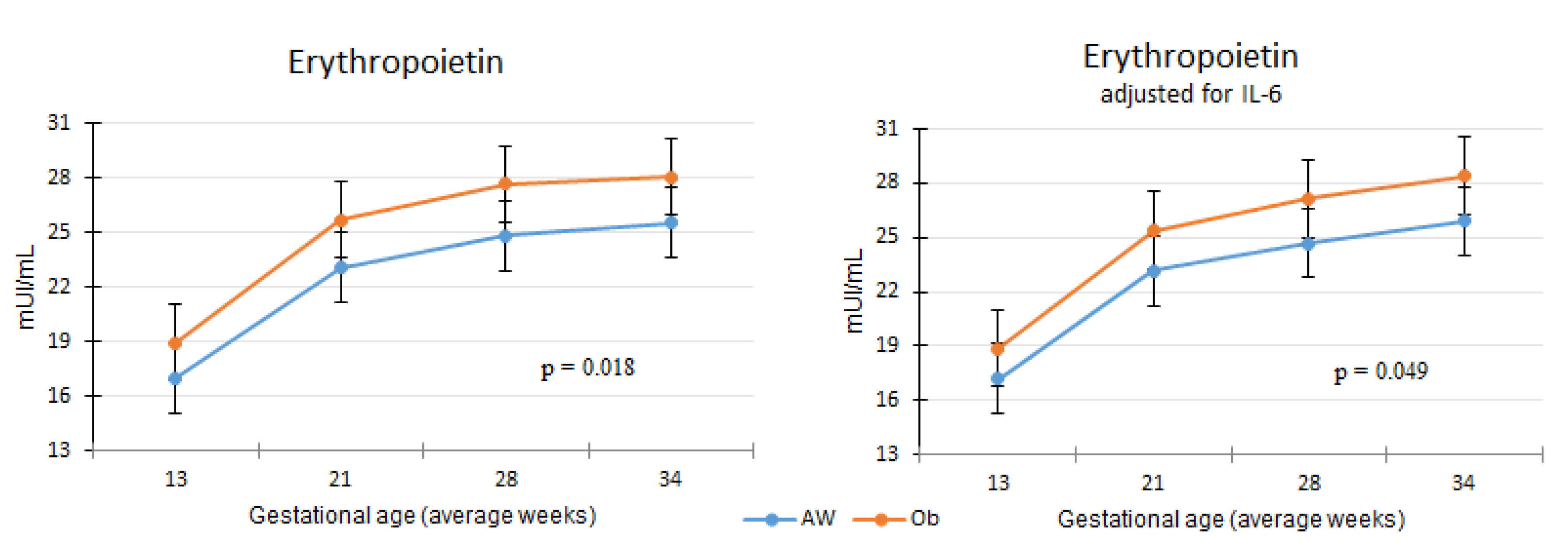
3.2. Erythropoietin Concentration Differences between Obesity and Normal Weight Study Groups
3.3. Differences in Red Cell Indices between Obesity and Normal Weight Study Groups
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bothwell, T.H. Iron requirements in pregnancy and strategies to meet them. Am. J. Clin. Nutr. 2000, 72, 257S–264S. [Google Scholar] [CrossRef]
- Fisher, A.L.; Nemeth, E. Iron homeostasis during pregnancy. Am. J. Clin. Nutr. 2017, 106, 1567S–1574S. [Google Scholar] [CrossRef]
- Tussing-Humphreys, L.; Pusatcioglu, C.; Nemeth, E.; Braunschweig, C. Rethinking iron regulation and assessment in iron deficiency, anemia of chronic disease, and obesity: Introducing hepcidin. J. Acad. Nutr. Diet. 2012, 112, 391–400. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta 2012, 1823, 1434–1443. [Google Scholar] [CrossRef]
- Conrad, K.P.; Benyo, D.F.; Westerhausen-Larsen, A.; Miles, T.M. Expression of erythropoietin by the human placenta. FASEB J. 1996, 10, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S. Changes in age distribution of erythrocytes during pregnancy: A longitudinal study. Gynecol. Obstet. Investig 1993, 36, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S.; Mamet, Y. Red blood cell survival and kinetics during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 93, 185–192. [Google Scholar] [CrossRef]
- McKenzie, S.B. Hematología Clínica, 2nd ed.; Manual Moderno: Mexico City, México, 2000. [Google Scholar]
- Bolton, F.G.; Street, M.J.; Pace, A.J. Changes in Erythrocyte Volume and Shape in Pregnancy. Obstet. Gynecol. Surv. 1983, 38, 461. [Google Scholar] [CrossRef]
- Koenig, M.; Tussing-Humphreys, L.; Day, J.; Cadwell, B.; Nemeth, E. Hepcidin and Iron Homeostasis during Pregnancy. Nutrients 2014, 6, 3062–3083. [Google Scholar] [CrossRef]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology E-Book; Elsevier Health Sciences: London, UK, 2015; ISBN 9780323389303. [Google Scholar]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Yanoff, L.B.; Menzie, C.M.; Denkinger, B.; Sebring, N.G.; McHugh, T.; Remaley, A.T.; Yanovski, J.A. Inflammation and iron deficiency in the hypoferremia of obesity. Int. J. Obes. 2007, 31, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Flores-Quijano, M.E.; Vega-Sánchez, R.; Tolentino-Dolores, M.C.; López-Alarcón, M.G.; Flores-Urrutia, M.C.; López-Olvera, A.D.; Talavera, J.O. Obesity Is Associated with Changes in Iron Nutrition Status and Its Homeostatic Regulation in Pregnancy. Nutrients 2019, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 28 August 2018).
- Crosby, W.H. Reticulocyte counts. Arch. Intern. Med. 1981, 141, 1747–1748. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S. Principles of Nutritional Assessment; Oxford University Press: New York, NY, USA, 2005; ISBN 9780195171693. [Google Scholar]
- Schulze, K.J.; Christian, P.; Ruczinski, I.; Ray, A.L.; Nath, A.; Wu, L.S.-F.; Semba, R.D. Hepcidin and iron status among pregnant women in Bangladesh. Asia Pac. J. Clin. Nutr. 2008, 17, 451–456. [Google Scholar] [PubMed]
- Cao, C.; Pressman, E.K.; Cooper, E.M.; Guillet, R.; Westerman, M.; O’Brien, K.O. Prepregnancy Body Mass Index and Gestational Weight Gain Have No Negative Impact on Maternal or Neonatal Iron Status. Reprod. Sci. 2016, 23, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Flores-Quijano, M.E.; Montalvo-Velarde, I.; Vital-Reyes, V.S.; Rodríguez-Cruz, M.; Rendón-Macías, M.E.; López-Alarcón, M. Longitudinal Analysis of the Interaction between Obesity and Pregnancy on Iron Homeostasis: Role of Hepcidin. Arch. Med. Res. 2016, 47, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Villarroel, P.; Arredondo, M.; Olivares, M. Anemia de las enfermedades crónicas asociada a obesidad: Papel de la hepcidina como mediador central. Rev. Méd. Chile 2013, 141, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Kańka, A.; Maciejewski, T.; Niemiec, K.T. The role and regulation of secretion of erythropoietin in pregnancy. Med. Wieku Rozwoj. 2013, 17, 270–275. [Google Scholar] [PubMed]
- El-Kannishy, G.M.; Megahed, A.F.; Tawfik, M.M.; El-Said, G.; Zakaria, R.T.; Mohamed, N.A.; Taha, E.M.; Ammar, A.A.; Abd Eltawab, A.M.; Sayed-Ahmed, N.A. Obesity may be erythropoietin dose-saving in hemodialysis patients. Kidney Res. Clin. Pract. 2018, 37, 148–156. [Google Scholar] [CrossRef] [PubMed]
- WHO. Serum Transferrin Receptor Levels for the Assessment of Iron Status and Iron Deficiency in Populations; Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Peyrin-Biroulet, L.; Williet, N.; Cacoub, P. Guidelines on the diagnosis and treatment of iron deficiency across indications: A systematic review. Am. J. Clin. Nutr. 2015, 102, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.E.; Sharif, M.U.; Stack, A.G. Transferrin Saturation: A Body Iron Biomarker. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 75, pp. 71–97. [Google Scholar]


| Adequate Weight (n = 53) | Obese (n = 40) | p | |
|---|---|---|---|
| Erythropoietin | 16.00 (12.95, 19.10) | 18.05 (13.65, 23.07) | 0.19 |
| Reticulocytes (% of total erythrocytes, corrected with hematocrit) | 1.20 (1.00, 1.87) | 1.40 (1.00, 1.80) | 0.77 |
| Erythrocytes (106/µL) | 4.50 (4.34, 4.73) | 4.54 (4.40, 4.98) | 0.20 |
| MCV (fL) | 90.86 (88.30, 94.23) | 88.88 (85.42, 92.54) | 0.05 |
| MCH (pg) | 30.30 (29.28, 31.42) | 29.31 (27.93, 30.80) | <0.01 |
| MCHC (g/dL) | 33.32 (32.76, 34.03) | 33.01 (32.43, 33.80) | 0.14 |
| RDW | 11.69 (11.06, 12.60) | 12.23 (11.51, 13.28) | 0.02 |
| Hemoglobin (g/dL) | 13.55 (13.18, 14.45) | 13.39 (13.08, 13.99) | 0.36 |
| Ferritin (ng/mL) | 39.30 (27.60, 65.05) | 40.60 (19.40, 96.15) | 0.89 |
| sTfr (mg/L) | 1.00 (0.84, 1.20) | 1.04 (0.82, 1.44) | 0.41 |
| Serum iron (μg/dL) | 162.45 (129.8, 199.5) | 149.76 (113.6, 199.7) | 0.47 |
| Hepcidin (ng/mL) | 8.04 (5.88, 11.86) | 9.58 (6.21, 15.67) | 0.23 |
| Serum folate (ng/mL) | 31.90 (24.30, 38.30) | 27.90 (24.32, 41.75)) | 0.28 |
| <3 ng/mL * | 0 | 0 | |
| Erythrocyte folate (pg/mL) | 567.0 (464.5, 748.65) | 523.0 (368.5, 778.0) | 0.58 |
| <120 pg/mL * | 0 | 0 | |
| Vitamin B12 (pg/mL) | 344.50 (263.75, 494.50) | 258.50 (186.75, 352.25) | <0.01 |
| <200 pg/mL * | 5 (10.5%) | 10 (26.3%) | 0.05 |
| IL–6 (pg/mL) | 1.79 (1.63, 2.10) | 2.15 (1.81, 2.43) | <0.01 |
| Leptin (pg/mL) | 21.50 (15.11, 26.25) | 44.48 (32.14, 61.57) | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Sánchez, R.; Tolentino-Dolores, M.C.; Cerezo-Rodríguez, B.; Chehaibar-Besil, G.; Flores-Quijano, M.E. Erythropoiesis and Red Cell Indices Undergo Adjustments during Pregnancy in Response to Maternal Body Size but not Inflammation. Nutrients 2020, 12, 975. https://doi.org/10.3390/nu12040975
Vega-Sánchez R, Tolentino-Dolores MC, Cerezo-Rodríguez B, Chehaibar-Besil G, Flores-Quijano ME. Erythropoiesis and Red Cell Indices Undergo Adjustments during Pregnancy in Response to Maternal Body Size but not Inflammation. Nutrients. 2020; 12(4):975. https://doi.org/10.3390/nu12040975
Chicago/Turabian StyleVega-Sánchez, Rodrigo, Mari Cruz Tolentino-Dolores, Blanca Cerezo-Rodríguez, Georgette Chehaibar-Besil, and María Eugenia Flores-Quijano. 2020. "Erythropoiesis and Red Cell Indices Undergo Adjustments during Pregnancy in Response to Maternal Body Size but not Inflammation" Nutrients 12, no. 4: 975. https://doi.org/10.3390/nu12040975
APA StyleVega-Sánchez, R., Tolentino-Dolores, M. C., Cerezo-Rodríguez, B., Chehaibar-Besil, G., & Flores-Quijano, M. E. (2020). Erythropoiesis and Red Cell Indices Undergo Adjustments during Pregnancy in Response to Maternal Body Size but not Inflammation. Nutrients, 12(4), 975. https://doi.org/10.3390/nu12040975





