Curcumin Enhances the Antitumoral Effect Induced by the Recombinant Vaccinia Neu Vaccine (rV-neuT) in Mice with Transplanted Salivary Gland Carcinoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Peptides, Cells, and Antibodies
2.2. Poxviruses
2.3. Transgenic BALB-neuT Mouse Colony
2.4. Recombinant Vaccinia neu Vaccination and Analysis of Antitumor Activity In Vivo
2.5. Antibody Immunity Following Vaccination with rV-neuT
2.6. IL-2 and IFN-γ Release Assay
2.7. Histological Examination and Immunohistochemistry
2.8. ErbB2 Expression in Human Cell Lines
2.9. Sulforhodamine B (SRB) Assay
2.10. Statistical Methods
3. Results
3.1. CUR Potentiated the Effect of the rV-neuT Vaccination in Inhibiting the In Vivo Growth of SALTO-5 Cells Transplanted in BALB-neuT Mice
3.2. CUR Increased the Anti-Neu Humoral Response Induced by the rV-neuT Vaccination
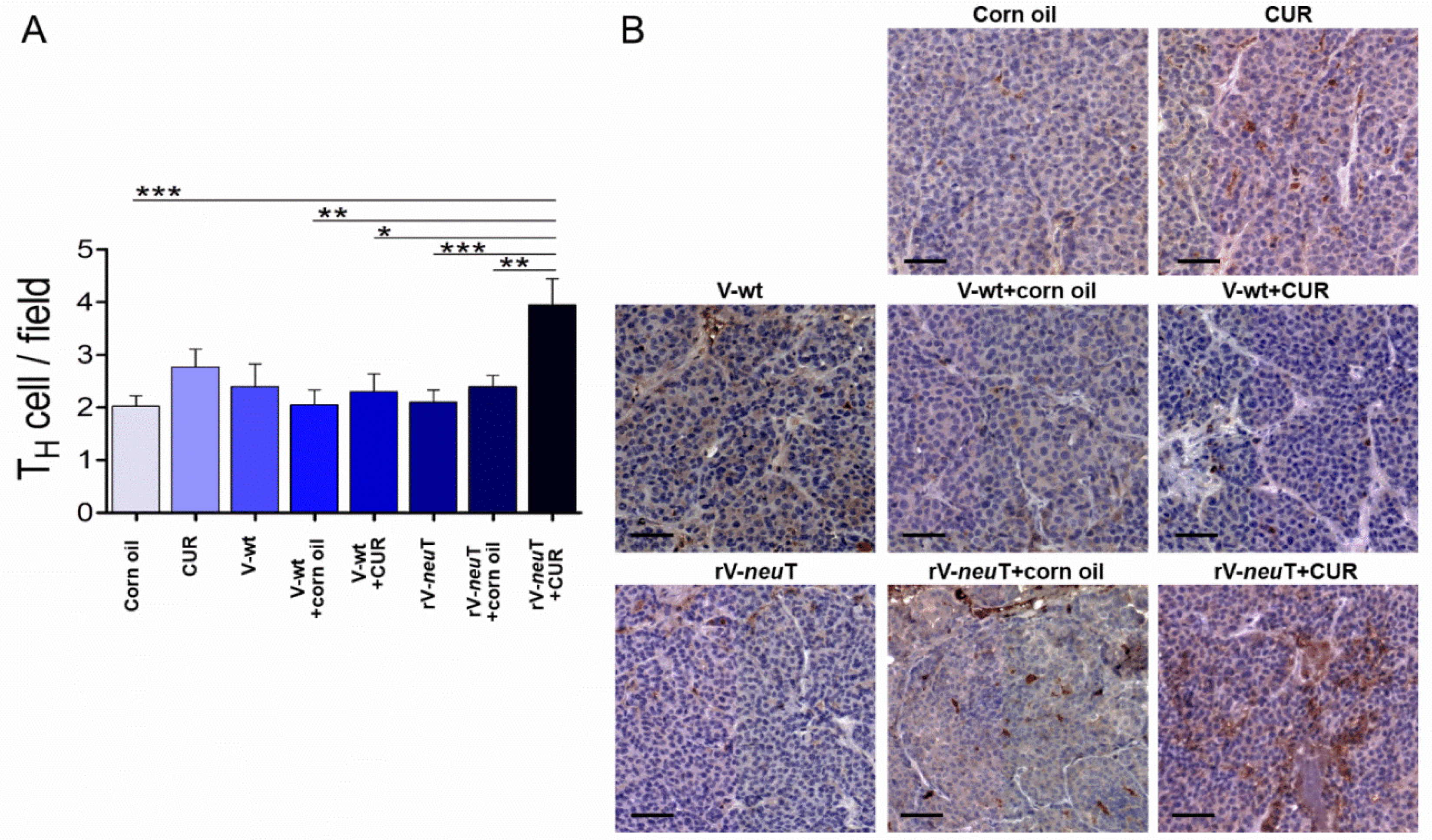
3.3. CUR Increased T-Cell Immune Response Induced by rV-neuT Vaccination
3.4. CUR Increased Necrotic Areas and Inflammatory Cell Infiltration into SALTO-5 Tumors of rV-neuT-Vaccinated Mice
3.5. Biological Effects of mAb 4D5 on HNC Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Landis, S.H.; Murray, T.; Bolden, S.; Wingo, P.A. Cancer statistics, 1999. CA Cancer J. Clin. 1999, 49, 8–31. [Google Scholar] [CrossRef]
- Masuelli, L.; Fantini, M.; Benvenuto, M.; Sacchetti, P.; Giganti, M.G.; Tresoldi, I.; Lido, P.; Lista, F.; Cavallo, F.; Nanni, P.; et al. Intratumoral delivery of recombinant vaccinia virus encoding for ErbB2/Neu inhibits the growth of salivary gland carcinoma cells. J. Transl. Med. 2014, 12, 122. [Google Scholar] [CrossRef] [Green Version]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Chen, W.S.; Xiao, N.; Bender, R.; Ghazalpour, A.; Tan, Z.; Swensen, J.; Millis, S.Z.; Basu, G.; Gatalica, Z.; et al. Mutations in the Kinase Domain of the HER2/ERBB2 Gene Identified in a Wide Variety of Human Cancers. J. Mol. Diagn. 2015, 17, 487–495. [Google Scholar] [CrossRef]
- Gerson, J.N.; Skariah, S.; Denlinger, C.S.; Astsaturov, I. Perspectives of HER2-targeting in gastric and esophageal cancer. Expert Opin. Investig. Drugs 2017, 26, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Hung, M.C. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene 2000, 19, 6115–6121. [Google Scholar] [CrossRef] [Green Version]
- Essajee, S.; Kaufman, H.L. Poxvirus vaccines for cancer and HIV therapy. Expert Opin. Biol. Ther. 2004, 4, 575–588. [Google Scholar] [CrossRef]
- Lechleider, R.J.; Arlen, P.M.; Tsang, K.Y.; Steinberg, S.M.; Yokokawa, J.; Cereda, V.; Camphausen, K.; Schlom, J.; Dahut, W.L.; Gulley, J.L. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin. Cancer. Res. 2008, 14, 5284–5291. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, H.L.; Kim-Schulze, S.; Manson, K.; DeRaffele, G.; Mitcham, J.; Seo, K.S.; Kim, D.W.; Marshall, J. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J. Transl. Med. 2007, 5, 60. [Google Scholar] [CrossRef] [Green Version]
- Gulley, J.; Chen, A.P.; Dahut, W.; Arlen, P.M.; Bastian, A.; Steinberg, S.M.; Tsang, K.; Panicali, D.; Poole, D.; Schlom, J.; et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate 2002, 53, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, J.L.; Hoyer, R.J.; Toomey, M.A.; Faraguna, K.; Chang, P.; Richmond, E.; Pedicano, J.E.; Gehan, E.; Peck, R.A.; Arlen, P.; et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J. Clin. Oncol. 2000, 18, 3964–3973. [Google Scholar] [CrossRef] [PubMed]
- Scholl, S.M.; Balloul, J.M.; Le Goc, G.; Bizouarne, N.; Schatz, C.; Kieny, M.P.; von Mensdorff-Pouilly, S.; Vincent-Salomon, A.; Deneux, L.; Tartour, E.; et al. Recombinant vaccinia virus encoding human MUC1 and IL2 as immunotherapy in patients with breast cancer. J. Immunother. 2000, 23, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, M.J.; Maguire, H.C.; Lattime, E.C. Intralesional vaccinia/GM-CSF recombinant virus in the treatment of metastatic melanoma. Adv. Exp. Med. Biol. 2000, 465, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Conry, R.M.; Khazaeli, M.B.; Saleh, M.N.; Allen, K.O.; Barlow, D.L.; Moore, S.E.; Craig, D.; Arani, R.B.; Schlom, J.; LoBuglio, A.F. Phase I trial of a recombinant vaccinia virus encoding carcinoembryonic antigen in metastatic adenocarcinoma: Comparison of intradermal versus subcutaneous administration. Clin. Cancer Res. 1999, 5, 2330–2337. [Google Scholar] [PubMed]
- Conry, R.M.; Allen, K.O.; Lee, S.; Moore, S.E.; Shaw, D.R.; LoBuglio, A.F. Human autoantibodies to carcinoembryonic antigen (CEA) induced by a vaccinia-CEA vaccine. Clin. Cancer Res. 2000, 6, 34–41. [Google Scholar]
- Masuelli, L.; Marzocchella, L.; Focaccetti, C.; Lista, F.; Nardi, A.; Scardino, A.; Mattei, M.; Turriziani, M.; Modesti, M.; Forni, G.; et al. Local delivery of recombinant vaccinia virus encoding for neu counteracts growth of mammary tumors more efficiently than systemic delivery in neu transgenic mice. Cancer Immunol. Immunother. 2010, 59, 1247–1258. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Gulley, J.L. Poxviral vectors for cancer immunotherapy. Expert Opin. Biol. Ther. 2012, 12, 463–478. [Google Scholar] [CrossRef] [Green Version]
- Izzi, V.; Buler, M.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Bei, R. Poxvirus-based vaccines for cancer immunotherapy: New insights from combined cytokines/co-stimulatory molecules delivery and “uncommon” strains. Anticancer Agents Med. Chem. 2014, 14, 183–189. [Google Scholar] [CrossRef]
- Al Yaghchi, C.; Zhang, Z.; Alusi, G.; Lemoine, N.R.; Wang, Y. Vaccinia virus, a promising new therapeutic agent for pancreatic cancer. Immunotherapy 2015, 7, 1249–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Fan, W.; Ru, G.; Huang, F.; Lu, X.; Zhang, X.; Mou, X.; Wang, S. Gemcitabine combined with an engineered oncolytic vaccinia virus exhibits a synergistic suppressive effect on the tumor growth of pancreatic cancer. Oncol. Rep. 2019, 41, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.S.; Bartlett, D.L. Oncolytic viruses as platform for multimodal cancer therapeutics: A promising land. Cancer Gene Ther. 2014, 21, 261–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bommareddy, P.K.; Shettigar, M.; Kaufman, H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Masuelli, L.; Di Stefano, E.; Fantini, M.; Mattera, R.; Benvenuto, M.; Marzocchella, L.; Sacchetti, P.; Focaccetti, C.; Bernardini, R.; Tresoldi, I.; et al. Resveratrol potentiates the in vitro and in vivo anti-tumoral effects of curcumin in head and neck carcinomas. Oncotarget 2014, 5, 10745–10762. [Google Scholar] [CrossRef] [Green Version]
- Masuelli, L.; Marzocchella, L.; Quaranta, A.; Palumbo, C.; Pompa, G.; Izzi, V.; Canini, A.; Modesti, A.; Galvano, F.; Bei, R. Apigenin induces apoptosis and impairs head and neck carcinomas EGFR/ErbB2 signaling. Front. Biosci. (Landmark Ed.) 2011, 16, 1060–1068. [Google Scholar] [CrossRef] [Green Version]
- Masuelli, L.; Benvenuto, M.; Mattera, R.; Di Stefano, E.; Zago, E.; Taffera, G.; Tresoldi, I.; Giganti, M.G.; Frajese, G.V.; Berardi, G.; et al. In Vitro and In Vivo Anti-tumoral Effects of the Flavonoid Apigenin in Malignant Mesothelioma. Front. Pharmacol. 2017, 8, 373. [Google Scholar] [CrossRef]
- Masuelli, L.; Benvenuto, M.; Fantini, M.; Marzocchella, L.; Sacchetti, P.; Di Stefano, E.; Tresoldi, I.; Izzi, V.; Bernardini, R.; Palumbo, C.; et al. Curcumin induces apoptosis in breast cancer cell lines and delays the growth of mammary tumors in neu transgenic mice. J. Biol. Regul. Homeost. Agents 2013, 27, 105–119. [Google Scholar]
- Masuelli, L.; Granato, M.; Benvenuto, M.; Mattera, R.; Bernardini, R.; Mattei, M.; d’Amati, G.; D’Orazi, G.; Faggioni, A.; Bei, R.; et al. Chloroquine supplementation increases the cytotoxic effect of curcumin against Her2/neu overexpressing breast cancer cells. Oncoimmunology 2017, 6, e1356151. [Google Scholar] [CrossRef] [Green Version]
- Masuelli, L.; Benvenuto, M.; Di Stefano, E.; Mattera, R.; Fantini, M.; De Feudis, G.; De Smaele, E.; Tresoldi, I.; Giganti, M.G.; Modesti, A.; et al. Curcumin blocks autophagy and activates apoptosis of malignant mesothelioma cell lines and increases the survival of mice intraperitoneally transplanted with a malignant mesothelioma cell line. Oncotarget 2017, 8, 34405–34422. [Google Scholar] [CrossRef] [Green Version]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [Green Version]
- Mbese, Z.; Khwaza, V.; Aderibigbe, B.A. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules 2019, 24, 4386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, B.L.; Norhaizan, M.E. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Panda, A.K.; Mukherjee, S.; Sa, G. Curcumin and tumor immune-editing: Resurrecting the immune system. Cell. Div. 2015, 10, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Molecules 2018, 23, 2778. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, S.; Md Sakib Hossain, D.; Mohanty, S.; Sankar Sen, G.; Chattopadhyay, S.; Banerjee, S.; Chakraborty, J.; Das, K.; Sarkar, D.; Das, T.; et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell. Mol. Immunol. 2010, 7, 306–315. [Google Scholar] [CrossRef]
- Mohammadi, A.; Blesso, C.N.; Barreto, G.E.; Banach, M.; Majeed, M.; Sahebkar, A. Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent. J. Nutr. Biochem. 2019, 66, 1–16. [Google Scholar] [CrossRef]
- Larussa, T.; Gervasi, S.; Liparoti, R.; Suraci, E.; Marasco, R.; Imeneo, M.; Luzza, F. Downregulation of Interleukin- (IL-) 17 through enhanced Indoleamine 2,3-Dioxygenase (IDO) induction by curcumin: A potential mechanism of tolerance towards Helicobacter pylori. J. Immunol. Res. 2018, 2018, 3739593. [Google Scholar] [CrossRef] [Green Version]
- Afolayan, F.I.D.; Erinwusi, B.; Oyeyemi, O.T. Immunomodulatory activity of curcumin-entrapped poly d,l-lactic-. Integr. Med. Res. 2018, 7, 168–175. [Google Scholar] [CrossRef]
- Boozari, M.; Butler, A.E.; Sahebkar, A. Impact of curcumin on toll-like receptors. J. Cell. Physiol. 2019, 234, 12471–12482. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Liu, L.; Luo, E.; Hu, J. Curcumin enhances anti-tumor immune response in tongue squamous cell carcinoma. Arch. Oral Biol. 2018, 92, 32–37. [Google Scholar] [CrossRef]
- Mukherjee, S.; Hussaini, R.; White, R.; Atwi, D.; Fried, A.; Sampat, S.; Piao, L.; Pan, Q.; Banerjee, P. TriCurin, a synergistic formulation of curcumin, resveratrol, and epicatechin gallate, repolarizes tumor-associated macrophages and triggers an immune response to cause suppression of HPV+ tumors. Cancer Immunol. Immunother. 2018, 67, 761–774. [Google Scholar] [CrossRef]
- Singh, M.; Ramos, I.; Asafu-Adjei, D.; Quispe-Tintaya, W.; Chandra, D.; Jahangir, A.; Zang, X.; Aggarwal, B.B.; Gravekamp, C. Curcumin improves the therapeutic efficacy of Listeria(at)-Mage-b vaccine in correlation with improved T-cell responses in blood of a triple-negative breast cancer model 4T1. Cancer Med. 2013, 2, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhu, W.; Da, J.; Xu, M.; Wang, Y.; Zhou, J.; Wang, Z. Bisdemethoxycurcumin in combination with α-PD-L1 antibody boosts immune response against bladder cancer. OncoTargets Ther. 2017, 10, 2675–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Miao, L.; Wang, Y.; Xu, Z.; Zhao, Y.; Shen, Y.; Xiang, G.; Huang, L. Curcumin Micelles Remodel Tumor Microenvironment and Enhance Vaccine Activity in an Advanced Melanoma Model. Mol. Ther. 2016, 24, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Luo, F.; Song, X.; Zhang, Y.; Chu, Y. Low-dose curcumin leads to the inhibition of tumor growth via enhancing CTL-mediated antitumor immunity. Int. Immunopharmacol. 2011, 11, 1234–1240. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Tian, K.; Chen, X.; Zhang, R.; Mu, X.; Wu, Y.; Wang, D.; Wang, S.; Liu, F.; et al. Apigenin suppresses PD-L1 expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects. J. Exp. Clin. Cancer Res. 2018, 37, 261. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.Y.; Su, C.H.; Luo, H.H.; Lei, Y.Y.; Zeng, B.; Zhu, H.S.; Chen, Z.G. Curcumin converts Foxp3+ regulatory T cells to T helper 1 cells in patients with lung cancer. J. Cell Biochem. 2018, 119, 1420–1428. [Google Scholar] [CrossRef]
- Xu, B.; Yu, L.; Zhao, L.Z. Curcumin up regulates T helper 1 cells in patients with colon cancer. Am. J. Transl. Res. 2017, 9, 1866–1875. [Google Scholar]
- Porzia, A.; Lanzardo, S.; Citti, A.; Cavallo, F.; Forni, G.; Santoni, A.; Galandrini, R.; Paolini, R. Attenuation of PI3K/Akt-mediated tumorigenic signals through PTEN activation by DNA vaccine-induced anti-ErbB2 antibodies. J. Immunol. 2010, 184, 4170–4177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bargmann, C.I.; Hung, M.C.; Weinberg, R.A. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature 1986, 319, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Masuelli, L.; Focaccetti, C.; Cereda, V.; Lista, F.; Vitolo, D.; Trono, P.; Gallo, P.; Amici, A.; Monaci, P.; Mattei, M.; et al. Gene-specific inhibition of breast carcinoma in BALB-neuT mice by active immunization with rat Neu or human ErbB receptors. Int. J. Oncol. 2007, 30, 381–392. [Google Scholar]
- Di Marco, E.; Pierce, J.H.; Knicley, C.L.; Di Fiore, P.P. Transformation of NIH 3T3 cells by overexpression of the normal coding sequence of the rat neu gene. Mol. Cell. Biol. 1990, 10, 3247–3252. [Google Scholar] [CrossRef] [Green Version]
- Pannellini, T.; Spadaro, M.; Di Carlo, E.; Ambrosino, E.; Iezzi, M.; Amici, A.; Lollini, P.L.; Forni, G.; Cavallo, F.; Musiani, P. Timely DNA vaccine combined with systemic IL-12 prevents parotid carcinomas before a dominant-negative p53 makes their growth independent of HER-2/neu expression. J. Immunol. 2006, 176, 7695–7703. [Google Scholar] [CrossRef] [Green Version]
- Rovero, S.; Amici, A.; Di Carlo, E.; Bei, R.; Nanni, P.; Quaglino, E.; Porcedda, P.; Boggio, K.; Smorlesi, A.; Lollini, P.L.; et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J. Immunol. 2000, 165, 5133–5142. [Google Scholar] [CrossRef] [Green Version]
- Gallo, P.; Dharmapuri, S.; Nuzzo, M.; Maldini, D.; Cipriani, B.; Forni, G.; Monaci, P. Adenovirus vaccination against neu oncogene exerts long-term protection from tumorigenesis in BALB/neuT transgenic mice. Int. J. Cancer 2007, 120, 574–584. [Google Scholar] [CrossRef]
- Scimeca, M.; Bonfiglio, R.; Urbano, N.; Cerroni, C.; Anemona, L.; Montanaro, M.; Fazi, S.; Schillaci, O.; Mauriello, A.; Bonanno, E. Programmed death ligand 1 expression in prostate cancer cells is associated with deep changes of the tumor inflammatory infiltrate composition. Urol. Oncol. 2019, 37, e219–e231. [Google Scholar] [CrossRef]
- Benvenuto, M.; Mattera, R.; Sticca, J.I.; Rossi, P.; Cipriani, C.; Giganti, M.G.; Volpi, A.; Modesti, A.; Masuelli, L.; Bei, R. Effect of the BH3 Mimetic Polyphenol (-)-Gossypol (AT-101) on the in vitro and in vivo growth of malignant mesothelioma. Front. Pharmacol. 2018, 9, 1269. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, E.; Momtazi, A.A.; Johnston, T.P.; Sahebkar, A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: A nature-made jack-of-all-trades? J. Cell. Physiol. 2018, 233, 830–848. [Google Scholar] [CrossRef]
- Yadav, V.S.; Mishra, K.P.; Singh, D.P.; Mehrotra, S.; Singh, V.K. Immunomodulatory effects of curcumin. Immunopharmacol. Immunotoxicol. 2005, 27, 485–497. [Google Scholar] [CrossRef]
- Ranjan, D.; Johnston, T.D.; Wu, G.; Elliott, L.; Bondada, S.; Nagabhushan, M. Curcumin blocks cyclosporine A-resistant CD28 costimulatory pathway of human T-cell proliferation. J. Surg. Res. 1998, 77, 174–178. [Google Scholar] [CrossRef]
- Li, X.; Liu, X. Effect of curcumin on immune function of mice. J. Huazhong Univ. Sci. Technol. Med. Sci. 2005, 25, 137–140. [Google Scholar] [CrossRef]
- Churchill, M.; Chadburn, A.; Bilinski, R.T.; Bertagnolli, M.M. Inhibition of intestinal tumors by curcumin is associated with changes in the intestinal immune cell profile. J. Surg. Res. 2000, 89, 169–175. [Google Scholar] [CrossRef]
- Han, S.S.; Chung, S.T.; Robertson, D.A.; Ranjan, D.; Bondada, S. Curcumin causes the growth arrest and apoptosis of B cell lymphoma by downregulation of egr-1, c-myc, bcl-XL, NF-kappa B, and p53. Clin. Immunol. 1999, 93, 152–161. [Google Scholar] [CrossRef]
- Bhaumik, S.; Jyothi, M.D.; Khar, A. Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett. 2000, 483, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Jagetia, G.C.; Aggarwal, B.B. “Spicing up” of the immune system by curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef]
- Zhao, H.M.; Xu, R.; Huang, X.Y.; Cheng, S.M.; Huang, M.F.; Yue, H.Y.; Wang, X.; Zou, Y.; Lu, A.P.; Liu, D.Y. Curcumin improves regulatory T cells in gut-associated lymphoid tissue of colitis mice. World J. Gastroenterol. 2016, 22, 5374–5383. [Google Scholar] [CrossRef]
- Oh, J.G.; Hwang, D.J.; Heo, T.H. Direct regulation of IL-2 by curcumin. Biochem. Biophys. Res. Commun. 2018, 495, 300–305. [Google Scholar] [CrossRef]
- Ranjan, D.; Chen, C.; Johnston, T.D.; Jeon, H.; Nagabhushan, M. Curcumin inhibits mitogen stimulated lymphocyte proliferation, NFkappaB activation, and IL-2 signaling. J. Surg. Res. 2004, 121, 171–177. [Google Scholar] [CrossRef]
- Forward, N.A.; Conrad, D.M.; Power Coombs, M.R.; Doucette, C.D.; Furlong, S.J.; Lin, T.J.; Hoskin, D.W. Curcumin blocks interleukin (IL)-2 signaling in T-lymphocytes by inhibiting IL-2 synthesis, CD25 expression, and IL-2 receptor signaling. Biochem. Biophys. Res. Commun. 2011, 407, 801–806. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, H.G.; Choi, J.M. Curcumin Elevates TFH Cells and Germinal Center B Cell Response for Antibody Production in Mice. Immune Netw. 2019, 19, e35. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Han, Y.; Kayahara, M.; Watanabe, T.; Arishige, H.; Kato, N. Consumption of curcumin elevates fecal immunoglobulin A, an index of intestinal immune function, in rats fed a high-fat diet. J. Nutr. Sci. Vitaminol. (Tokyo) 2010, 56, 68–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanni, P.; Landuzzi, L.; Nicoletti, G.; De Giovanni, C.; Rossi, I.; Croci, S.; Astolfi, A.; Iezzi, M.; Di Carlo, E.; Musiani, P.; et al. Immunoprevention of mammary carcinoma in HER-2/neu transgenic mice is IFN-gamma and B cell dependent. J. Immunol. 2004, 173, 2288–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.M.; Terabe, M.; Sakai, Y.; Munasinghe, J.; Forni, G.; Morris, J.C.; Berzofsky, J.A. Early role of CD4+ Th1 cells and antibodies in HER-2 adenovirus vaccine protection against autochthonous mammary carcinomas. J. Immunol. 2005, 174, 4228–4236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montgomery, R.B.; Makary, E.; Schiffman, K.; Goodell, V.; Disis, M.L. Endogenous anti-HER2 antibodies block HER2 phosphorylation and signaling through extracellular signal-regulated kinase. Cancer Res. 2005, 65, 650–656. [Google Scholar]
- Brodowicz, T.; Kandioler, D.; Tomek, S.; Ludwig, C.; Rudas, M.; Kunstfeld, R.; Koestler, W.; Hejna, M.; Budinsky, A.; Wiltschke, C.; et al. Anti-Her-2/neu antibody induces apoptosis in Her-2/neu overexpressing breast cancer cells independently from p53 status. Br. J. Cancer 2001, 85, 1764–1770. [Google Scholar] [CrossRef]
- Yakes, F.M.; Chinratanalab, W.; Ritter, C.A.; King, W.; Seelig, S.; Arteaga, C.L. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002, 62, 4132–4141. [Google Scholar]
- Cuello, M.; Ettenberg, S.A.; Clark, A.S.; Keane, M.M.; Posner, R.H.; Nau, M.M.; Dennis, P.A.; Lipkowitz, S. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res. 2001, 61, 4892–4900. [Google Scholar]
- South, E.H.; Exon, J.H.; Hendrix, K. Dietary curcumin enhances antibody response in rats. Immunopharmacol. Immunotoxicol. 1997, 19, 105–119. [Google Scholar] [CrossRef]
- Antony, S.; Kuttan, R.; Kuttan, G. Immunomodulatory activity of curcumin. Immunol. Investig. 1999, 28, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Yip, Y.L.; Ward, R.L. Anti-ErbB-2 monoclonal antibodies and ErbB-2-directed vaccines. Cancer Immunol. Immunother. 2002, 50, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, E.; Campiglio, M.; Pupa, S.M.; Ménard, S.; Balsari, A. Activity and resistance of trastuzumab according to different clinical settings. Cancer Treat. Rev. 2012, 38, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Blankenstein, T.; Qin, Z. The role of IFN-gamma in tumor transplantation immunity and inhibition of chemical carcinogenesis. Curr. Opin. Immunol. 2003, 15, 148–154. [Google Scholar] [CrossRef]
- Liu, D.; You, M.; Xu, Y.; Li, F.; Zhang, D.; Li, X.; Hou, Y. Inhibition of curcumin on myeloid-derived suppressor cells is requisite for controlling lung cancer. Int. Immunopharmacol. 2016, 39, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Reilly, R.T.; Machiels, J.P.; Emens, L.A.; Ercolini, A.M.; Okoye, F.I.; Lei, R.Y.; Weintraub, D.; Jaffee, E.M. The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu-expressing tumors. Cancer Res. 2001, 61, 880–883. [Google Scholar]
- Musiani, P.; Allione, A.; Modica, A.; Lollini, P.L.; Giovarelli, M.; Cavallo, F.; Belardelli, F.; Forni, G.; Modesti, A. Role of neutrophils and lymphocytes in inhibition of a mouse mammary adenocarcinoma engineered to release IL-2, IL-4, IL-7, IL-10, IFN-alpha, IFN-gamma, and TNF-alpha. Lab. Investig. 1996, 74, 146–157. [Google Scholar]
- Yan, M.; Parker, B.A.; Schwab, R.; Kurzrock, R. HER2 aberrations in cancer: Implications for therapy. Cancer Treat. Rev. 2014, 40, 770–780. [Google Scholar] [CrossRef]
- Chung, C.H.; Germain, A.; Subramaniam, R.M.; Heilmann, A.M.; Fedorchak, K.; Ali, S.M.; Miller, V.A.; Palermo, R.A.; Fakhry, C. Genomic alterations in human epidermal growth factor receptor 2 (HER2/ERBB2) in head and neck squamous cell carcinoma. Head Neck 2017, 39, E15–E19. [Google Scholar] [CrossRef]
- De Block, K.; Vander Poorten, V.; Dormaar, T.; Nuyts, S.; Hauben, E.; Floris, G.; Deroose, C.M.; Schöffski, P.; Clement, P.M. Metastatic HER-2-positive salivary gland carcinoma treated with trastuzumab and a taxane: A series of six patients. Acta Clin. Belg. 2016, 71, 383–388. [Google Scholar] [CrossRef]
- Thorpe, L.M.; Schrock, A.B.; Erlich, R.L.; Miller, V.A.; Knost, J.; Le-Lindqwister, N.; Jujjavarapu, S.; Ali, S.M.; Liu, J.J. Significant and durable clinical benefit from trastuzumab in 2 patients with HER2-amplified salivary gland cancer and a review of the literature. Head Neck 2017, 39, E40–E44. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Ma, T.M.; Rooper, L.; Hembrough, T.; Foss, R.D.; Schmitt, N.C.; Sawhney, R.; Flanders, A.; Kang, H. Exceptional responses to pertuzumab, trastuzumab, and docetaxel in human epidermal growth factor receptor-2 high expressing salivary duct carcinomas. Head Neck 2018, 40, E100–E106. [Google Scholar] [CrossRef] [PubMed]







| Time (Week) | Estimated Tumor Volume Over Time (mm3) | Standard Error | |||
| V-wt | 1 | 7.54 | 2.69 | ||
| 2 | 103.11 | 14.72 | |||
| 3 | 191.62 | 32.08 | |||
| 4 | 556.47 | 57.88 | |||
| 5 | 1737.03 | 196.42 | |||
| 6 | 3019.57 | 295.22 | |||
| Expected Reduction of Tumor Volume (mm3) | Standard Error | t-Statistic | p-Value | ||
| CUR | 1 | 2.14 | 3.01 | 0.72 | 0.4793 |
| 2 | −19.56 | 16.46 | −1.19 | 0.2435 | |
| 3 | −54.09 | 35.87 | −1.51 | 0.1417 | |
| 4 | −186.18 | 64.71 | −2.88 | 0.0072 | |
| 5 | −857.72 | 219.60 | −3.91 | 0.0005 | |
| 6 | −1576.84 | 289.38 | −5.45 | <0.0001 | |
| rV-neuT | 1 | 2.65 | 3.06 | 0.87 | 0.3930 |
| 2 | −75.01 | 16.75 | −4.48 | 0.0001 | |
| 3 | −106.11 | 36.52 | −2.91 | 0.0067 | |
| 4 | −356.26 | 65.87 | −5.41 | <0.0001 | |
| 5 | −1035.72 | 223.56 | −4.63 | <0.0001 | |
| 6 | −1205.78 | 312.21 | −3.86 | 0.0005 |
| HR a | 95% CI b | p-Value | |||
|---|---|---|---|---|---|
| V-wt | vs. | rV-neuT | 6.447 | (2.013, 20.642) | 0.0017 |
| rV-neuT | vs. | rV-neuT+CUR | 11.848 | (3.561, 39.414) | <0.0001 |
| V-wt | vs. | rV-neuT+CUR | 76.377 | (9.690, 602.00) | <0.0001 |
| Group | Number of Pooled Sera | Serum Titer (SD) a | p-Value |
|---|---|---|---|
| Corn Oil | 4 | Neg | |
| CUR | 4 | Neg | |
| V-wt | 4 | Neg | |
| V-wt+Corn Oil | 3 | Neg | |
| V-wt+CUR | 4 | Neg | |
| rV-neuT | 6 | 2100 b (±0.012) | 0.000001 c |
| rV-neuT+Corn Oil | 8 | 2400 (±0.020) | 0.00001 c |
| rV-neuT+CUR | 8 | 5000 (±0.019) |
| Immunoglobulin Isotype Against Neu | ||||||
|---|---|---|---|---|---|---|
| IgM | IgG1 | IgG2a | IgG2b | IgG3 | IgA | |
| rV-neuT | 16.708 ± 1.454 a | 17.633 ± 0.621 | 25.874 ± 0.210 | 24.534 ± 2.687 | 9.833 ± 0.058 | 5.418 ± 1.460 |
| rV-neuT+Corn Oil | 17.158 ± 0.407 | 16.210 ± 3.706 | 26.413 ± 0.216 | 25.511 ± 4.181 | 9.614 ± 2.103 | 5.094 ± 1.032 |
| rV-neuT+CUR | 16.372 ± 0.913 | 15.620 ± 0.439 | 25.226 ± 3.423 | 27.700 ± 1.520 | 9.516 ± 1.165 | 5.566 ± 0.834 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Focaccetti, C.; Benvenuto, M.; Ciuffa, S.; Fazi, S.; Scimeca, M.; Nardi, A.; Miele, M.T.; Battisti, A.; Bonanno, E.; Modesti, A.; et al. Curcumin Enhances the Antitumoral Effect Induced by the Recombinant Vaccinia Neu Vaccine (rV-neuT) in Mice with Transplanted Salivary Gland Carcinoma Cells. Nutrients 2020, 12, 1417. https://doi.org/10.3390/nu12051417
Focaccetti C, Benvenuto M, Ciuffa S, Fazi S, Scimeca M, Nardi A, Miele MT, Battisti A, Bonanno E, Modesti A, et al. Curcumin Enhances the Antitumoral Effect Induced by the Recombinant Vaccinia Neu Vaccine (rV-neuT) in Mice with Transplanted Salivary Gland Carcinoma Cells. Nutrients. 2020; 12(5):1417. https://doi.org/10.3390/nu12051417
Chicago/Turabian StyleFocaccetti, Chiara, Monica Benvenuto, Sara Ciuffa, Sara Fazi, Manuel Scimeca, Alessandra Nardi, Martino Tony Miele, Andrea Battisti, Elena Bonanno, Andrea Modesti, and et al. 2020. "Curcumin Enhances the Antitumoral Effect Induced by the Recombinant Vaccinia Neu Vaccine (rV-neuT) in Mice with Transplanted Salivary Gland Carcinoma Cells" Nutrients 12, no. 5: 1417. https://doi.org/10.3390/nu12051417
APA StyleFocaccetti, C., Benvenuto, M., Ciuffa, S., Fazi, S., Scimeca, M., Nardi, A., Miele, M. T., Battisti, A., Bonanno, E., Modesti, A., Masuelli, L., & Bei, R. (2020). Curcumin Enhances the Antitumoral Effect Induced by the Recombinant Vaccinia Neu Vaccine (rV-neuT) in Mice with Transplanted Salivary Gland Carcinoma Cells. Nutrients, 12(5), 1417. https://doi.org/10.3390/nu12051417











