Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review
Abstract
1. Introduction
2. Basis of Digestive System Mechanism
3. Digestion Models for Testing the Bioaccessibility of Secondary Plant Metabolites
3.1. Static Models
3.2. Dynamic Models
3.3. Colonic Models
4. In Vitro Digestion Stages
4.1. Mouth Stage
4.2. Gastric Stage
4.3. Small Intestinal Stage
4.4. Colonic Stage
5. Polyphenols: Structure–Antioxidant Activity Relationship
5.1. The Antioxidant Activity of Phenolic Acids and Flavonoids
5.1.1. Phenolic Acids
5.1.2. Flavonoids
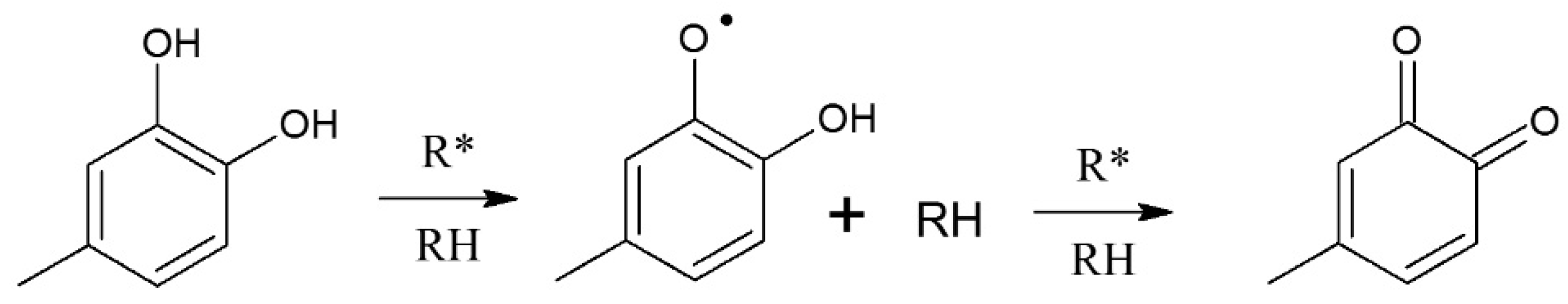
5.2. Mechanisms of Free Radical Scavenging by Polyphenols and Influence of Reaction Conditions—General Information
5.2.1. Phenolic Acids
5.2.2. Flavonoids
6. Parameters Affecting the Chemical Changes in Phytochemicals during Digestion
6.1. Impact of the Plant Matrix
6.2. Influence of Food Processing and Interaction of Phytochemicals with Other Food Components
7. Effects of Simulated Digestion on Phenolic Composition and Their Antioxidant Activity in Food
7.1. Impact of Physiological Conditions Encountered in the Gastrointestinal Tract on Phenolic Composition
7.2. Impact of Physiological Conditions Encountered in the Gastrointestinal Tract on the Antioxidant Activity of Polyphenols
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Galanakis, C.M. Bioavailability, bioaccessibility and bioactivity of food components. In Nutraceutical and Functional Food Components: Effects of Innovative Processing Techniques, 1st ed.; Galankis, C.M., Ed.; Elsevier Inc.: Athens, Greece, 2017; pp. 1–14. [Google Scholar]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K. Aprotosoaie AC1, Trifan A, Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Vladimir-Knežević, S.; Blažeković, B.; Bival Štefan, M.; Babac, M. Plant polyphenols as antioxidants influencing the human health. In Phytochemicals as Nutraceuticals—Global Approaches to Their Role in Nutrition and Health; In Tech: London, UK, 2012; pp. 155–177. [Google Scholar] [CrossRef]
- Grigore, A.; Pirvu, L.; Bubueanu, C.; Panteli, M.; Rasit, I. Influence of chemical composition on the antioxidant and anti-inflammatory activity of Rosmarinus Officinalis extracts. Rom. Biotech. Lett. 2015, 20, 10047–10054. [Google Scholar]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The antimicrobial and antiviral activity of polyphenols from almond (Prunus dulcis L.) Skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, G.; Martel, F. The effect of dietary polyphenols on intestinal absorption of glucose and fructose: Relation with obesity and type 2 diabetes. Food Rev. Int. 2019, 35, 390–406. [Google Scholar] [CrossRef]
- Somaratne, G.; Ferrua, M.J.; Yea, A.; Naud, F.; Flouryd, J.; Dupontd, D.; Singh, J. Food material properties as determining factors in nutrient release during human gastric digestion: A review. Crit. Rev. Food Sci. Nutr. 2020, 1–17. [Google Scholar] [CrossRef]
- Li, C.; Yu, W.; Wue, P.; Chen, X.D. Current in vitro digestion systems for understanding food digestion in human upper gastrointestinal tract. Trends Food Sci. Technol. 2020, 96, 114–126. [Google Scholar] [CrossRef]
- Todorovic, T.; Dozic, I.; Barrero, M.V.; Ljuskovic, B.; Pejovic, J.; Marjanovic, M.; Knezevic, M. Salivary enzymes and periodontal disease. Med. Oral Patol. Oral Cir. Bucal 2006, 11, 115–119. [Google Scholar]
- Peyrot des Gachons, C.; Breslin, P.A. Salivary amylase: Digestion and metabolic syndrome. Curr. Diab. Rep. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Kulkarni, B.V.; Mattes, R.D. Lingual lipase activity in the orosensory detection of fat by humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 30, 879–885. [Google Scholar] [CrossRef]
- Lamb, P.J.; Griffin, S.M. The Anatomy and physiology of the Oesophagus. In Upper Gastrointestinal Surgery; Fielding, J.W.L., Hallissey, M., Eds.; Springer: London, UK, 2005; pp. 1–16. [Google Scholar]
- Seeley, R.R.; Stephens, T.D.; Tate, P. Anatomy and Physiology, 6th ed.; McGraw Hill: Boston, MA, USA, 2003. [Google Scholar]
- Collins, J.T.; Badireddy, M. Anatomy, abdomen and pelvis, small intestine. In StatPearls [Internet]. Treasure Island; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459366/ (accessed on 14 April 2020).
- Kiela, P.R.; Ghishan, F.K. Physiology of intestinal absorption and secretion. Best Pract Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef]
- Kopáčová, M.; Rejchrt, S.; Bureš, J.; Tachecí, I. Small intestinal tumours. Gastroenterol Res. Pract. 2013, 2013, 702536. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, T.C.; Fossmark, R.; Waldum, H.L. The phylogeny and biological function of gastric juice—Microbiological consequences of removing gastric scid. Int. J. Mol. Sci. 2019, 20, 6031. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, L.L.; Sharma, S. Physiology, large Intestine. In StatPearls, Treasure Island; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507857/ (accessed on 24 March 2020).
- Bayliak, M.M.; Burdyliuk, N.I.; Lushchak, V.I. Effects of pH on antioxidant and pro-oxidant properties of common medicinal herbs. Open Life Sci. 2016, 11, 298–307. [Google Scholar] [CrossRef]
- Sun, H.N.; Mu, T.H.; Xi, L.S. Effect of pH, heat, and light treatments on the antioxidant activity of sweet potato leaf polyphenols. Int. J. Food Prop. 2017, 20, 318–332. [Google Scholar] [CrossRef]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martίnez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Minekus, M. The TNO Gastro-Intestinal Model (TIM). In The Impact of Food Bioactives on Health. In Vitro and Ex Vivo Models, 1st ed.; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing AG: Cham, Switzerland, 2015; pp. 37–46. [Google Scholar]
- Vardakou, M.; Mercuri, A.; Barker, S.A.; Craig, D.Q.M.; Faulks, R.M.; Wickham, M.S.J. Achieving antral grinding forces in biorelevant in vitro models: Comparing the USP Dissolution Apparatus II and the Dynamic Gastric Model with human in vivo data. AAPS Pharm. Sci. Technol. 2011, 12, 620–626. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Egger, L.; Ménard, O.; Delgado-Andrade, C.; Alvito, P.; Assunção, R.; Balance, S.; Barberá, R.; Brodkorb, A.; Cattenoz, T.; Clemente, A.; et al. The harmonized INFOGEST in vitro digestion method: From knowledge to action. Food Res. Int. 2016, 88, 217–225. [Google Scholar] [CrossRef]
- Eggera, L.; Schlegelb, P.; Baumanna, C.; Stoffersa, H.; Guggisberga, D.; Brüggera, C.; Dürra, D.; Stollb, P.; Vergèresa, G.; Portmanna, R. Physiological comparability of the harmonized INFOGEST in vitro digestion method to in vivo pig digestion. Food Res. Int. 2017, 102, 567–574. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Golding, M.; Wooster, T.J. The influence of emulsion structure and stability on lipid digestion. Curr. Opin. Colloid Interface Sci. 2010, 15, 90–101. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Zajac, N. Digestion and absorption of phenolic compounds assessed by in vitro simulation methods. A review. Rocz. Panstw. Zakl. Hig. 2013, 64, 79–84. [Google Scholar]
- Minekus, M.; Marteau, P.; Havenaar, R.; Huisint Veldt, J.H.J. A multicompartmental dynamic computer-controlled model simulating the stomach and small intestine. Altern. Lab. Anim. 1995, 2, 197–209. [Google Scholar]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A (13)C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Paz de Pena, M.; Concepcion, C.; Alan, C. Catabolism of coffee chlorogenic acids by human colonic microbiota. Biofactors 2013, 39, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.; Alric, M.; Blanquet-Diot, S.; Bornhorst, G.; Cueva, C.; Deglaire, A.; Denis, S.; Ferrua, M.; Havenaar, R.; Lelieveld, J.; et al. Can dynamic in vitro digestion systems mimic the physiological reality? Crit. Rev. Food Sci. Nutr. USA 2017. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Singh, R.P. A human gastric simulator (HGS) to study food digestion in human stomach. J. Food Sci. 2010, 75, 627–635. [Google Scholar] [CrossRef]
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; van Duynhoven, J.; Vaughan, E.E.; van de Wiele, T. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. J. Agric. Food Chem. 2010, 58, 10236–10246. [Google Scholar] [CrossRef]
- Fogliano, V.; Corollaro, M.L.; Vitaglione, P.; Napolitano, A.; Ferracane, R.; Travaglia, F.; Arlorio, M.; Costabile, A.; Klinder, A.; Gibson, G. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol. Nutr. Food Res. 2011, 55, 44–55. [Google Scholar] [CrossRef]
- Van Dorsten, F.A.; Peters, S.; Gross, G.; Gomez-Roldan, V.; Klinkenberg, M.; de Vos, R.C.; Vaughan, E.E.; van Duynhoven, J.P.; Possemiers, S.; van de Wiele, T.; et al. Gut microbial metabolism of polyphenols from black tea and red wine/grape juice is source-specific and colon-region dependent. J. Agric. Food Chem. 2012, 60, 11331–11342. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, Y. Review of in vitro digestion models for rapid screening of emulsion-based systems. Food Funct. 2010, 1, 32–59. [Google Scholar] [CrossRef] [PubMed]
- Engelen, L.; de Wijk, R.A.; Prinz, J.F.; van der Bilt, A.; Bosman, F. The relation between saliva flow after different stimulations and the perception of flavor and texture attributes in custard desserts. Physiol. Behav. 2003, 78, 165–169. [Google Scholar] [CrossRef]
- Lemmens, L.; Van Buggenhout, S.; Van Loey, A.M.; Hendrickx, M.E. Particle size reduction leading to cell wall rupture is more important for the β-carotene bioaccessibility of raw compared to thermally processed carrots. J. Agric. Food Chem. 2010, 58, 12769–12776. [Google Scholar] [CrossRef] [PubMed]
- Sergent, T.; Dupont, I.; Jassogne, C.; Ribonnet, L.; van der Heiden, E.; Scippo, M.L.; Muller, M.; McAlister, D.; Pussemier, L.; Larondelle, Y.; et al. CYP1A1 induction and CYP3A4 inhibition by the fungicide imazalil in the human intestinal Caco-2 cells-comparison with other conazole pesticides. Toxicol. Lett. 2009, 184, 159–168. [Google Scholar] [CrossRef]
- Schulze, K. Imaging and modelling of digestion in the stomach and the duodenum. Neurogastroenterol. Motil. 2006, 18, 172–183. [Google Scholar] [CrossRef]
- Blanquet-Diot, S.; Soufi, M.; Rambeau, M.; Rock, E.; Alric, M. Digestive stability of xanthophylls exceeds that of carotenes as studied in a dynamic in vitro gastrointestinal system. J. Nutr. 2009, 139, 876–883. [Google Scholar] [CrossRef]
- Clarysse, S.; Tack, J.; Lammert, F.; Duchateau, G.; Reppas, C.; Augustijns, P. Postprandial evolution in composition and characteristics of human duodenal fluids in different nutritional states. J. Pharm. Sci. 2009, 98, 1177–1192. [Google Scholar] [CrossRef]
- Day, A.J.; Cañada, F.J.; Díaz, J.C.; Kroon, P.A.; McLauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.A.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef]
- Gayoso, L.; Claerbout, A.S.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D. Bioaccessibility of rutin, caffeic acid and rosmarinic acid: Influence of the in vitro gastrointestinal digestion models. J. Func. Foods 2016, 26, 428–438. [Google Scholar] [CrossRef]
- Possemiers, S.; Bolca, S.; Verstraete, W.; Heyerick, A. The intestinal microbiome: A separate organ inside the body with the metabolic potential to influence the bioactivity of botanicals. Fitoterapia 2011, 82, 53–66. [Google Scholar] [CrossRef]
- Scalbert, A.; Morand, C.; Manach, C.; Remesy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Mateo Anson, N.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioavailability of ferulic acid is determined by its bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Aura, A.M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Aura, A.M.; O’Leary, K.A.; Williamson, G.; Ojala, M.; Bailey, M.; Puupponen-Pimia, R.; Nuutila, A.M.; Oksman-Caldentey, K.M.; Poutanen, K. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J. Agric. Food Chem. 2002, 50, 725–730. [Google Scholar] [CrossRef]
- Myint, K.Z.; Wu, K.; Xia, Y.; Fan, Y.; Shen, J.; Zhang, P.; Gu, J. Polyphenols from Stevia Rebaudiana (Bertoni) leaves and their functional properties. J. Food Sci. 2020, 85, 240–248. [Google Scholar] [CrossRef]
- Miller, N.; Ruiz-Larrea, M. Flavonoids and other plant phenols in the diet: Their significance as antioxidants. J. Nutr. Environ. Med. 2002, 12, 39–51. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and glycemic control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef]
- Kumamoto, M.; Sonda, T.; Nagayama, K.; Tabata, M. Effects of pH and metal ions on antioxidative activities of catechins. Biosci. Biotechnol. Biochem. 2001, 65, 126–132. [Google Scholar] [CrossRef]
- Khokhar, S.; Owusu Apenten, R.K. Iron binding characteristics of phenolic compounds: Some tentative structure–activity relations. Food Chem. 2003, 81, 133–140. [Google Scholar] [CrossRef]
- Yordi, G.E.; Pérez, E.M.; Matos, M.J.; Uriarte Villares, E. Antioxidant and pro-oxidant effects of polyphenolic compounds and structure-activity relationship evidence. In Nutrition, Well-Being and Health; Bouayed, J., Bohn, T., Eds.; IntechOpen: Rijeka, Croatia, 2012; pp. 23–28. [Google Scholar]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Świerczek-Zięba, G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules 2014, 19, 78–101. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E. Phenolic acids—Their properties, occurrence in plant materials, absorption and metabolism. Bromat. Chem. Toksykol. 2006, 2, 103–110. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acid. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Makowska-Wąs, J.; Janeczko, Z. Bioavailability of plant polyphenols. Post. Fitoter. 2004, 3, 126–137. [Google Scholar]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Ostrowska, J.; Skrzydlewska, E. The biological activity of flavonoids. Post. Fitoter. 2005, 3–4, 71–79. [Google Scholar]
- Masuoka, N.; Matsuda, M.; Kubo, I. Characterisation of the antioxidant activity of flavonoids. Food Chem. 2012, 131, 541–545. [Google Scholar] [CrossRef]
- Bubols, G.B.; Vianna Dda, R.; Medina-Remon, A.; von Poser, G.; Lamuela-Raventos, R.M.; Eifler-Lima, V.L.; Garcia, S.C. The antioxidant activity of coumarins and flavonoids. Mini Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar]
- Zhou, L.; Elias, R.J. Antioxidant and pro-oxidant activity of (-)-epigallocatechin-3-gallate in food emulsions: Influence of pH and phenolic concentration. Food Chem. 2013, 138, 1503–1509. [Google Scholar] [CrossRef]
- Košinová, P.; Di Meo, F.; Anouar, E.H.; Duroux, J.L.; Trouillas, P. H-atom acceptor capacity of free radicals used in antioxidant measurements. Int. J. Quantum Chem. 2011, 111, 1131–1142. [Google Scholar] [CrossRef]
- Milenkovi, D.; Yorovi, J.; Jeremi, T.; Dimitri Markovi, J.M.; Avdovi, E.H.; Markovi, Z. Free radical scavenging potency of dihydroxybenzoic acids. J. Chem. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Di Majo, D.; La Neve, L.; La Guardia, M.; Casuccio, A.; Giammanco, M. The influence of two different pH levels on the antioxidant properties of flavonols, flavan-3-ols, phenolic acids and aldehyde compounds analysed in synthetic wine and in a phosphate buffer. J. Food Compos. Anal. 2011, 24, 265–269. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Torreggiani, A.; Trinchero, A.; Tamba, M.; Taddei, P. Raman and pulse radiolysis studies of the antioxidant properties of quercetin: Cu (II) chelation and oxidizing radical scavenging. J. Raman Spectrosc. 2005, 36, 380–388. [Google Scholar] [CrossRef]
- Sadik, C.D.; Sies, H.; Schewe, T. Inhibition of 15-lipoxygenases by flavonoids: Structure–Activity relations and mode of action. Biochem. Pharmacol. 2003, 65, 773–781. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, 21–32. [Google Scholar] [CrossRef]
- Mandalari, G.; Tomaino, A.; Rich, G.T.; Lo Curto, R.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Saija, A.; Parker, M.L.; Waldron, K.W.; et al. Polyphenol and nutrient release from skin of almonds during simulated human digestion. Food Chem. 2010, 122, 1083–1088. [Google Scholar] [CrossRef]
- Arranz, S.; Manuel Silvan, J.; Saura-Calixto, F. Nonextractable polyphenols, usually ignored, are the major part of dietary polyphenols: A study on the Spanish diet. Mol. Nutr. Food Res. 2010, 54, 1646–1658. [Google Scholar] [CrossRef]
- Hemery, Y.M.; Anson, N.M.; Havenaar, R.; Haenen, G.R.M.M.; Noort, M.W.J.; Rouau, X. Dry-fractionation of wheat bran increases the bioaccessibility of phenolic acids in breads made from processed bran fractions. Food Res. Intl. 2010, 43, 1429–1438. [Google Scholar] [CrossRef]
- Eastwood, M.; Morris, E. Physical properties of dietary fiber that influence physiological function: A model for polymers along the gastrointestinal tract. Am. J. Clin. Nutr. 1992, 55, 436–442. [Google Scholar] [CrossRef]
- Aherne, S.A.; Daly, T.; Jiwan, M.A.; O’Sullivan, L.; O’Brien, N.M. Bioavailability of β-carotene isomers from raw and cooked carrots using an in vitro digestion model coupled with a human intestinal Caco-2 cell model. Food Res. Intl. 2010, 43, 1449–1454. [Google Scholar] [CrossRef]
- Ortega, N.; Reguant, J.; Romero, M.P.; Macia, A.; Motilva, M.J. Effect of fat content on the digestibility and bioaccessibility of cocoa polyphenol by an in vitro digestion model. J. Agric. Food Chem. 2009, 57, 5743–5749. [Google Scholar] [CrossRef]
- Bohin, M.C.; Vincken, J.P.; van der Hijden, H.; Gruppen, H. Efficacy of food proteins as carriers for flavonoids. J. Agric. Food Chem. 2012, 60, 4136–4143. [Google Scholar] [CrossRef]
- Dupas, C.; Baglieri, A.M.; Ordonaud, C.; Tome, D.; Maillard, M.N. Chlorogenic acid is poorly absorbed, independently of the food matrix: A Caco-2 cells and rat chronic absorption study. Mol. Nutr. Food Res. 2006, 50, 1053–1060. [Google Scholar] [CrossRef]
- Haratifar, S.; Corredig, M. Interactions between tea catechins and casein micelles and their impact on renneting functionality. Food Chem. 2014, 143, 27–32. [Google Scholar] [CrossRef]
- Green, R.J.; Murphy, A.S.; Schulz, B.; Watkins, B.A.; Ferruzzi, M.G. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol. Nutr. Food Res. 2007, 51, 1152–1162. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Hallund, J.; Talbot, D.; Schroot, J.; Williams, C.M.; Bugel, S.; Cassidy, A. Absorption of isoflavones in humans: Effects of food matrix and processing. J. Nutr. Biochem. 2006, 17, 257–264. [Google Scholar] [CrossRef]
- Neilson, A.P.; George, J.C.; Janle, E.M.; Mattes, R.D.; Rudolph, R.; Matusheski, N.V.; Ferruzzi, M.G. Influence of chocolate matrix composition on cocoa flavan-3-ol bioaccessibility in vitro and bioavailability in humans. J. Agric. Food Chem. 2009, 57, 9418–9426. [Google Scholar] [CrossRef]
- Sanz, T.; Luyten, H. Release, partitioning and stability of isoflavones from enriched custards during mouth, stomach and intestine in vitro simulations. Food Hydrocoll. 2006, 20, 892–900. [Google Scholar] [CrossRef]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Ginsburg, I.; Koren, E.; Shalish, M.; Kanner, J.; Kohen, R. Saliva increases the availability of lipophilic polyphenols as antioxidants and enhances their retention in the oral cavity. Arch. Oral Biol. 2012, 57, 1327–1334. [Google Scholar] [CrossRef]
- Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 2002, 13, 184–196. [Google Scholar] [CrossRef]
- Wroblewski, K.; Muhandiram, R.; Chakrabartty, A.; Bennick, A. The molecular interaction of human salivary histatins with polyphenolic compounds. Eur. J. Biochem. 2001, 268, 4384–4397. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2011, 52, 213–248. [Google Scholar] [CrossRef]
- Spencer, J. Metabolism of tee flavonoids in the gastrointestinal tract. J. Nutr. 2003, 133, 3255–3261. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem. 2013, 136, 206–212. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J. Agricand. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef]
- Perez-Vicente, A.; Gil-Izquierdo, A.; Garcia-Viguera, C. In vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins, and vitamin C. J. Agric. Food Chem. 2002, 50, 2308–2312. [Google Scholar] [CrossRef]
- Talavera, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.L.; Remesy, C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J. Nutr. 2003, 133, 4178–4182. [Google Scholar] [CrossRef]
- Boyer, J.; Brown, D.; Liu, R.H. In vitro digestion and lactase treatment influence uptake of quercetin and quercetin glucoside by the Caco-2 cell monolayer. Nutr. J. 2005, 4. [Google Scholar] [CrossRef]
- Rios, R.Y.; Bennett, R.N.; Lazarus, S.A.; Rémésy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar] [CrossRef]
- Lafay, S.; Gil-Izquierdo, A.; Manach, C.; Morand, C.; Besson, C.; Scalbert, A. Chlorogenic acid is absorbed in its intact form in the stomach of rats. J. Nutr. 2006, 136, 1192–1197. [Google Scholar] [CrossRef]
- Pinacho, R.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D.; Calvo, M.I. Phenolic compounds of blackthorn (Prunus Spinosa L.) and influence of in vitro digestion on their antioxidant capacity. J. Funct. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar]
- Pineda-Vadillo, C.; Nau, F.; Dubiard, C.G.; Cheynier, V.; Meudec, E.; Sanz-Buenhombre, M.; Guadarrama, A.; Tóth, T.; Csavajda, É.; Hingyi, H.; et al. In vitro digestion of dairy and egg products enriched with grape extracts: Effect of the food matrix on polyphenol bioaccessibility and antioxidant activity. Food Res. Int. 2016, 88, 284–292. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Wall-Medrano, A.; Velderrain-Rodríguez, G.R.; Chen, C.Y.O.; Salazar-López, N.J.; Robles-Sánchez, M.; GonzálezAguilar, G.A. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. 2017, 8, 15–38. [Google Scholar] [CrossRef]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red cabbage—Stability to simulated gastrointestinal digestion. Phytochemistry 2007, 68, 1285–1294. [Google Scholar] [CrossRef]
- Cruz-Trinidad, B.; Sanchez-Burgos, J.A.; Juscelino, T.; Sayago-Ayerdi, S.G.; Zamora-Gasga, V.M. In vitro gastrointestinal digestion of mango by-product snacks: Potential absorption of polyphenols and antioxidant capacity. Intl. J. Food Sci. Technol. 2019, 54, 3091–3098. [Google Scholar]
- Gil-Izquierdo, A.; Zafrilla, P.; Tomás-Barberán, F.A. An in vitro method to simulate phenolic compound release from the food matrix in the gastrointestinal tract. Europ. Food Res. Technl. 2002, 214, 155–159. [Google Scholar] [CrossRef]
- Baeza, G.; Sarriá, B.; Bravo, L.; Mateos, R. Polyphenol content, in vitro bioaccessibility and antioxidant capacity of widely consumed beverages. J. Sci. Food Agric. 2018, 98, 1397–1406. [Google Scholar] [CrossRef]
- Vallejo, F.; Gil-Izquierdo, A.; Pérez-Vicente, A.; García-Viguera, C. In vitro gastrointestinal digestion study of broccoli inflorescence phenolic compounds, glucosinolates, and vitamin C. J. Agric. Food Chem. 2004, 52, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ying, D.; Guo, B.; Cheng, L.J.; May, B.; Bird, T.; Sanguansri, L.; Caa, Y.; Augustin, M.A. Extrusion of apple pomace increases antioxidant activity upon in vitro digestion. Food Funct. 2019, 10, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.R.; Zhang, Y.C.; Vodovotz, Y.; Scwartz, S.J.; Failla, M.L. Stability and bioaccessibility of isoflavones from soy bread during in vitro digestion. J. Agric. Food Chem. 2003, 51, 4603–4609. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.O.; Pinheiro, A.C.B.; Brígida, A.I.S.; Genisheva, Z.A.; Vicente, A.A.M.O.S.; Teixeira, J.A.C.; de Matta, V.M.; Freitas, S.P. In vitro gastrointestinal evaluation of a juçara-based smoothie: Effect of processing on phenolic compounds bioaccessibility. J. Food Sci. Technol. 2019, 56, 5017–5026. [Google Scholar] [CrossRef]
- Altunkaya, A.; Gokmen, V.; Skibsted, L.H. pH dependent antioxidant activity of lettuce (L. Sativa) and synergism with added phenolic antioxidants. Food Chem. 2016, 190, 25–32. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, Y.; Gao, J.; Feng, J.; Shang, Y.; Wei, Z. Phenolics and antioxidant activity of bamboo leaves soup as affected by in vitro digestion. Food Chem. Toxicol. 2020, 135, 110941. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E.; Bilen, F.D.; Gonzales, G.B.; Grootaert, C.; Van de Wiele, T.; Van Camp, J. Bioaccessibility of polyphenols from plant-processing byproducts of black carrot (Daucus Carota L.). J. Agric. Food Chem. 2016, 64, 2450–2458. [Google Scholar] [CrossRef]
- Siracusa, L.; Kulisic-Bilusic, T.; Politeo, O.; Krause, I.; Dejanovic, B.; Ruberto, G. Phenolic composition and antioxidant activity of aqueous infusions from Capparis Spinosa L. and Crithmum Maritimum L. before and after submission to a twostep in vitro digestion model. J. Agric. Food Chem. 2011, 59, 12453–12459. [Google Scholar] [CrossRef] [PubMed]
- 120Costa, P.; Grevenstuk, T.; da Costa, A.M.R.; Gonçalves, S.; Romano, A. Antioxidant and anti-cholinesterase activities of Lavandula viridis L’Her extracts after in vitro gastrointestinal digestion. Ind. Crops Prod. 2014, 55, 83–89. [Google Scholar]
- Cristea, E.; Sturza, R.; Jauregi, P.; Niculaua, M.; Ghendov-Moșanu, A.; Patras, A. Influence of pH and ionic strength on the color parameters and antioxidant properties of an ethanolic red grape marc extract. J. Food Biochem. 2019, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Materska, M.; Olszówka, K.; Chilczuk, B.; Stochmal, A.; Pecio, Ł.; Pacholczyk-Sienicka, B.; Piacente, S.; Pizza, C.; Masullo, M. Polyphenolic profiles in lettuce (Lactuca Sativa L.) after CaCl2 treatment and cold storage. Eur. Food Res. Technol. 2019, 245, 733–744. [Google Scholar] [CrossRef]
- Roy, L.G.; Urooj, A. Antioxidant potency, pH and heat stability of selected plant extracts. J. Food Biochem. 2013, 37, 336–342. [Google Scholar] [CrossRef]
- Nayik, G.A.; Nanda, V. Effect of thermal treatment and pH on antioxidant activity of saffron honey using response surface methodology. J. Food Meas. Charact. 2016, 10, 64–70. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Sui, X.; Dong, X.; Zhou, W. Combined effect of pH and high temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. Food Chem. 2014, 163, 163–170. [Google Scholar] [CrossRef]





| Part of the Digestive Tract | pH | Substrates (Nutrient) | Enzymes | Digestion Products | References |
|---|---|---|---|---|---|
| Mouth | neutral | Starch, fats | salivary amylase (ptyalin), lingual lipase | maltose and dextrins, non-esterified fatty acids | [9,10,11] |
| Esophagus | neutral | moving food to stomach after initial enzymatic and mechanistic processes in mouth | [12] | ||
| Stomach | 1.5–2.0 | Peptides, emulsified lipids casein | Pepsin, lipase rennet | amino acids, glycerol, fatty acids, glycerides, curdle casein | [12,13] |
| Small Intestine | light alkaline, approx. 8 | Polypeptides, starch sucrose, fats, proteins, starch/glycogen | Aminopeptidase, amylase, sucrose, lipase, chymotrypsin, pancreatic amylase | amino acids, maltose and dextrins, glucose and fructose, glycerol and fatty acids, amino acids, maltose and isomaltose | [13,14,15,16] |
| Large Intestine | neutral | absorption of water and salts, production and absorption of vitamins, propelling feces for elimination from organism | [17,18] | ||
| Product | Phenolic Compounds | In Vitro Gastric Conditions | Results | In vitro Intestinal Conditions | Results | References |
|---|---|---|---|---|---|---|
| Mango by-Product Snacks | gallic acid, magniferin | pepsin, HCl, pH 1.5, 2 h | Small increase in polyphenols | pancreatin, buffer, pH 7.5, 6 h | 90%–95% decrease in gallic acid, 95%–98% decrease in mangiferin | [109] |
| Orange Juice | flavanones | pepsin, HCl, pH 2.0, 2 h | No changes | pancreatin, bile, NaHCO3, pH 7.5, 2 h | 50%–60% conversion into chalcones | [110] |
| Pomegranate Juice | anthocyanins | pepsin, HCl, pH 2.0, 2 h | 10% increase | pancreatin, bile, NaHCO3, pH 7.5, 2 h | approximately 80% decrease | [99] |
| Coffee Blend (65% Roasted, 35% Green) | monohydroxy-cinnamoylquinic acids, dihydroxycinnamoyl-quinic acids, lactones, caffeoylshikimic acids, cinnamoyl amino acids | pepsin, HCl, pH 2.0, 2 h | recovery of the initial amount: monohydroxy-cinnamoylquinic acids 97%, dihydroxycinnamoyl-quinic acids 101%, lactones 39%, caffeoylshikimic acids 80%, cinnamoyl amino acids 74% | pancreatin, Britton-Robinson buffer, pH 7.5, 2 h | recovery of the initial amount: monohydroxy-cinnamoylquinic acids 67%, dihydroxycinnamoyl-quinic acids 108%, lactones 36%, caffeoylshikimic acids 55%, cinnamoyl amino acids 63% | [111] |
| Broccoli | flavonoids, hydroxycinnamoyl derivatives | pepsin, HCl, pH 2.0, 2 h | flavonoids stable, 6%–25% losses of cinnamics | pancreatin–bile, NaHCO3, pH 7.5, 2 h | approximately 80%–85% losses | [112] |
| Apple Pomace | flavanols, phenolic acids dihydrochalones flavonoids | pepsin, HCl, pH 2.0, 30 min | marked increase in flavanols, phenolic acids and dihydrochalones, no changes/small changes in flavonoids | pancreatin, buffer, pH 6.0, N2, 5 h | significant degradation of epicatechin, procyanidin, quercetin-3-o-galactoside, chlorogenic acid, phloridzin | [113] |
| Soy Bread | isoflavonoids | pepsin, HCl, pH 2.0, 1 h, N2 | no changes | pancreatin, bile, NaHCO3, pH 6.9, N2, 2 h | isoflavonoids mostly stable; some conversion to aglycones | [114] |
| Juçara-Based Smoothie | anthocyanins, total polyphenols (TPC) | pepsin, HCl, pH 3.0, 2 h | the bioaccessibility of the anthocyanins was approximately 25%, the bioaccessibility of TPC was approximately 20% | pancreatin, bile, NaHCO3, pH 7.0, 2 h | the bioaccessibility of the anthocyanins was in the range of 7%–12%, the bioaccessibility of (TPC) was in the range of 40%–47% | [115] |
| Raspberry | anthocyanins | pepsin, HCl, pH 2.0, 2 h | no changes | pancreatin, bile, NaHCO3, pH 7.5, 2 h | 30% losses of anthocyanins | [98] |
| Onions, Apples | quercetin, quercetin-3-glucoside | pepsin, HCl, pH 2.0, 30 min | no changes | pancreatin, bile, NaHCO3, pH 6.5, 1 h | 50%–75% loss of quercetin, 10% loss of quercetin-3-glucoside | [101] |
| Bamboo Leaves Soup | total polyphenols (TPC) | pepsin, HCl, pH 2.0, 1 h | TPC increased by 1.64% | pancreatin, bile, NaHCO3, pH 7.4, 2 h | TPC decreased by 19.97% | [116] |
| Yerba Mate | caffeoyl glycosides, monohydroxy-cinnamoylquinic acids, dihydroxycinnamoyl-quinic acids, lactones, flavonoids | pepsin, HCl, pH 2.0, 2 h | recovery of the initial amount: caffeoyl glycosides 92%, monohydroxy-cinnamoylquinic acids 93%, dihydroxycinnamoyl-quinic acids 92%, lactones 99%, flavonoids 97% | pancreatin, Britton-Robinson buffer, pH 7.5, 2 h | recovery of the initial amount: caffeoyl glycosides 57%, monohydroxycinna-moylquinic acids 58%, dihydroxycinnamoyl-quinic acids 48%, lactones 45%, flavonoids 54% | [117] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. https://doi.org/10.3390/nu12051401
Wojtunik-Kulesza K, Oniszczuk A, Oniszczuk T, Combrzyński M, Nowakowska D, Matwijczuk A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients. 2020; 12(5):1401. https://doi.org/10.3390/nu12051401
Chicago/Turabian StyleWojtunik-Kulesza, Karolina, Anna Oniszczuk, Tomasz Oniszczuk, Maciej Combrzyński, Dominika Nowakowska, and Arkadiusz Matwijczuk. 2020. "Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review" Nutrients 12, no. 5: 1401. https://doi.org/10.3390/nu12051401
APA StyleWojtunik-Kulesza, K., Oniszczuk, A., Oniszczuk, T., Combrzyński, M., Nowakowska, D., & Matwijczuk, A. (2020). Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients, 12(5), 1401. https://doi.org/10.3390/nu12051401








