Urinary Metabolomic Profiling Analysis and Evaluation of the Effect of Ecklonia cava Extract Intake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Seapolynol Supplement
2.2. Urine Sample Collection
2.3. Materials and Chemicals
2.4. Sample Preparation
2.5. Metabolomic Analysis Using LC–MS
2.6. Statistical Analysis
3. Results
3.1. Alteration of Clinical Characteristics after 12 Weeks of Intervention
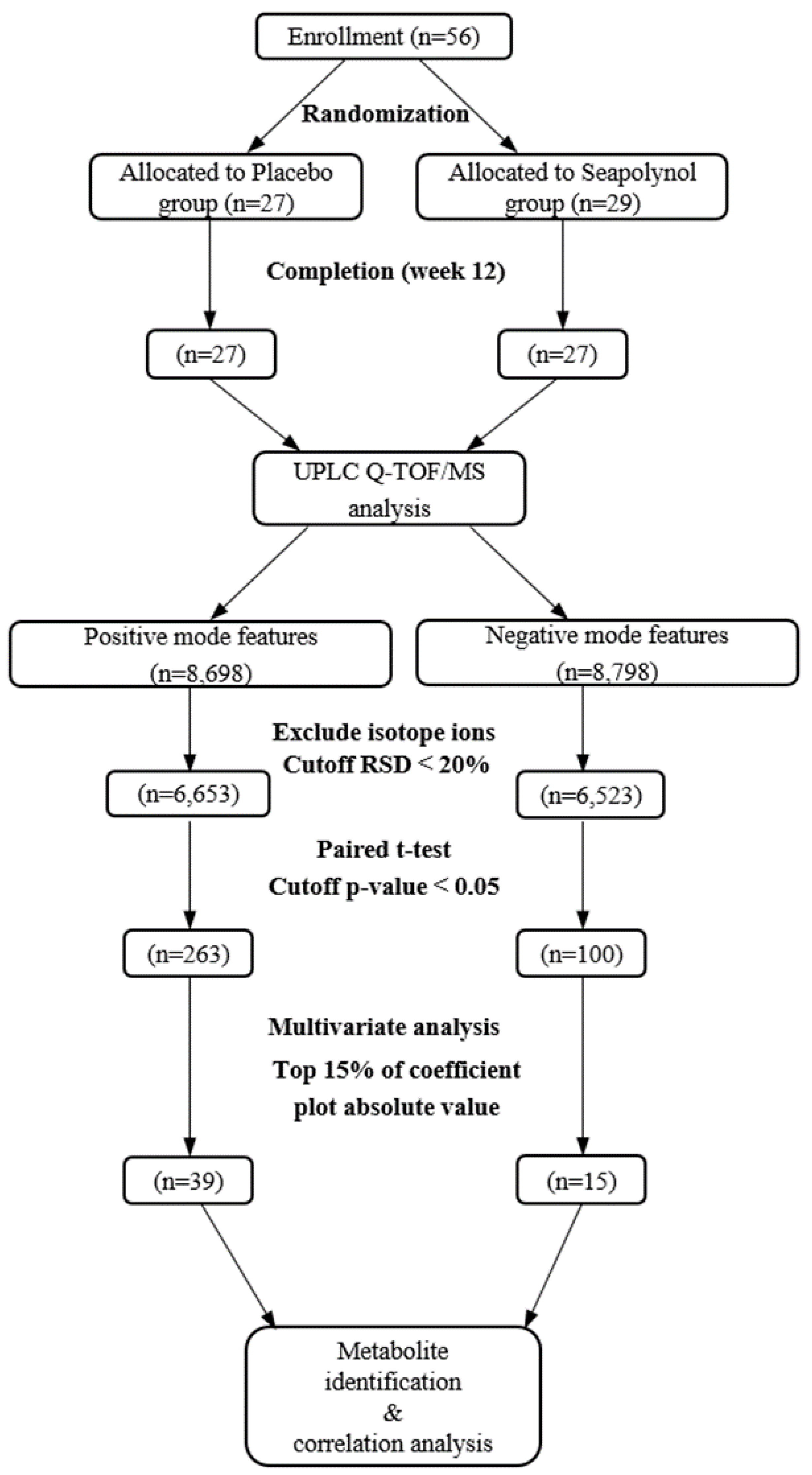
3.2. Global Profiling of Human Urine Using UPLC/QTOF MS
3.3. Identification of Urine Metabolites
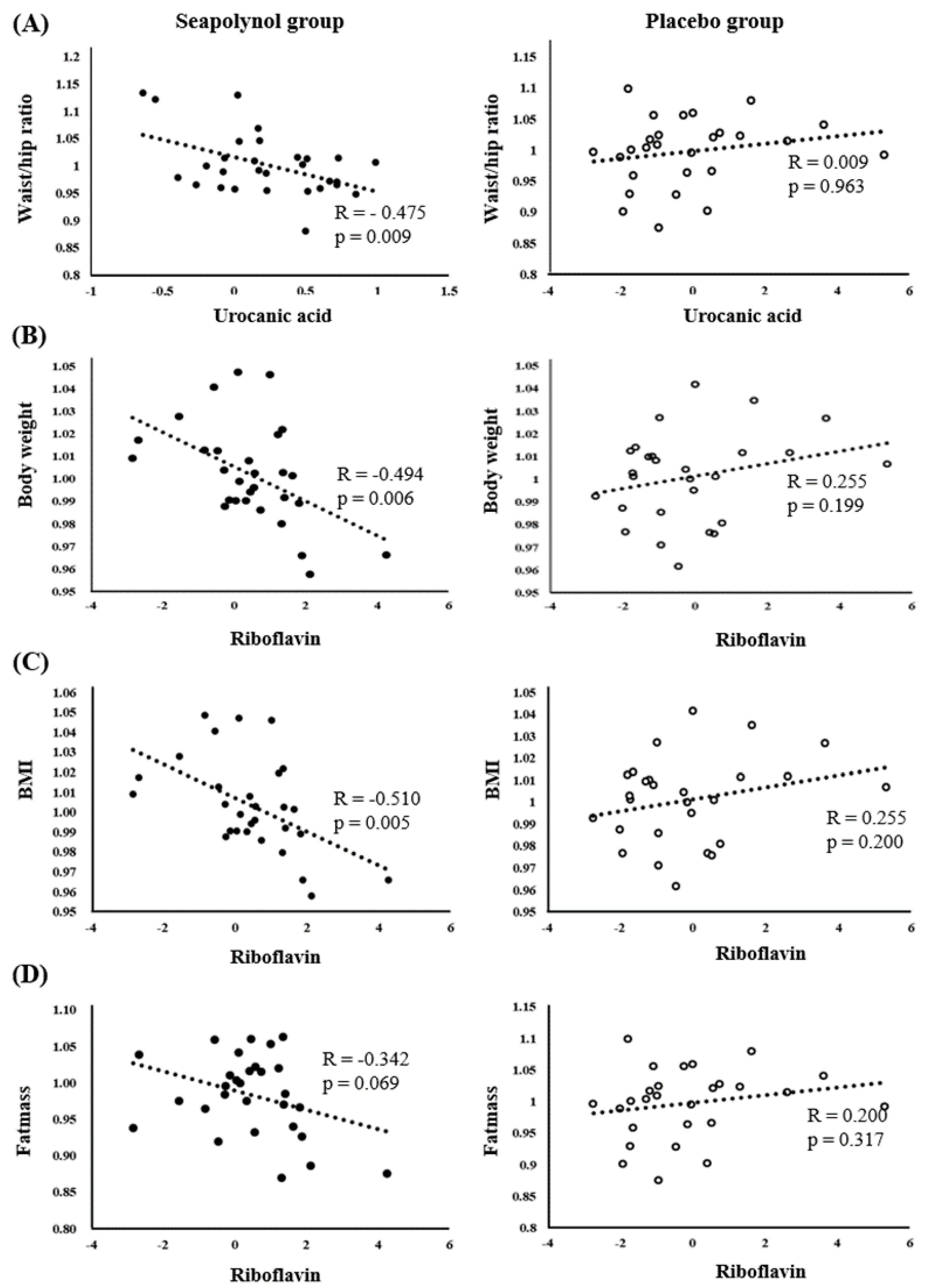
3.4. Correlation between the Levels of Significant Metabolites and Body Fat-Related Clinical Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ahn, G.-N.; Kim, K.-N.; Cha, S.-H.; Song, C.B.; Lee, J.H.; Heo, M.-S.; Yeo, I.-K.; Lee, N.-H.; Jee, Y.-H.; Kim, J.-S.; et al. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.-J.; Ryu, B.M.; Lee, S.-H.; Kim, M.-M.; Kim, S.-K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorgan. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-C.; Kim, S.H.; Park, Y.J.; Lee, B.H.; Hwang, H.J. Effects of 12-week oral supplementation of Ecklonia cava polyphenols on anthropometric and blood lipid parameters in overweight Korean individuals: A double-blind randomized clinical trial. Phytother. Res. 2012, 26, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kwon, O.R.; Kim, J.Y. Supplementation of a polyphenol extract from Ecklonia cava reduces body fat, oxidative and inflammatory stress in overweight healthy subjects with abdominal obesity: A randomized, placebo-controlled, double-blind trial. J. Funct. Foods 2018, 46, 356–364. [Google Scholar] [CrossRef]
- Hamilton, D.; Dee, A.; Perry, I.J. The lifetime costs of overweight and obesity in childhood and adolescence: A systematic review. Obes. Rev. 2018, 19, 452–463. [Google Scholar] [CrossRef]
- Bouchard, C. Defining the genetic architecture of the predisposition to obesity: A challenging but not insurmountable task. Am. J. Clin. Nutr. 2010, 91, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Herber, D. An integrative view of obesity. Am. J. Clin. Nutr. 2010, 91, 280S–283S. [Google Scholar] [CrossRef] [Green Version]
- Park, E.Y.; Kim, E.H.; Kim, M.H.; Seo, Y.W.; Lee, J.I.; Jun, H.S. Polyphenol-rich fraction of brown alga Ecklonia cava collected from Gijang, Korea, reduces obesity and glucose levels in high-fat diet-induced obese mice. Evid. Based Complementary Altern. Med. 2012, 2012, 418912. [Google Scholar]
- McGhie, T.K.; Rowan, D.D. Metabolomics for measuring phytochemicals, and assessing human and animal responses to phytochemicals, in food science. Mol. Nutr. Food Res. 2012, 56, 147–158. [Google Scholar] [CrossRef]
- Herrero, M.; Simó, C.; García-Cañas, V.; Ibáñez, E.; Cifuentes, A. Foodomics: MS-based strategies in modern food science and nutrition. Mass Spectrom. Rev. 2012, 31, 49–69. [Google Scholar] [CrossRef] [Green Version]
- Brennan, L. Metabolomics in nutrition research: Current status and perspectives. Biochem. Soc. Trans. 2013, 41, 670–673. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, A.; Brennan, L. Metabolomic applications in nutritional research: A perspective. J. Sci. Food Agric. 2015, 95, 2567–2570. [Google Scholar] [CrossRef] [PubMed]
- Ibero-Baraibar, I.; Romo-Hualde, A.; Gonzalez-Navarro, C.J.; Zulet, M.A.; Martinez, J.A. Urinary metabolomic profile following the intake of meals supplemented with cocoa extract in middle-aged obese subjects. Food Funct. 2016, 7, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Gross, A.L.; Esquivel, P. Impact of Cocoa products intake on plasma and urine metabolites: A review of targeted and non-targeted studies in humans. Nutrients 2019, 11, 1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickson, L.; Tenon, M.; Svilar, L.; Fança-Berthon, P.; Lugan, R.; Martin, J.-C.; Vaillant, F.; Rogez, H. Main human urinary metabolites after genipap (Genipa americana L.) juice intake. Nutrients 2018, 10, 1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, M.S.; Choi, M.-S.; Choi, J.-Y.; Kim, N.I.; Kim, M.-S.; Jung, S.H.; Ryu, D.H.; Hwang, G.-S. Effect of green tea on hepatic lipid metabolism in mice fed a high-fat diet. J. Nutr. Biochem. 2018, 51, 1–7. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, M.Y.; Park, H.M.; Park, Y.K.; Shon, J.C.; Liu, K.-H.; Lee, C.H. Urine and serum metabolite profiling of rats fed a high-fat diet and the anti-obesity effects of caffeine consumption. Molecules 2015, 20, 3107–3128. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, M.; Matz, A.; Klemashevich, C.; Rosenberg, D.W. Dietary walnut supplementation alters mucosal metabolite profiles during DSS-induced colonic ulceration. Nutrients 2019, 11, 1118. [Google Scholar] [CrossRef] [Green Version]
- Rådjursöga, M.; Lindqvist, H.M.; Pedersen, A.; Karlsson, B.G.; Malmodin, D.; Ellegård, L.; Winkvist, A. Nutritional metabolomics: Postprandial response of meals relating to vegan, lacto-ovo vegetarian, and omnivore Diets. Nutrients 2018, 10, 1063. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.-H.; Jung, S.; Kim, S.-A.; Kang, M.-S.; Kim, M.-S.; Joung, H.; Hwang, G.-S.; Shin, S.-M. Differential effects of typical Korean versus American-style diets on gut microbial composition and metabolic profile in healthy overweight Koreans: A randomized crossover trial. Nutrients 2019, 11, 2450. [Google Scholar] [CrossRef] [Green Version]
- Coyne, T.; Ibiebele, T.I.; Baade, P.D.; McClintock, C.S.; Shaw, J.E. Metabolic syndrome and serum carotenoids: Findings of a cross-sectional study in Queensland, Australia. Br. J. Nutr. 2009, 102, 1668–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vioque, J.; Weinbrenner, T.; Asensio, L.; Castelló, A.; Young, I.S.; Fletcher, A. Plasma concentrations of carotenoids and vitamin C are better correlated with dietary intake in normal weight than overweight and obese elderly subjects. Br. J. Nutr. 2007, 97, 977–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schectman, G.; Byrd, J.C.; Gruchow, H.W. The influence of smoking on vitamin C status in adults. Am. J. Public Health 1989, 79, 158–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öhrvall, M.; Tengblad, S.; Vessby, B. Lower tocopherol serum levels in subjects with abdominal adiposity. J. Intern. Med. 1993, 234, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Galan, P.; Viteri, F.E.; Bertrais, S.; Czernichow, S.; Faure, H.; Arnaud, J.; Ruffieux, D.; Chenal, S.; Arnault, N.; Favier, A.; et al. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur. J. Clin. Nutr. 2005, 59, 1181–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barile, M.; Giancaspero, T.A.; Leone, P.; Galluccio, M.; Indiveri, C. Riboflavin transport and metabolism in humans. J. Inherit. Metab. Dis. 2016, 39, 545–557. [Google Scholar] [CrossRef]
- Bárbara, J.H.; Tânia, G.L.; Cláudio, M.G. Therapeutic approaches using riboflavin in mitochondrial energy metabolism disorders. Curr. Drug Targets 2016, 17, 1527–1534. [Google Scholar]
- Eo, H.Y.; Jeon, Y.-J.; Lee, M.S.; Lim, Y.S. Brown alga Ecklonia cava polyphenol extract ameliorates hepatic lipogenesis, oxidative stress, and inflammation by activation of AMPK and SIRT1 in high-fat diet-induced obese mice. J. Agric. Food Chem. 2015, 63, 349–359. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, A.-G.; Zheng, M.-C.; Wang, Q.-Z.; Liang, H.; Han, X.-X.; Schouten, E.G. B Vitamins can reduce body weight gain by increasing metabolism-related enzyme activities in rats fed on a high-fat diet. Curr. Med. Sci. 2018, 38, 174–183. [Google Scholar] [CrossRef]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef] [Green Version]
- Mazur-Bialy, A.I.; Pocheć, E. Vitamin B2 deficiency enhances the pro-inflammatory activity of adipocyte, consequences for insulin resistance and metabolic syndrome development. Life Sci. 2017, 178, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Itabe, H. Oxidized low-density lipoprotein as a biomarker of in vivo oxidative stress: From atherosclerosis to periodontitis. J. Clin. Biochem. Nutr. 2012, 51, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saho, X.; Wang, K.; Liu, X.; Gu, C.; Zhang, P.; Xie, J.; Liu, W.; Chen, T.; Li, Y. Screening and verifying endometrial carcinoma diagnostic biomarkers based on a urine metabolomic profiling study using UPLC-Q-TOF/MS. Clin. Chim. Acta 2016, 463, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.L.; Lim, K.-M.; Joo, K.-M.; Park, H.-S.; Leung, D.Y.M.; Ye, Y.-M. Increased cis-to-trans urocanic acid ratio in the skin of chronic spontaneous urticaria patients. Sci. Rep. 2017, 7, 1318. [Google Scholar] [CrossRef] [Green Version]


| Variables | Placebo (n = 27) | Seapolynol (n = 29) | ||||
|---|---|---|---|---|---|---|
| Baseline | After 12 Weeks | p-Value | Baseline | After 12 Weeks | p-Value | |
| Gender | 7/20 | - | 10/19 | - | ||
| Age (years) | 38.66 ± 11.32 | - | 36.00 ± 9.9 | - | ||
| Body weight (kg) | 74.12 ± 9.33 | 74.21 ± 9.62 | 0.74 | 76.70 ± 8.24 | 76.77 ± 7.56 | 0.83 |
| BMI (kg/m2) | 27.53 ± 1.62 | 27.56 ± 1.73 | 0.79 | 27.20 ± 1.22 | 27.28 ± 7.56 | 0.50 |
| Waist circumference (cm) | 89.24 ± 6.91 | 89.95 ± 8.18 | 0.46 | 89.57 ± 6.02 | 90.71 ± 7.32 | 0.23 |
| Waist/hip ratio | 0.88 ± 0.06 | 0.88 ± 0.06 | 0.74 | 0.88 ± 0.06 | 0.88 ± 0.06 | 0.96 |
| Fat mass (kg) | 26.59 ± 3.41 | 26.53 ± 3.80 | 0.83 | 26.35 ± 4.09 | 25.92 ± 4.32 | 0.11 |
| Percent body fat (%) | 35.90 ± 4.74 | 35.70 ± 4.90 | 0.52 | 34.55 ± 6.29 | 33.88 ± 6.54 | 0.02 |
| Fat/lean mass ratio | 0.57 ± 0.12 | 0.56 ± 0.12 | 0.53 | 0.54 ± 0.14 | 0.53 ± 0.14 | 0.02 |
| FMI (kg/m2) | 9.95 ± 1.42 | 9.92 ± 1.53 | 0.83 | 9.44 ± 1.73 | 9.31 ± 1.91 | 0.17 |
| TAT (cm2) | 405.93 ± 69.02 | 402.58 ± 75.99 | 0.61 | 396.07 ± 72.44 | 389.17 ± 74.56 | 0.15 |
| VAT (cm2) | 125.50 ± 50.14 | 126.12 ± 57.66 | 0.89 | 106.92 ± 35.82 | 105.56 ± 31.10 | 0.68 |
| TG (mg/dL) | 131.37 ± 101.66 | 143.96 ± 104.27 | 0.20 | 110.00 ± 70.80 | 114.40 ± 74.81 | 0.69 |
| TC (mg/dL) | 201.52 ± 37.13 | 200.04 ± 39.96 | 0.72 | 193.00 ± 30.20 | 190.70 ± 27.85 | 0.51 |
| LDL-C (mg/dL) | 132.30 ± 32.06 | 131.22 ± 35.44 | 0.78 | 126.00 ± 24.90 | 121.30 ± 25.06 | 0.21 |
| Correlation Coefficient r | Body Weight | BMI | Waist/Hip Ratio | Fat Mass | Percent Bodyfat | Fat/Lean Ratio | FMI | TAT | VAT | SAT | TC | LDL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | −0.117 | −0.162 | −0.475 *** | 0.061 | 0.135 | 0.156 | 0.059 | −0.212 | −0.149 | −0.049 | 0.347 | 0.247 |
| b | 0.121 | 0.116 | 0.081 | 0.034 | −0.050 | −0.014 | 0.033 | 0.386 | 0.243 | 0.189 | −0.050 | −0.067 |
| c | −0.082 | −0.081 | 0.304 | −0.137 | −0.156 | −0.155 | −0.136 | 0.217 | 0.106 | 0.163 | −0.230 | −0.127 |
| d | −0.494 *** | −0.510 *** | −0.082 | −0.342 | −0.259 | −0.221 | −0.343 | −0.033 | −0.129 | 0.097 | −0.113 | −0.123 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Jung, Y.; Lee, E.; Jang, S.; Ryu, D.H.; Kwon, O.; Hwang, G.-S. Urinary Metabolomic Profiling Analysis and Evaluation of the Effect of Ecklonia cava Extract Intake. Nutrients 2020, 12, 1407. https://doi.org/10.3390/nu12051407
Kim J, Jung Y, Lee E, Jang S, Ryu DH, Kwon O, Hwang G-S. Urinary Metabolomic Profiling Analysis and Evaluation of the Effect of Ecklonia cava Extract Intake. Nutrients. 2020; 12(5):1407. https://doi.org/10.3390/nu12051407
Chicago/Turabian StyleKim, Juyeon, Youngae Jung, Eunok Lee, Seoyeong Jang, Do Hyun Ryu, Oran Kwon, and Geum-Sook Hwang. 2020. "Urinary Metabolomic Profiling Analysis and Evaluation of the Effect of Ecklonia cava Extract Intake" Nutrients 12, no. 5: 1407. https://doi.org/10.3390/nu12051407
APA StyleKim, J., Jung, Y., Lee, E., Jang, S., Ryu, D. H., Kwon, O., & Hwang, G.-S. (2020). Urinary Metabolomic Profiling Analysis and Evaluation of the Effect of Ecklonia cava Extract Intake. Nutrients, 12(5), 1407. https://doi.org/10.3390/nu12051407






