Long-Term Feeding of a High-Fat Diet Ameliorated Age-Related Phenotypes in SAMP8 Mice
Abstract
1. Introduction
2. Materials and Methods
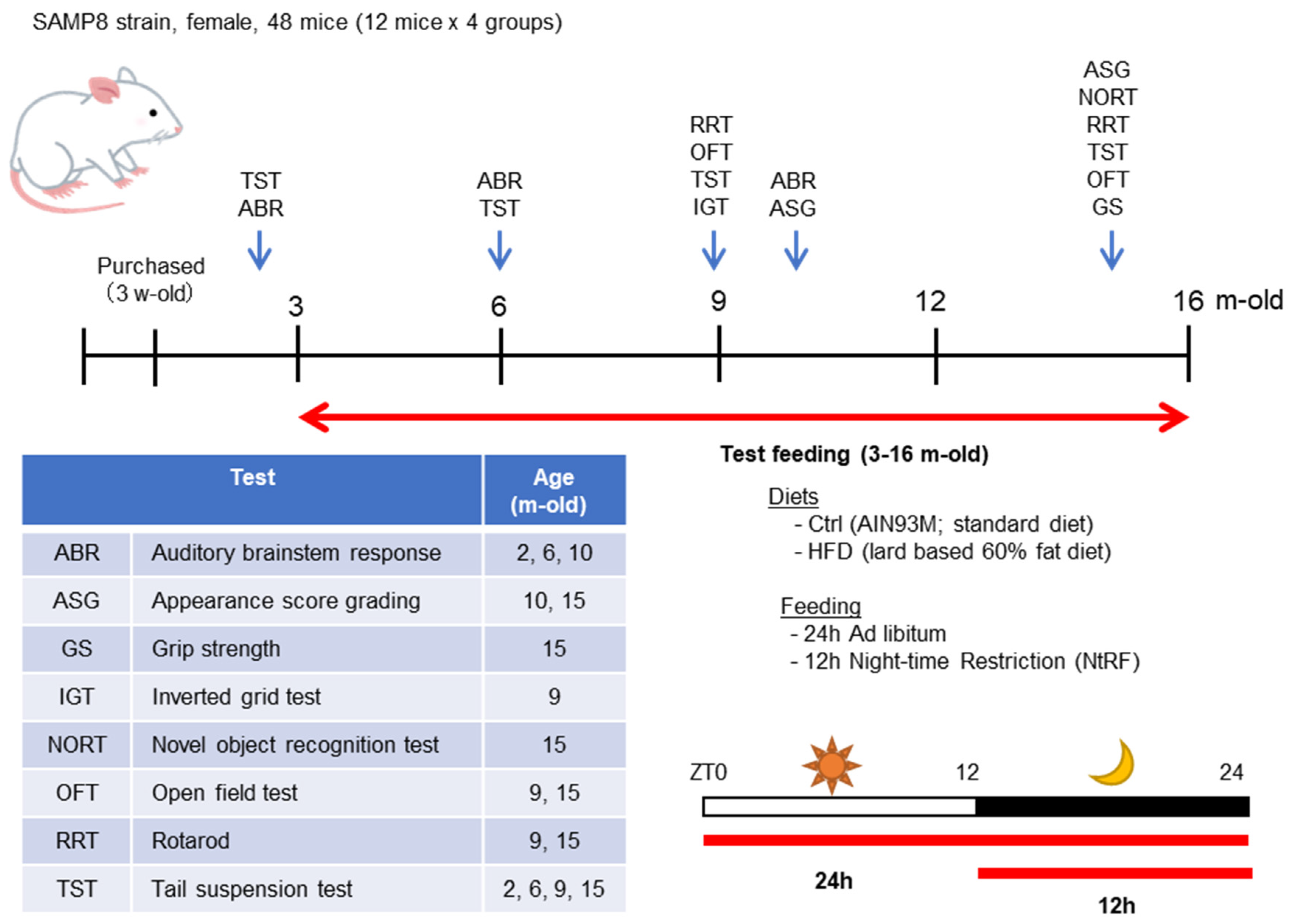
2.1. Animals
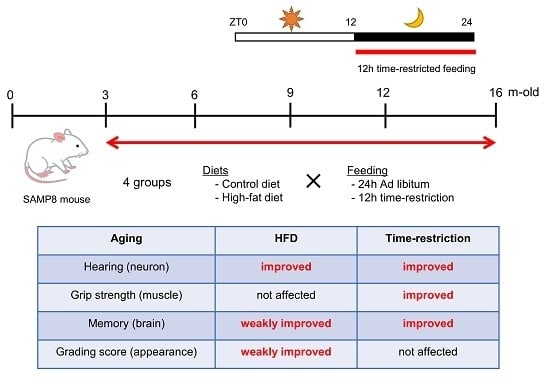
2.2. Experimental Design and Time Course
2.3. Hearing Measurement
2.4. Other Aging Evaluation Tests
2.4.1. Grip Strength
2.4.2. Grading Score
2.5. Blood Biochemical Analysis and Bone Density Measurement
2.6. Gene-Expression Analysis
2.7. Statistical Analysis
3. Results
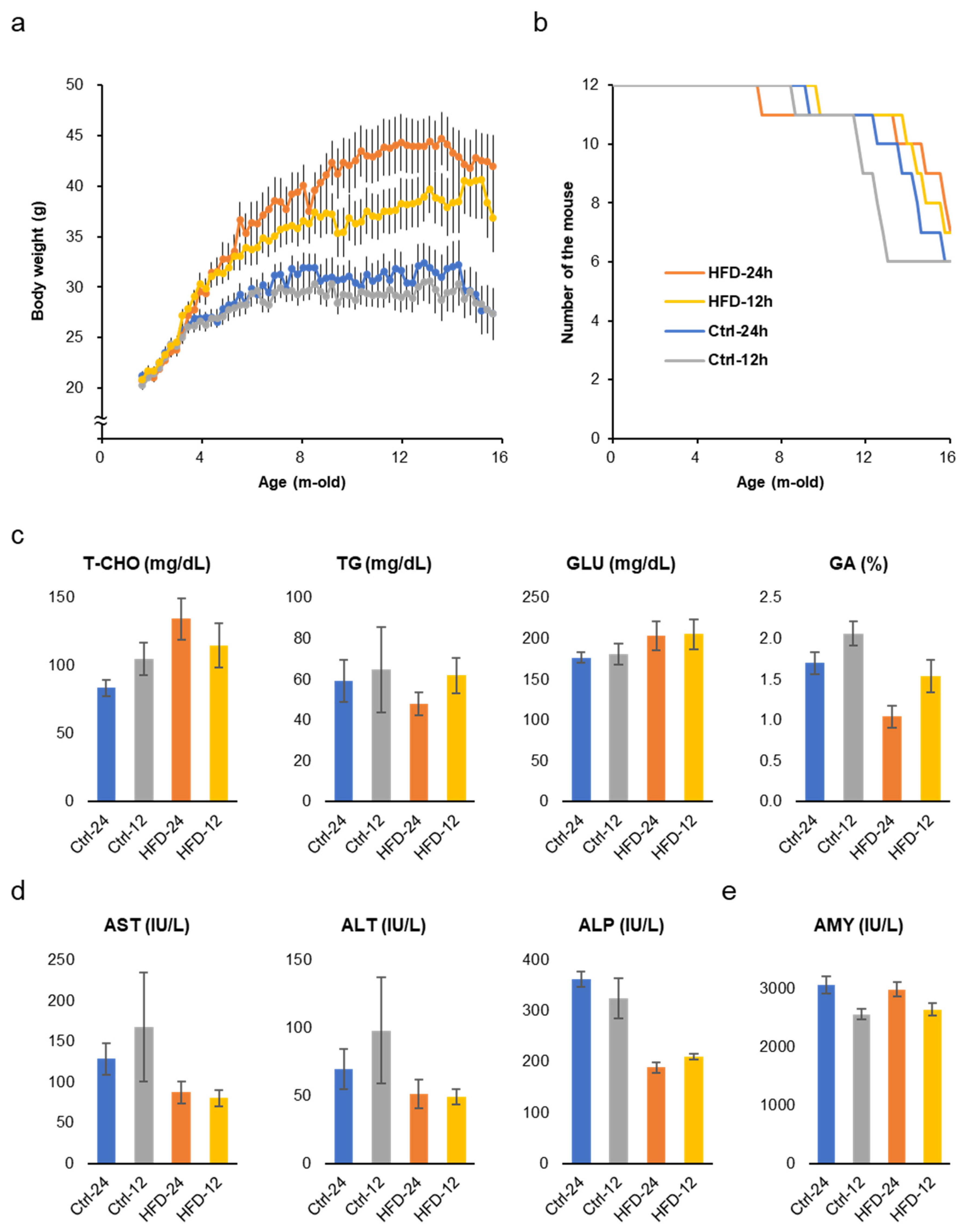
3.1. HFD Did Not Induce Metabolic Disorders in SAMP8 Mice
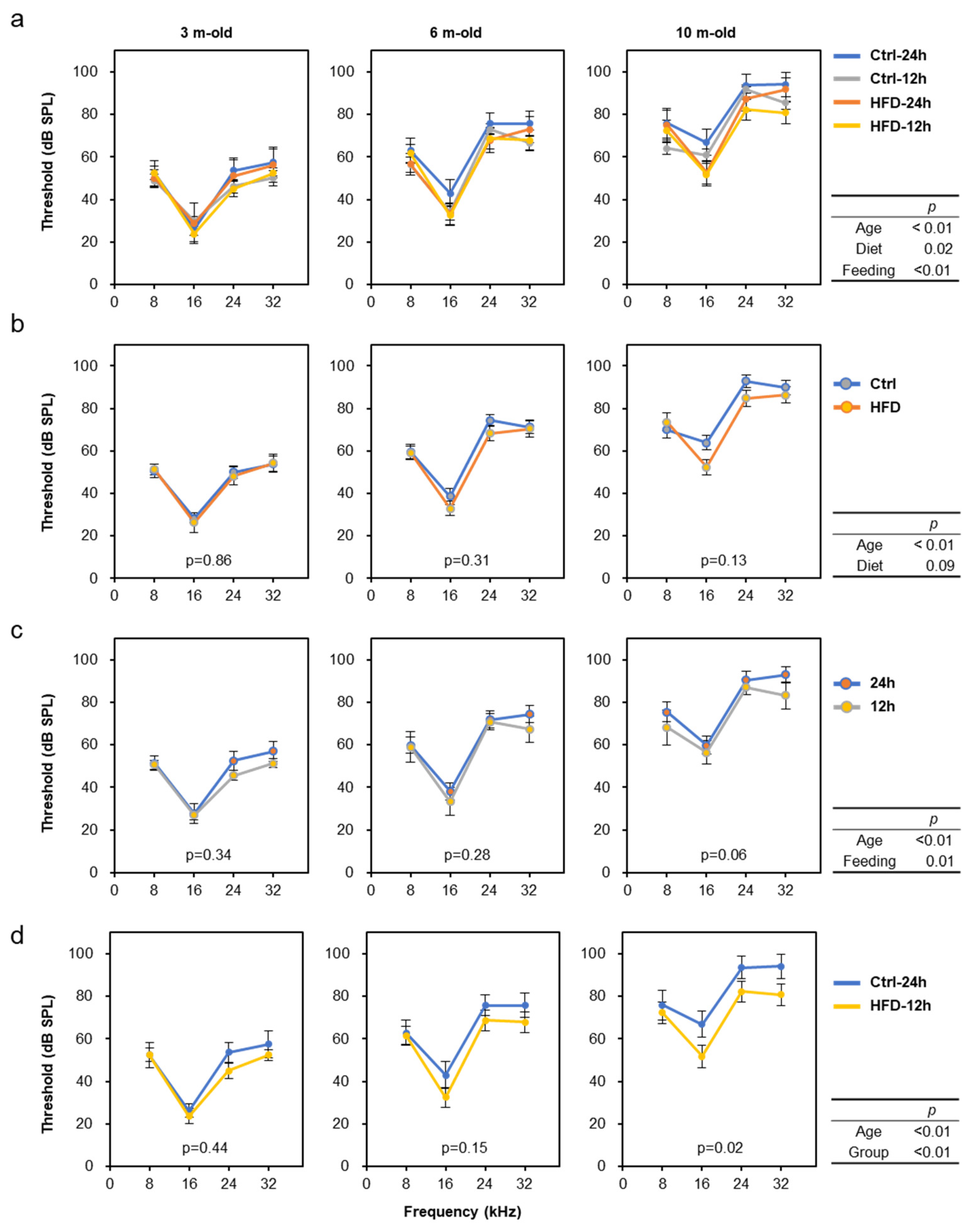
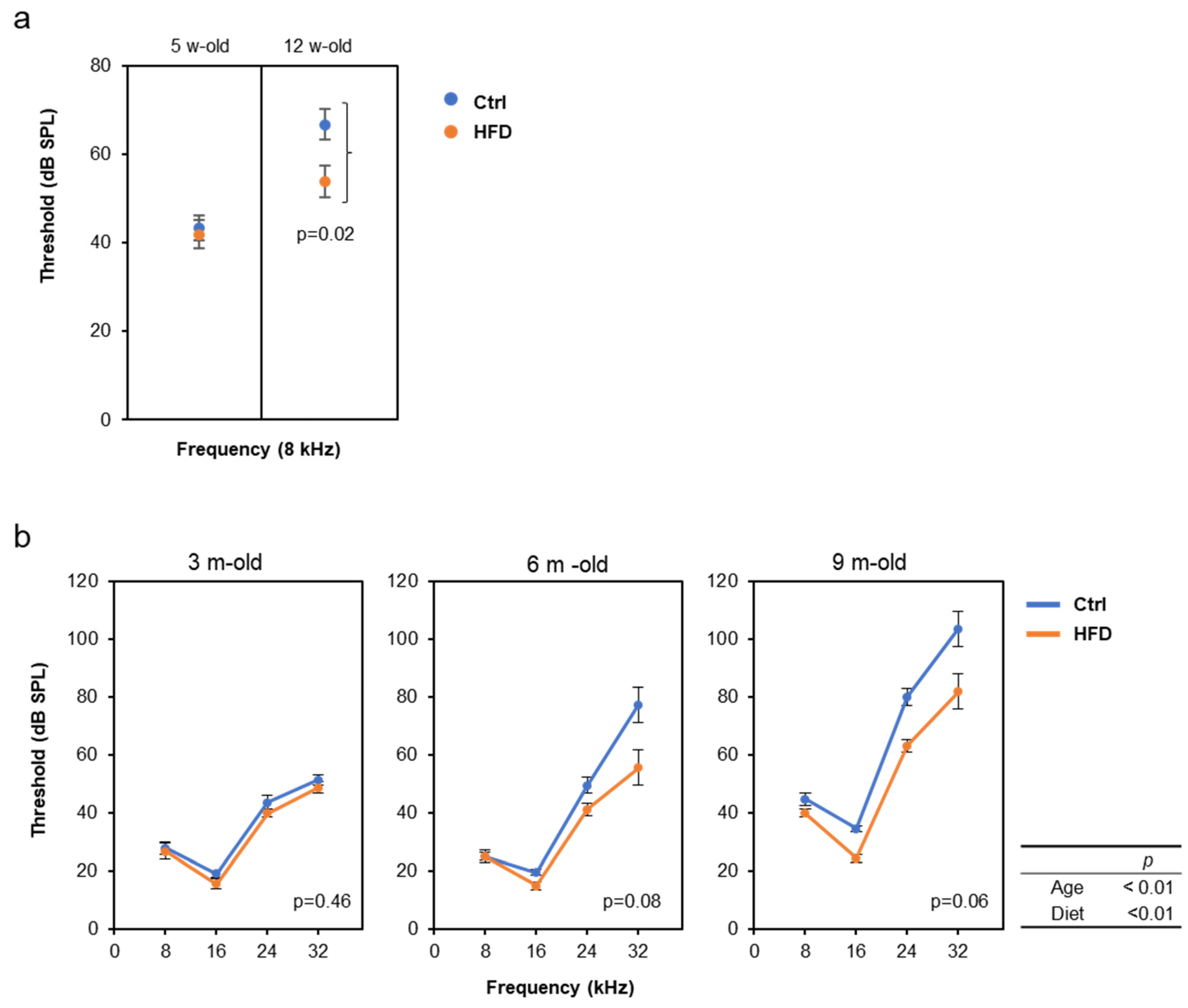
3.2. HFD Retarded AHL in SAMP8, DBA/2J, and C57BL/6J Mice
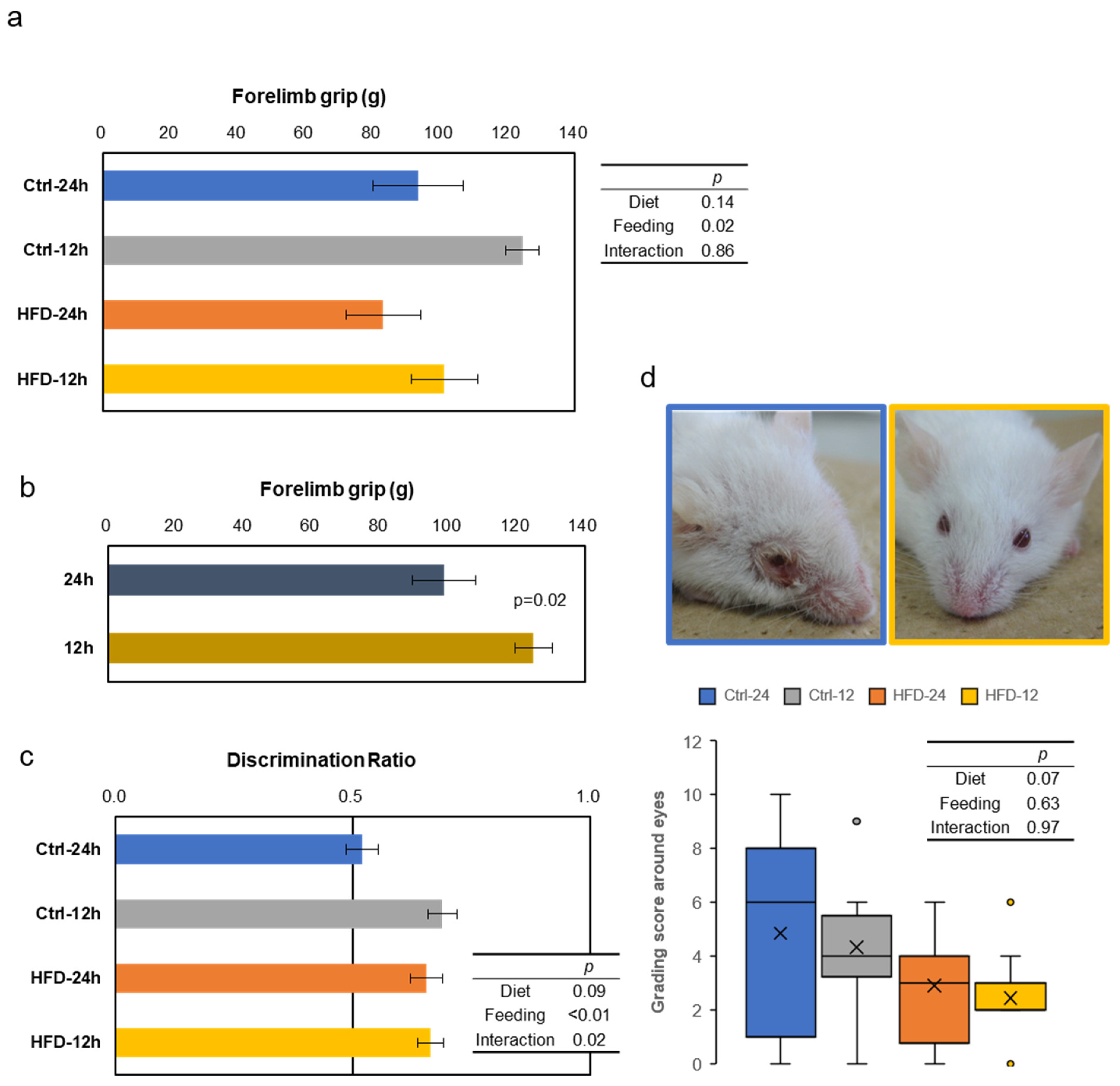
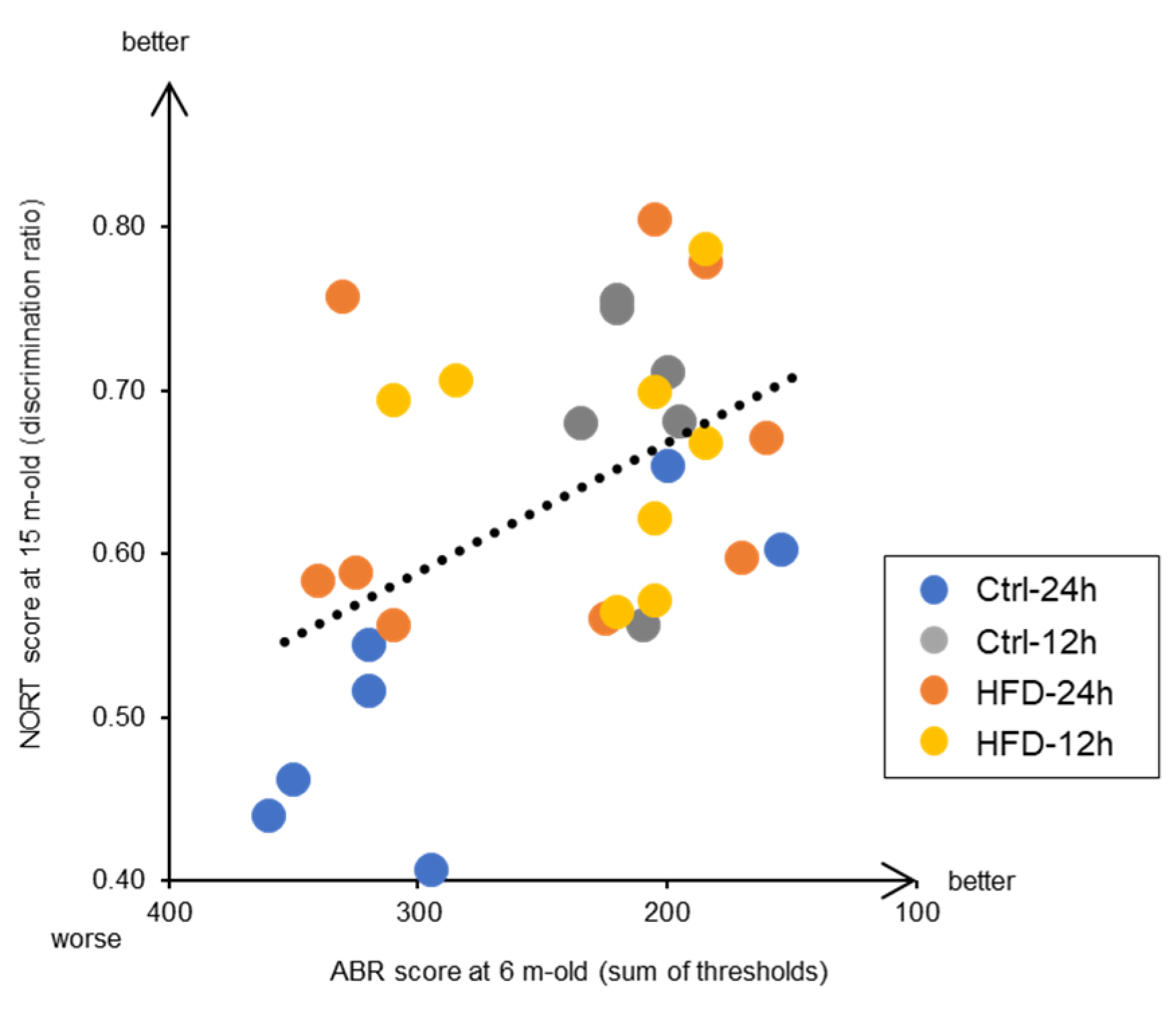
3.3. Effects of HFD and NtRF on Other Age-Related Phenotypes in SAMP8 Mice
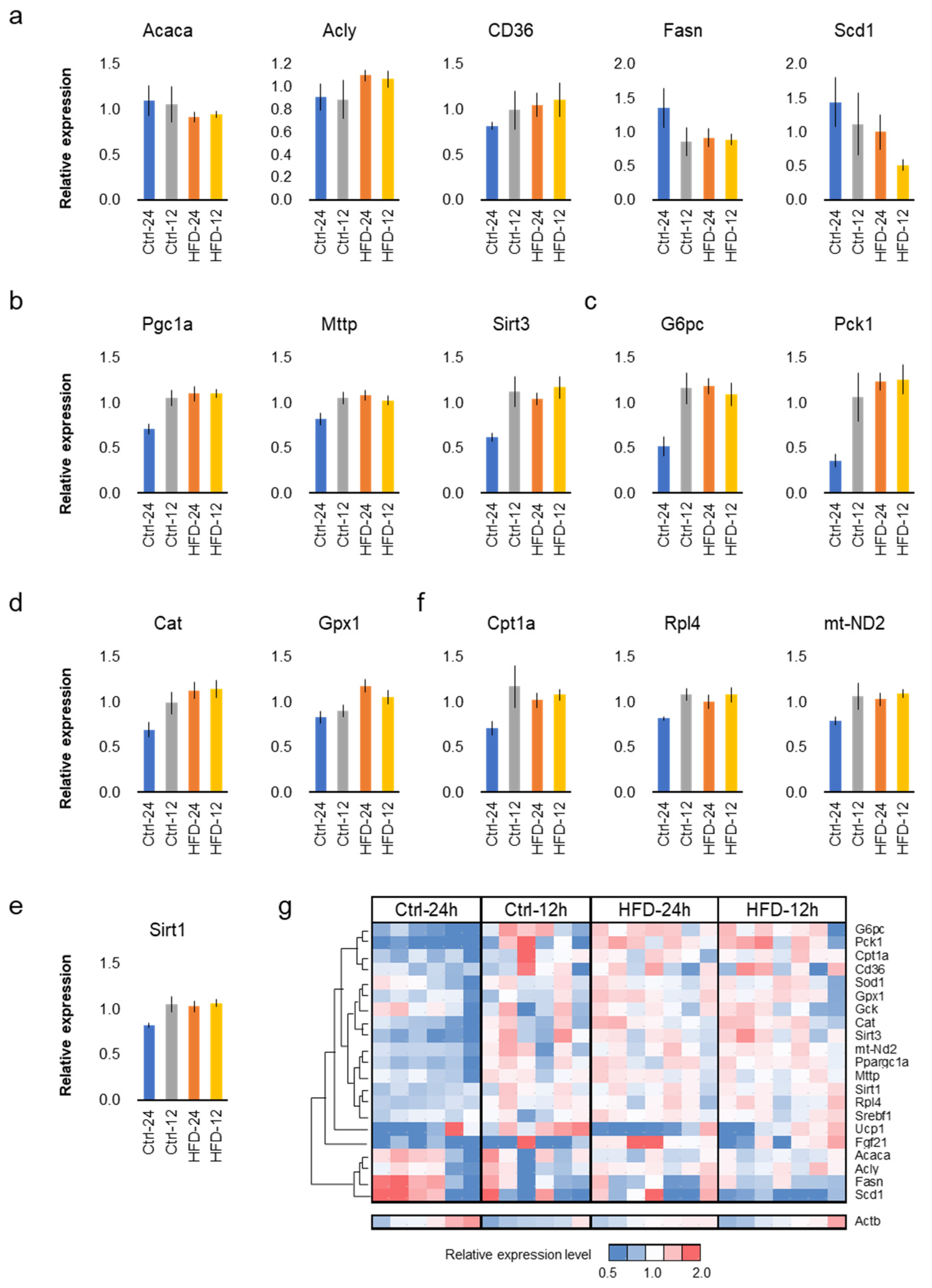
3.4. HFD and NtRF Affected Hepatic Gene Expressions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Yimin; Furumaki, H.; Matsuoka, S.; Sakurai, T.; Kohanawa, M.; Zhao, S.; Kuge, Y.; Tamaki, N.; Chiba, H. A novel murine model for non-alcoholic steatohepatitis developed by combination of a high-fat diet and oxidized low-density lipoprotein. Lab. Investig. J. Tech. Methods Pathol. 2012, 92, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Recena Aydos, L.; Aparecida do Amaral, L.; Serafim de Souza, R.; Jacobowski, A.C.; Freitas Dos Santos, E.; Rodrigues Macedo, M.L. Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in C57bl/6 Models. Nutrients 2019, 11, 3067. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e1213. [Google Scholar] [CrossRef]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef]
- Xie, K.; Neff, F.; Markert, A.; Rozman, J.; Aguilar-Pimentel, J.A.; Amarie, O.V.; Becker, L.; Brommage, R.; Garrett, L.; Henzel, K.S.; et al. Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice. Nat. Commun. 2017, 8, 155. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Bernier, M.; Mattison, J.A.; Aon, M.A.; Kaiser, T.A.; Anson, R.M.; Ikeno, Y.; Anderson, R.M.; Ingram, D.K.; de Cabo, R. Daily Fasting Improves Health and Survival in Male Mice Independent of Diet Composition and Calories. Cell Metab. 2018, 29, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Hahn, O.; Drews, L.F.; Nguyen, A.; Tatsuta, T.; Gkioni, L.; Hendrich, O.; Zhang, Q.; Langer, T.; Pletcher, S.; Wakelam, M.J.O.; et al. A nutritional memory effect counteracts benefits of dietary restriction in old mice. Nat. Metab. 2019, 1, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Hosokawa, M.; Takeshita, S.; Irino, M.; Higuchi, K.; Matsushita, T.; Tomita, Y.; Yasuhira, K.; Hamamoto, H.; Shimizu, K.; et al. A new murine model of accelerated senescence. Mech. Ageing Dev. 1981, 17, 183–194. [Google Scholar] [CrossRef]
- Takeda, T.; Akiguchi, I.; Higuchi, K.; Hosokawa, M.; Hosokawa, T.; Nomura, Y. The Senescence-Accelerated Mouse (SAM): Achievements and Future Directions, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; p. 580. [Google Scholar]
- Morley, J.E.; Farr, S.A.; Kumar, V.B.; Armbrecht, H.J. The SAMP8 mouse: A model to develop therapeutic interventions for Alzheimer’s disease. Curr. Pharm. Des. 2012, 18, 1123–1130. [Google Scholar] [CrossRef]
- Takeda, T. Senescence-accelerated mouse (SAM): With special reference to age-associated pathologies and their modulation. Nihon Eiseigaku Zasshi. Jpn. J. Hyg. 1996, 51, 569–578. [Google Scholar] [CrossRef]
- Someya, S.; Yu, W.; Hallows, W.C.; Xu, J.; Vann, J.M.; Leeuwenburgh, C.; Tanokura, M.; Denu, J.M.; Prolla, T.A. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 2010, 143, 802–812. [Google Scholar] [CrossRef]
- Someya, S.; Xu, J.; Kondo, K.; Ding, D.; Salvi, R.J.; Yamasoba, T.; Rabinovitch, P.S.; Weindruch, R.; Leeuwenburgh, C.; Tanokura, M.; et al. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc. Natl. Acad. Sci. USA 2009, 106, 19432–19437. [Google Scholar] [CrossRef]
- Tian, G.; Sawashita, J.; Kubo, H.; Nishio, S.Y.; Hashimoto, S.; Suzuki, N.; Yoshimura, H.; Tsuruoka, M.; Wang, Y.; Liu, Y.; et al. Ubiquinol-10 supplementation activates mitochondria functions to decelerate senescence in senescence-accelerated mice. Antioxid. Redox Signal. 2014, 20, 2606–2620. [Google Scholar] [CrossRef]
- Fujita, T.; Yamashita, D.; Uehara, N.; Inokuchi, G.; Hasegawa, S.; Otsuki, N.; Nibu, K. A high-fat diet delays age-related hearing loss progression in C57BL/6J mice. PLoS ONE 2015, 10, e0117547. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Robinson, L.; Spruijt, B.; Riedel, G. Between and within laboratory reliability of mouse behaviour recorded in home-cage and open-field. J. Neurosci. Methods 2018, 300, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M. Measuring the strength of mice. J. Vis. Exp. JoVE 2013. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M. Measuring motor coordination in mice. J. Vis. Exp. JoVE 2013, e2609. [Google Scholar] [CrossRef] [PubMed]
- Oike, H.; Aoki-Yoshida, A.; Kimoto-Nira, H.; Yamagishi, N.; Tomita, S.; Sekiyama, Y.; Wakagi, M.; Sakurai, M.; Ippoushi, K.; Suzuki, C.; et al. Dietary intake of heat-killed Lactococcus lactis H61 delays age-related hearing loss in C57BL/6J mice. Sci. Rep. 2016, 6, 23556. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Takagi, Y.; Kaneko, S.; Kurosawa, T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 2011, 60, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Okauchi, H.; Higo-Yamamoto, S.; Sowa, T.; Oike, H.; Yamamoto, S.; Wada, N.; Sakamoto, K.; Oishi, K. Chronically skipping breakfast impairs hippocampal memory-related gene expression and memory function accompanied by reduced wakefulness and body temperature in mice. Biochem. Biophys. Res. Commun. 2020, 524, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Kasai, R.; Higuchi, K.; Takeshita, S.; Shimizu, K.; Hamamoto, H.; Honma, A.; Irino, M.; Toda, K.; Matsumura, A.; et al. Grading score system: A method for evaluation of the degree of senescence in senescence accelerated mouse (SAM). Mech. Ageing Dev. 1984, 26, 91–102. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Oike, H.; Ogawa, Y.; Oishi, K. Simple and Quick Visualization of Periodical Data Using Microsoft Excel. Methods Protoc. 2019, 2, 81. [Google Scholar] [CrossRef]
- Zheng, Q.Y.; Johnson, K.R.; Erway, L.C. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 1999, 130, 94–107. [Google Scholar] [CrossRef]
- Shoji, H.; Takao, K.; Hattori, S.; Miyakawa, T. Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol. Brain 2016, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Oike, H.; Sakurai, M.; Ippoushi, K.; Kobori, M. Time-fixed feeding prevents obesity induced by chronic advances of light/dark cycles in mouse models of jet-lag/shift work. Biochem. Biophys. Res. Commun. 2015, 465, 556–561. [Google Scholar] [CrossRef]
- Yasumoto, Y.; Hashimoto, C.; Nakao, R.; Yamazaki, H.; Hiroyama, H.; Nemoto, T.; Yamamoto, S.; Sakurai, M.; Oike, H.; Wada, N.; et al. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism 2016, 65, 714–727. [Google Scholar] [CrossRef]
- Kim, E.; Kim, E.J.; Seo, S.W.; Hur, C.G.; McGregor, R.A.; Choi, M.S. Meta-review of protein network regulating obesity between validated obesity candidate genes in the white adipose tissue of high-fat diet-induced obese C57BL/6J mice. Crit. Rev. Food Sci. Nutr. 2014, 54, 910–923. [Google Scholar] [CrossRef]
- Baur, J.; Pearson, K.; Price, N.; Jamieson, H.; Lerin, C.; Kalra, A.; Prabhu, V.; Allard, J.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, M.; Takeda, T.; Kogishi, K.; Higuchi, K.; Matushita, T.; Wang, J.; Chiba, T.; Hosokawa, M. Serum lipid concentrations and mean life span are modulated by dietary polyunsaturated fatty acids in the senescence-accelerated mouse. J. Nutr. 2000, 130, 221–227. [Google Scholar] [CrossRef][Green Version]
- de Magalhaes, J.P.; Muller, M.; Rainger, G.E.; Steegenga, W. Fish oil supplements, longevity and aging. Aging 2016, 8, 1578–1582. [Google Scholar] [CrossRef]
- Gutiérrez-Casado, E.; Khraiwesh, H.; López-Domínguez, J.A.; Montero-Guisado, J.; López-Lluch, G.; Navas, P.; de Cabo, R.; Ramsey, J.J.; González-Reyes, J.A.; Villalba, J.M. The impact of aging, calorie restriction and dietary fat on autophagy markers and mitochondrial ultrastructure and dynamics in mouse skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 74, 760–769. [Google Scholar] [CrossRef]
- Newman, J.C.; Covarrubias, A.J.; Zhao, M.; Yu, X.; Gut, P.; Ng, C.P.; Huang, Y.; Haldar, S.; Verdin, E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017, 26, 547–557.e548. [Google Scholar] [CrossRef]
- Someya, S.; Prolla, T.A. Mitochondrial oxidative damage and apoptosis in age-related hearing loss. Mech. Ageing Dev. 2010, 131, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Someya, S.; Yamasoba, T.; Prolla, T.A.; Tanokura, M. Genes encoding mitochondrial respiratory chain components are profoundly down-regulated with aging in the cochlea of DBA/2J mice. Brain Res. 2007, 1182, 26–33. [Google Scholar] [CrossRef]
- Someya, S.; Yamasoba, T.; Weindruch, R.; Prolla, T.A.; Tanokura, M. Caloric restriction suppresses apoptotic cell death in the mammalian cochlea and leads to prevention of presbycusis. Neurobiol. Aging 2007, 28, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Yamasoba, T.; Lin, F.R.; Someya, S.; Kashio, A.; Sakamoto, T.; Kondo, K. Current concepts in age-related hearing loss: Epidemiology and mechanistic pathways. Hear. Res. 2013, 303, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Inokawa, H.; Umemura, Y.; Shimba, A.; Kawakami, E.; Koike, N.; Tsuchiya, Y.; Ohashi, M.; Minami, Y.; Cui, G.; Asahi, T.; et al. Chronic circadian misalignment accelerates immune senescence and abbreviates lifespan in mice. Sci. Rep. 2020, 10, 2569. [Google Scholar] [CrossRef]
- Dawes, P.; Emsley, R.; Cruickshanks, K.J.; Moore, D.R.; Fortnum, H.; Edmondson-Jones, M.; McCormack, A.; Munro, K.J. Hearing loss and cognition: The role of hearing AIDS, social isolation and depression. PLoS ONE 2015, 10, e0119616. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Manczak, M.; Calkins, M.J.; Truong, Q.; Reddy, T.P.; Reddy, A.P.; Shirendeb, U.; Lo, H.H.; Rabinovitch, P.S.; Reddy, P.H. Mitochondria-targeted catalase reduces abnormal APP processing, amyloid beta production and BACE1 in a mouse model of6 Alzheimer’s disease: Implications for neuroprotection and lifespan extension. Hum. Mol. Genet. 2012, 21, 2973–2990. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- Fritze, T.; Teipel, S.; Ovari, A.; Kilimann, I.; Witt, G.; Doblhammer, G. Hearing Impairment Affects Dementia Incidence. An Analysis Based on Longitudinal Health Claims Data in Germany. PLoS ONE 2016, 11, e0156876. [Google Scholar] [CrossRef]
- Hung, S.C.; Liao, K.F.; Muo, C.H.; Lai, S.W.; Chang, C.W.; Hung, H.C. Hearing Loss is Associated with Risk of Alzheimer’s Disease: A Case-Control Study in Older People. J. Epidemiol. 2015, 25, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Metter, E.J.; O’Brien, R.J.; Resnick, S.M.; Zonderman, A.B.; Ferrucci, L. Hearing loss and incident dementia. Arch. Neurol. 2011, 68, 214–220. [Google Scholar] [CrossRef] [PubMed]
| Ingredient (g/100 g Diet) | AIN93M (Control Diet) | HFD-60 (High-Fat Diet) |
|---|---|---|
| Casein | 14.0 | 25.6 |
| L-Cystine | 0.18 | 0.36 |
| Corn starch | 46.6 | - |
| α-Corn starch | 15.5 | 16.0 |
| Sucrose | 10.0 | 5.5 |
| Soybean oil | 4.0 | 2.0 |
| Lard | - | 33.0 |
| Maltodextrin | - | 6.0 |
| Cellulose | 5.0 | 6.61 |
| AIN93G mineral mix | 3.5 | 3.5 |
| AIN93 vitamin mix | 1.0 | 1.0 |
| Calcium carbonate | - | 0.18 |
| Choline bitartrate | 0.25 | 0.25 |
| Tert-butylhydroquinone | 0.0008 | - |
| Total | 100.0 | 100.0 |
| Calorie (kcal/100 g diet) | 360 | 493 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oike, H.; Ogawa, Y.; Azami, K. Long-Term Feeding of a High-Fat Diet Ameliorated Age-Related Phenotypes in SAMP8 Mice. Nutrients 2020, 12, 1416. https://doi.org/10.3390/nu12051416
Oike H, Ogawa Y, Azami K. Long-Term Feeding of a High-Fat Diet Ameliorated Age-Related Phenotypes in SAMP8 Mice. Nutrients. 2020; 12(5):1416. https://doi.org/10.3390/nu12051416
Chicago/Turabian StyleOike, Hideaki, Yukino Ogawa, and Kayo Azami. 2020. "Long-Term Feeding of a High-Fat Diet Ameliorated Age-Related Phenotypes in SAMP8 Mice" Nutrients 12, no. 5: 1416. https://doi.org/10.3390/nu12051416
APA StyleOike, H., Ogawa, Y., & Azami, K. (2020). Long-Term Feeding of a High-Fat Diet Ameliorated Age-Related Phenotypes in SAMP8 Mice. Nutrients, 12(5), 1416. https://doi.org/10.3390/nu12051416