Traditional Brazilian Diet and Olive Oil Reduce Cardiometabolic Risk Factors in Severely Obese Individuals: A Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Statements
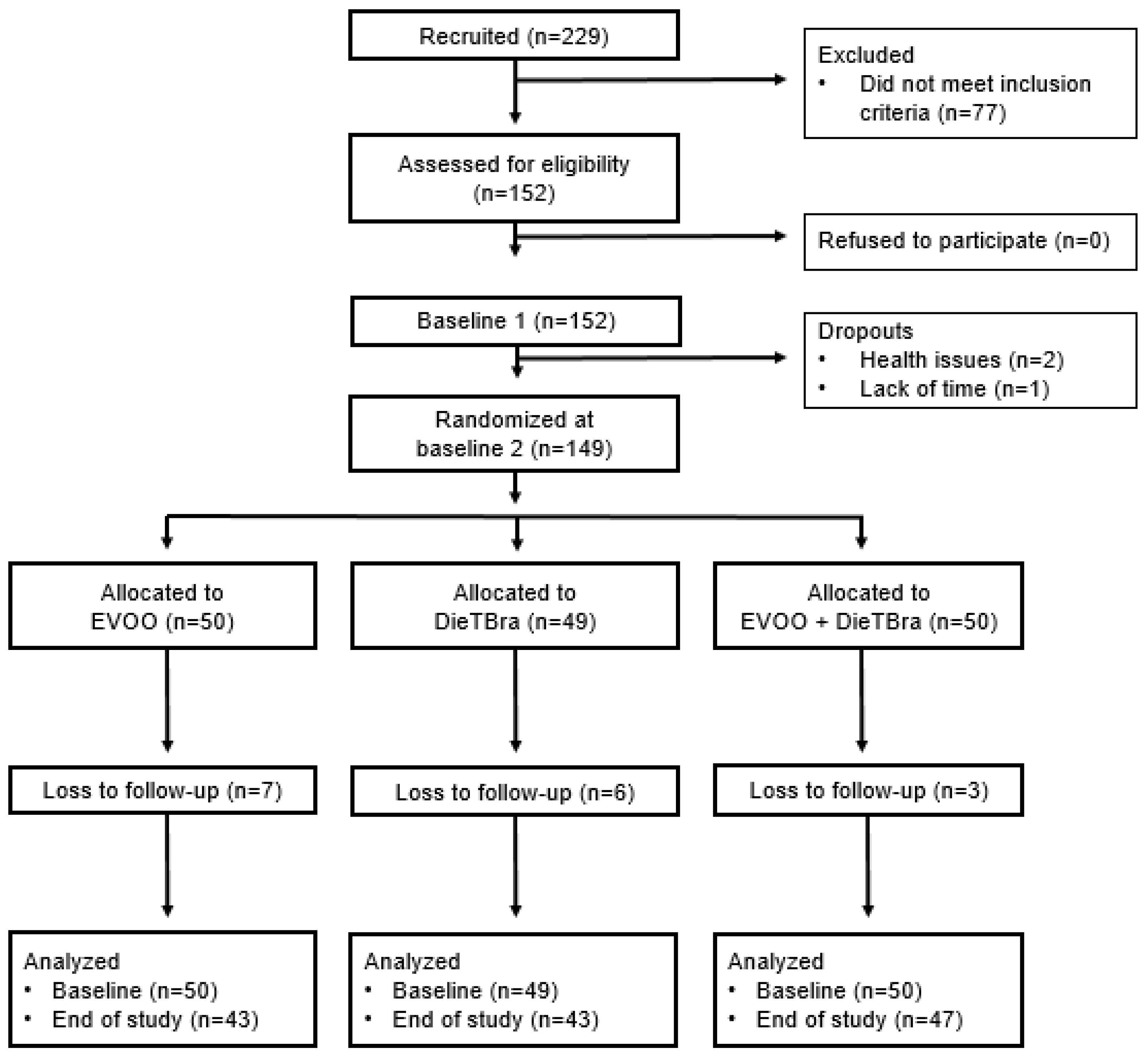
2.2. Participants
2.3. Baseline
2.4. Randomization
2.5. Interventions
2.6. Blinding
2.7. Follow-Up
2.8. Study Variables
2.9. Research Team and Quality Control
2.10. Statistical Analysis, Endpoints, and Sample Size
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Di Cesare, M.; Bentham, J.; Stevens, G.A.; Zhou, B.; Danaei, G.; Lu, Y.; Bixby, H.; Cowan, M.J.; Riley, L.M.; Hajifathalian, K.; et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar]
- Malta, D.C.; Santos, M.A.S.; Andrade, S.S.C.d.A.; Oliveira, T.P.; Stopa, S.R.; de Oliveira, M.M.; Jaime, P. Time trend in adult obesity indicators in Brazilian state capitals, 2006–2013. Cienc. Saude Coletiva 2016, 21, 1061–1069. [Google Scholar] [CrossRef]
- Crowe, C.; Gibson, I.; Cunningham, K.; Kerins, C.; Costello, C.; Windle, J.; O’Shea, P.M.; Hynes, M.; McGuire, B.; Kilkelly, K.; et al. Effects of an eight-week supervised, structured lifestyle modification programme on anthropometric, metabolic and cardiovascular risk factors in severely obese adults. BMC Endocr. Disord. 2015, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.N.; Columbus, M.L.; Shields, K.J.; Asubonteng, J.; Meyer, M.L.; Sutton-Tyrrell, K.; Goodpaster, B.H.; Delany, J.P.; Jakicic, J.M.; Barinas-Mitchell, E. Effects of an intensive behavioral weight loss intervention consisting of caloric restriction with or without physical activity on common carotid artery remodeling in severely obese adults. Metabolism 2012, 61, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Bigornia, S.J.; Mott, M.M.; Hess, D.T.; Apovian, C.M.; McDonnell, M.E.; Duess, M.A.; Kluge, M.A.; Fiscale, A.J.; Vita, J.A.; Gokce, N. Long-term successful weight loss improves vascular endothelial function in severely obese individuals. Obesity 2010, 18, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Stradling, C.; Hamid, M.; Taheri, S.; Thomas, G. A Review of Dietary Influences on Cardiovascular Health: Part 2: Dietary Patterns. Cardiovasc. Hematol. Disord. Targets 2014, 14, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Mancini, J.G.; Filion, K.B.; Atallah, R.; Eisenberg, M.J. Systematic Review of the Mediterranean Diet for Long-Term Weight Loss. Am. J. Med. 2016, 129, 407–415.e4. [Google Scholar] [CrossRef]
- Kastorini, C.M.; Milionis, H.J.; Goudevenos, J.A.; Panagiotakos, D.B. Mediterranean diet and coronary heart disease: Is obesity a link?—A systematic review. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 536–551. [Google Scholar] [CrossRef]
- Freeman, A.M.; Morris, P.B.; Barnard, N.; Esselstyn, C.B.; Ros, E.; Agatston, A.; Devries, S.; O’Keefe, J.; Miller, M.; Ornish, D.; et al. Trending Cardiovascular Nutrition Controversies. J. Am. Coll. Cardiol. 2017, 69, 1172–1187. [Google Scholar] [CrossRef]
- Buckland, G.; Gonzalez, C.A. The role of olive oil in disease prevention: A focus on the recent epidemiological evidence from cohort studies and dietary intervention trials. Br. J. Nutr. 2015, 113, S94–S101. [Google Scholar] [CrossRef]
- Nascimento, S.; Barbosa, F.S.; Sichieri, R.; Pereira, R.A. Dietary availability patterns of the brazilian macro-regions. Nutr. J. 2011, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Olinto, M.T.A.; Gigante, D.P.; Horta, B.; Silveira, V.; Oliveira, I.; Willett, W. Major dietary patterns and cardiovascular risk factors among young Brazilian adults. Eur. J. Nutr. 2012, 51, 281–291. [Google Scholar] [CrossRef]
- Rodrigues, A.P.S.; Rosa, L.P.S.; Silveira, E.A. PPARG2 Pro12Ala polymorphism influences body composition changes in severely obese patients consuming extra virgin olive oil: A randomized clinical trial. Nutr. Metab. 2018, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- dos Rodrigues, A.P.S.; Rosa, L.P.S.; da Silva, H.D.; de Silveira-Lacerda, E.P.; Silveira, E.A. The Single Nucleotide Polymorphism PPARG2 Pro12Ala Affects Body Mass Index, Fat Mass, and Blood Pressure in Severely Obese Patients. J. Obes. 2018, 2018, 2743081. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Webb, V.L.; Moran, C.H.; Bailer, B.A. Lifestyle Modification for Obesity. Circulation 2012, 125, 1157–1170. [Google Scholar] [CrossRef]
- Sartorelli, D.S.; Franco, L.J. Tendências do diabetes mellitus no Brasil: O papel da transição nutricional. Cad. Saude Publica 2003, 19, S29–S36. [Google Scholar] [CrossRef]
- Ministry of Health. Dietary Guidelines for the Brazilian Population; Ministério da Saúde: Brasília, DF, Brazil, 2015.
- Buckton, C.H.; Lean, M.E.J.; Combet, E. ‘Language is the source of misunderstandings’–impact of terminology on public perceptions of health promotion messages. BMC Public Health 2015, 15, 579. [Google Scholar] [CrossRef]
- Samuelson, G. Global strategy on diet, physical activity and health. Scand. J. Nutr. 2004, 48, 57. [Google Scholar] [CrossRef]
- Horie, L.M.; Gonzalez, M.C.; Torrinhas, R.S.; Cecconello, I.; Waitzberg, D.L. New specific equation to estimate resting energy expenditure in severely obese patients. Obesity 2011, 19, 1090–1094. [Google Scholar] [CrossRef]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef] [PubMed]
- WHO. The WHO STEPwise Approach to Chronic Disease Risk Factor Surveillance. In WHO STEPS Surveillance Manual; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Satija, A.; Yu, E.; Willett, W.C.; Hu, F.B. Understanding Nutritional Epidemiology and Its Role in Policy. Adv. Nutr. 2015, 6, 5–18. [Google Scholar] [CrossRef]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef] [PubMed]
- Frese, E.M.; Fick, A.; Sadowsky, S.H. Blood Pressure Measurement Guidelines for Physical Therapists. Cardiopulm. Phys. Ther. J. 2011, 22, 5–12. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, I.C.M.; Van hees, V.T.; Ramires, V.V.; Knuth, A.G.; Bielemann, R.M.; Ekelund, U.; Brage, S.; Hallal, P.C. Physical activity levels in three Brazilian birth cohorts as assessed with raw triaxial wrist accelerometry. Int. J. Epidemiol. 2014, 43, 1959–1968. [Google Scholar] [CrossRef]
- Laerd Statistics. One-way ANCOVA in SPSS. Available online: https://statistics.laerd.com/spss-tutorials/ancova-using-spss-statistics.php (accessed on 13 April 2020).
- Kwak, S.G.; Kim, J.H. Central limit theorem: The cornerstone of modern statistics. Korean J. Anesthesiol. 2017, 70, 144–156. [Google Scholar] [CrossRef]
- Jellinger, P.S.; Handelsman, Y.; Rosenblit, P.D.; Bloomgarden, Z.T.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; Guerin, C.K.; Bell, D.S.H.; Mechanick, J.I.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr. Pract. 2017, 23, 1–87. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Barbagallo, C.M.; Cicero, A.F.G.; Corsini, A.; Manzato, E.; Trimarco, B.; Bernini, F.; Visioli, F.; Bianchi, A.; Canzone, G.; et al. Nutraceuticals and functional foods for the control of plasma cholesterol levels. An intersociety position paper. Pharmacol. Res. 2018, 134, 51–60. [Google Scholar] [CrossRef]
- Surampudi, P.; Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipid Lowering with Soluble Dietary Fiber. Curr. Atheroscler. Rep. 2016, 18, 75. [Google Scholar] [CrossRef]
- Demonty, I.; Ras, R.T.; van der Knaap, H.C.M.; Duchateau, G.S.M.J.E.; Meijer, L.; Zock, P.L.; Geleijnse, J.M.; Trautwein, E.A. Continuous Dose-Response Relationship of the LDL-Cholesterol–Lowering Effect of Phytosterol Intake. J. Nutr. 2009, 139, 271–284. [Google Scholar] [CrossRef]
- Ghaffari, M.A.; Ghiasvand, T. Kinetic study of Low Density Lipoprotein oxidation by copper. Indian J. Clin. Biochem. 2010, 25, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Dunn, S.P.; Urbina, E.M. Review of clinical practice guidelines for the management of LDL-related risk. J. Am. Coll. Cardiol. 2014, 64, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Bozkurt, B.; Panjrath, G.; Aggarwal, B.; Ostfeld, R.J.; Barnard, N.D.; Gaggin, H.; Freeman, A.M.; Allen, K.; Madan, S.; et al. Lifestyle Modifications for Preventing and Treating Heart Failure. J. Am. Coll. Cardiol. 2018, 72, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.M.; Morris, P.B.; Aspry, K.; Gordon, N.F.; Barnard, N.D.; Esselstyn, C.B.; Ros, E.; Devries, S.; O’Keefe, J.; Miller, M.; et al. A Clinician’s Guide for Trending Cardiovascular Nutrition Controversies: Part II. J. Am. Coll. Cardiol. 2018, 72, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Berrougui, H.; Ikhlef, S.; Khalil, A. Extra Virgin Olive Oil Polyphenols Promote Cholesterol Efflux and Improve HDL Functionality. Evid. Based Complement. Altern. Med. 2015, 2015, 208062. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Atkinson, D. Lipid-free Apolipoprotein A-I Structure: Insights into HDL Formation and Atherosclerosis Development. Arch. Med. Res. 2015, 46, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.B.E.; Pourshahidi, L.K. Portion Size and Obesity. Adv. Nutr. 2014, 5, 829–834. [Google Scholar] [CrossRef]
- Willet, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Medicine, I.; Board, F.; Macronutrients, A.; Intakes, S.; Intakes, S. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); National Academies Press: Washington, DC, USA, 2005; ISBN 030908525X. [Google Scholar]
| Variables | Total n = 149 | EVOO n = 50 | DieTBra n = 49 | DieTBra + EVOO n = 50 |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Age, years | 39.63 ± 8.82 | 38.00 ± 8.00 | 39.00 ± 8.00 | 42.00 ± 10.00 |
| BMI, kg/m2 | 45.77 ± 6.41 | 45.52 ± 6.35 | 46.03 ± 6.20 | 45.78 ± 6.77 |
| LDL-c, mg/dL | 109.44 ± 35.48 | 106.94 ± 29.96 | 106.40 ± 32.56 | 114.76 ± 42.51 |
| Fasting blood glucose, mg/dL | 109.95 ± 45.24 | 104.44 ± 29.02 | 107.43 ± 35.15 | 117.94 ± 63.45 |
| Systolic BP, mmHg | 128.19 ± 17.88 | 127.59 ± 16.97 | 126.60 ± 17.86 | 130.36 ± 18.89 |
| Diastolic BP, mmHg | 85.65 ± 13.65 | 86.58 ± 17.84 | 84.34 ± 11.50 | 86.01 ± 10.58 |
| Fasting insulinemia, µUI/mL | 23.42 ± 14.86 | 25.24 ± 17.74 | 24.40 ± 14.63 | 20.65 ± 11.39 |
| Hemoglobin A1c, % | 6.30 ± 1.43 | 6.13 ± 1.27 | 6.26 ± 1.42 | 6.52 ± 1.58 |
| HOMA-IR | 6.40 ± 4.89 | 6.78 ± 5.87 | 6.68 ± 4.76 | 5.75 ± 3.87 |
| Total cholesterol, mg/dL | 189.12 ± 38.10 | 187.74 ± 37.59 | 184.31 ± 33.40 | 195.22 ± 42.61 |
| HDL-c, mg/dL | 47.62 ± 11.35 | 47.18 ± 10.32 | 48.45 ± 10.51 | 47.26 ± 13.16 |
| Triglycerides, mg/dL | 160.31 ± 78.40 | 160.46 ± 79.85 | 154.75 ± 87.76 | 165.60 ± 67.72 |
| Non-HDL-c, mg/dL | 141.49 ± 37.79 | 140.56 ± 36.73 | 135.86 ± 32.91 | 147.96 ± 42.74 |
| TG/HDL Ratio | 3.58 ± 2.12 | 3.61 ± 2.14 | 3.38 ± 2.19 | 3.76 ± 2.04 |
| Castelli I Index (TC/HDL) | 4.13 ± 1.13 | 4.13 ± 1.13 | 3.93 ± 0.93 | 4.33 ± 1.27 |
| Castelli II Index (LDL/HDL) | 2.42 ± 0.96 | 2.37 ± 0.88 | 2.29 ± 0.81 | 2.58 ± 1.15 |
| Homocysteine, mmol/L | 9.80 ± 8.36 | 9.91 ± 12.78 | 9.12 ± 3.84 | 10.36 ± 5.68 |
| Sedentary behavior, h/d | 19.61 ± 1.39 | 19.52 ± 1.22 | 19.52 ± 1.36 | 19.79 ± 1.57 |
| Endpoints | EVOO | DieTBra | DieTBra + EVOO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 50) | 12 Weeks (n = 43) | pa | Baseline (n = 49) | 12 Weeks (n = 43) | pa | Baseline (n = 50) | 12 Weeks (n = 47) | pa | |
| LDL-c, mg/dL | 106.94 ± 29.96 | 101.30 ± 29.76 | 0.131 | 106.40 ± 32.56 | 100.72 ± 32.55 | 0.258 | 114.76 ± 42.51 | 114.52 ± 30.30 | 0.698 |
| Fasting blood glucose, mg/dL | 104.44 ± 29.02 | 100.24 ± 21.39 | 0.651 | 107.43 ± 35.15 | 101.07 ± 25.57 | 0.129 | 117.94 ± 63.45 | 116.02 ± 55.12 | 0.505 |
| Systolic BP, mmHg | 127.59 ± 16.97 | 126.67 ± 16.43 | 0.806 | 126.60 ± 17.86 | 124.63 ± 15.40 | 0.171 | 130.36 ± 18.89 | 126.60 ± 13.31 | 0.072 |
| Diastolic BP, mmHg | 86.58 ± 17.84 | 83.91 ± 11.11 | 0.724 | 84.34 ± 11.50 | 82.33 ± 10.59 | 0.150 | 86.01 ± 10.58 | 83.50 ± 9.68 | 0.053 |
| Fasting insulinemia, µUI/mL | 25.24 ± 17.74 | 25.24 ± 11.67 | 0.927 | 24.40 ± 14.63 | 25.38 ± 12.96 | 0.747 | 20.65 ± 11.39 | 21.41 ± 12.13 | 0.505 |
| Hemoglobin A1c, % | 6.13 ± 1.27 | 6.09 ± 0.93 | 0.488 | 6.26 ± 1.42 | 6.08 ± 1.22 | 0.174 | 6.52 ± 1.58 | 6.51 ± 1.72 | 0.912 |
| HOMA-IR | 6.78 ± 5.87 | 6.38 ± 3.61 | 0.711 | 6.68 ± 4.76 | 6.53 ± 4.00 | 0.728 | 5.75 ± 3.87 | 6.26 ± 6.33 | 0.428 |
| Total cholesterol, mg/dL | 187.74 ± 37.59 | 180.69 ± 35.79 | 0.242 | 184.31 ± 33.40 | 178.28 ± 35.45 | 0.137 | 195.22 ± 42.61 | 195.06 ± 33.41 | 0.794 |
| HDL-c, mg/dL | 47.18 ± 10.32 | 47.18 ± 49.21 | 0.133 | 48.45 ± 10.51 | 48.45 ± 48.98 | 0.734 | 47.26 ± 13.16 | 49.51 ± 10.48 | 0.197 |
| Non-HDL-c, mg/dL | 138.91 ± 33.32 | 131.48 ± 32.24 | 0.069 | 136.32 ± 33.61 | 129.30 ± 32.62 | 0.081 | 148.55 ± 42.54 | 145.55 ± 34.13 | 0.566 |
| Triglycerides, mg/dL | 160.46 ± 79.85 | 151.35 ± 65.53 | 0.296 | 154.75 ± 87.76 | 142.46 ± 64.96 | 0.069 | 165.60 ± 67.72 | 160.83 ± 77.33 | 0.286 |
| TG/HDL Ratio | 3.61 ± 2.14 | 3.28 ± 1.88 | 0.114 | 3.38 ± 2.19 | 3.09 ± 1.69 | 0.116 | 3.76 ± 2.04 | 3.51 ± 2.32 | 0.181 |
| Castelli I Index (TC/HDL) | 4.13 ± 1.13 | 3.75 ± 0.79 | 0.002 * | 3.93 ± 0.93 | 3.76 ± 0.85 | 0.066 | 4.33 ± 1.27 | 4.08 ± 0.97 | 0.134 |
| Castelli II Index (LDL/HDL) | 2.37 ± 0.88 | 2.09 ± 0.65 | 0.005 * | 2.29 ± 0.81 | 2.14 ± 0.77 | 0.149 | 2.58 ± 1.15 | 2.39 ± 0.76 | 0.169 |
| Homocysteine, mmol/L | 9.91 ± 12.78 | 8.80 ± 2.84 | 0.477 | 9.12 ± 3.84 | 9.03 ± 2.55 | 0.873 | 10.36 ± 5.68 | 10.51 ± 6.38 | 0.214 |
| Endpoints (12 Weeks–Baseline) | EVOO | DieTBra | DieTBra + EVOO | All Groups | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Percentual Difference from Baseline | Mean ± SD | 95% CI | Percentual Difference from Baseline | Mean ± SD | 95% CI | Percentual Difference from Baseline | pa | |
| ∆ LDL-c | −5.11 ± 21.79 | −11.82–1.59 | −4.78 | −4.27 ± 23.84 | −11.79–3.26 | −4.01 | −1.93 ± 33.61 | −11.92–8.05 | −1.68 | 0.921 |
| ∆ Glycemia | −1.28 ± 18.27 | −6.98–4.41 | −1.22 | −5.53 ± 23.48 | −12.76–1.69 | −5.15 | −3.06 ± 31.29 | −12.25–6.12 | −2.59 | 0.668 |
| ∆ SBP | −0.53 ± 14.24 | −4.92–3.85 | −0.41 | −2.51 ± 1.80 | −6.16–1.13 | −1.98 | −3.64 ± 13.55 | −7.62–0.34 | −2.79 | 0.529 |
| ∆ BPB | −0.51 ± 9.43 | −3.41–2.39 | −0.59 | −1.90 ± 8.42 | −4.53–0.72 | −2.25 | −2.37 ± 8.20 | −4.78–0.04 | −2.75 | 0.693 |
| ∆ Insulinemia | −0.23 ± 16.69 | −5.37–4.90 | −0.91 | 0.67 ± 13.52 | −3.49–4.83 | 2.74 | 0.98 ± 10.07 | −1.97–3.94 | 4.75 | 0.809 |
| ∆ HbA1c | 0.09 ± 0.87 | −0.17–0.36 | 1.47 | −0.32 ± 1.52 | −0.79–0.15 | −5.11 | −0.02 ± 1.70 | −0.53–0.47 | −0.31 | 0.212 |
| ∆ HOMA-IR | −0.31 ± 5.39 | −1.96–1.35 | −4.57 | −0.20 ± 3.83 | −1.38–0.97 | −2.99 | 0.54 ± 4.63 | −0.83–1.92 | 9.39 | 0.709 |
| ∆ TC | −5.51 ± 30.44 | −14.88–3.86 | −2.93 | −6.55 ± 28.39 | −15.29–2.18 | −3.55 | −1.40 ± 36.66 | −12.17–9.36 | −0.72 | 0.686 |
| ∆ HDL-c | 1.90 ± 8.17 | −0.61–4.42 | 4.03 | 0.46 ± 8.90 | −2.27–3.21 | 0.95 | 1.59 ± 8.35 | −0.86–4.05 | 3.36 | 0.362 |
| ∆ Triglycerides | −6.67 ± 41.35 | −19.4–6.05 | −4.16 | −14.56 ± 51.19 b | −30.31–1.19 | −9.41 | −6.55 ± 41.59 | −18.76–5.66 | −3.95 | 0.717 |
| ∆ Non-HDL-c | −7.42 ± 26.13 | −15.46–0.62 | −5.34 | −7.02 ± 25.77 | −14.95–0.91 | −5.15 | −3.00 ± 35.57 | −13.44–7.44 | −2.02 | 0.724 |
| ∆ TG/HDL | −0.25 ± 1.03 | −0.57–0.06 | −3.61 | −0.33 ± 1.35 | −0.75–0.85 | −9.38 | −0.25 ± 1.29 | −0.63–0.12 | −6.65 | 0.975 |
| ∆ Castelli I | −0.33 ± 0.68 | −0.54–−0.12 | −7.99 | −0.20 ± 0.69 | −0.41–0.14 | −5.09 | −0.22 ± 0.99 | −0.51–0.07 | −5.08 | 0.550 |
| ∆ Castelli II | −0.26 ± 0.59 b | −0.44–−0.08 | −10.97 | −0.14 ± 0.62 | −0.33–0.05 | −6.11 | −0.18 ± 0.89 | −0.45–0.08 | −6.98 | 0.533 |
| ∆ Homocysteine | −1.48 ± 13.53 b | −5.64–2.68 | −14.93 | −0.07 ± 3.02 | −1.00–0.85 | −0.77 | 0.33 ± 1.78 | −0.19–0.85 | 3.18 | 0.992 |
| EVOO | DieTBra | DieTBra + EVOO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | p * | Baseline | 12 Weeks | p * | Baseline | 12 Weeks | p * | |
| Calories | 1615.33 ± 582.45 | 1733.00 ± 552.38 | 0.076 | 1687.9 ± 592.20 | 1267.47 ± 361.89 | <0.001 | 1771.32 ± 905.06 | 1440.36 ± 391.26 | 0.002 |
| Carbohydrates (%) | 53.84 ± 6.12 | 48.10 ± 6.72 | <0.001 | 53.16 ± 8.59 | 54.05 ± 6.86 | 0.483 | 52.04 ± 7.48 | 46.14 ± 6.36 | <0.001 |
| Proteins (%) | 17.62 ± 3.63 | 16.52 ± 3.40 | 0.077 | 17.66 ± 4.56 | 19.27 ± 4.46 | 0.062 | 18.81 ± 4.71 | 17.59 ± 5.40 | 0.360 |
| Total fat (%) | 28.54 ± 5.11 | 35.37 ± 7.13 | <0.001 | 29.17 ± 6.74 | 26.68 ± 5.55 | 0.032 | 29.15 ± 5.58 | 36.27 ± 7.45 | <0.001 |
| Saturated fat (g) | 15.09 ± 7.35 | 14.21 ± 7.95 | 0.516 | 16.74 ± 7.68 | 9.39 ± 5.87 | <0.001 | 16.13 ± 9.85 | 12.17 ± 5.42 | 0.002 |
| MUFA (g) | 15.25 ± 7.27 | 27.39 ± 11.30 | <0.001 | 14.30 ± 7.54 | 9.26 ± 5.67 | <0.001 | 15.79 ± 8.94 | 26.86 ± 12.70 | <0.001 |
| PUFA (g) | 8.35 ± 4.14 | 8.73 ± 3.08 | 0.269 | 7.92 ± 4.41 | 5.27 ± 3.14 | 0.002 | 9.08 ± 5.47 | 7.68 ± 4.59 | 0.084 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.S.e.A.d.C.; Rodrigues, A.P.d.S.; Rosa, L.P.d.S.; Noll, M.; Silveira, E.A. Traditional Brazilian Diet and Olive Oil Reduce Cardiometabolic Risk Factors in Severely Obese Individuals: A Randomized Trial. Nutrients 2020, 12, 1413. https://doi.org/10.3390/nu12051413
Santos ASeAdC, Rodrigues APdS, Rosa LPdS, Noll M, Silveira EA. Traditional Brazilian Diet and Olive Oil Reduce Cardiometabolic Risk Factors in Severely Obese Individuals: A Randomized Trial. Nutrients. 2020; 12(5):1413. https://doi.org/10.3390/nu12051413
Chicago/Turabian StyleSantos, Annelisa Silva e Alves de Carvalho, Ana Paula dos Santos Rodrigues, Lorena Pereira de Souza Rosa, Matias Noll, and Erika Aparecida Silveira. 2020. "Traditional Brazilian Diet and Olive Oil Reduce Cardiometabolic Risk Factors in Severely Obese Individuals: A Randomized Trial" Nutrients 12, no. 5: 1413. https://doi.org/10.3390/nu12051413
APA StyleSantos, A. S. e. A. d. C., Rodrigues, A. P. d. S., Rosa, L. P. d. S., Noll, M., & Silveira, E. A. (2020). Traditional Brazilian Diet and Olive Oil Reduce Cardiometabolic Risk Factors in Severely Obese Individuals: A Randomized Trial. Nutrients, 12(5), 1413. https://doi.org/10.3390/nu12051413








