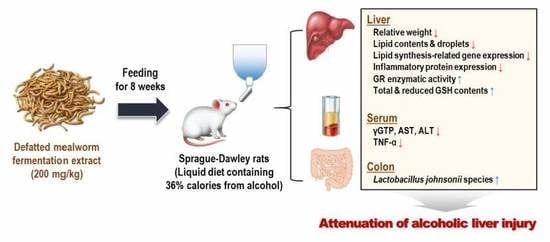
Defatted Tenebrio molitor Larva Fermentation Extract Modifies Steatosis, Inflammation and Intestinal Microflora in Chronic Alcohol-Fed Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Defatted Mealworm Fermentation Extract (MWF)
2.2. Animals and Experimental Design
2.3. Dosage Information
2.4. Biochemical Analysis in Serum and Liver
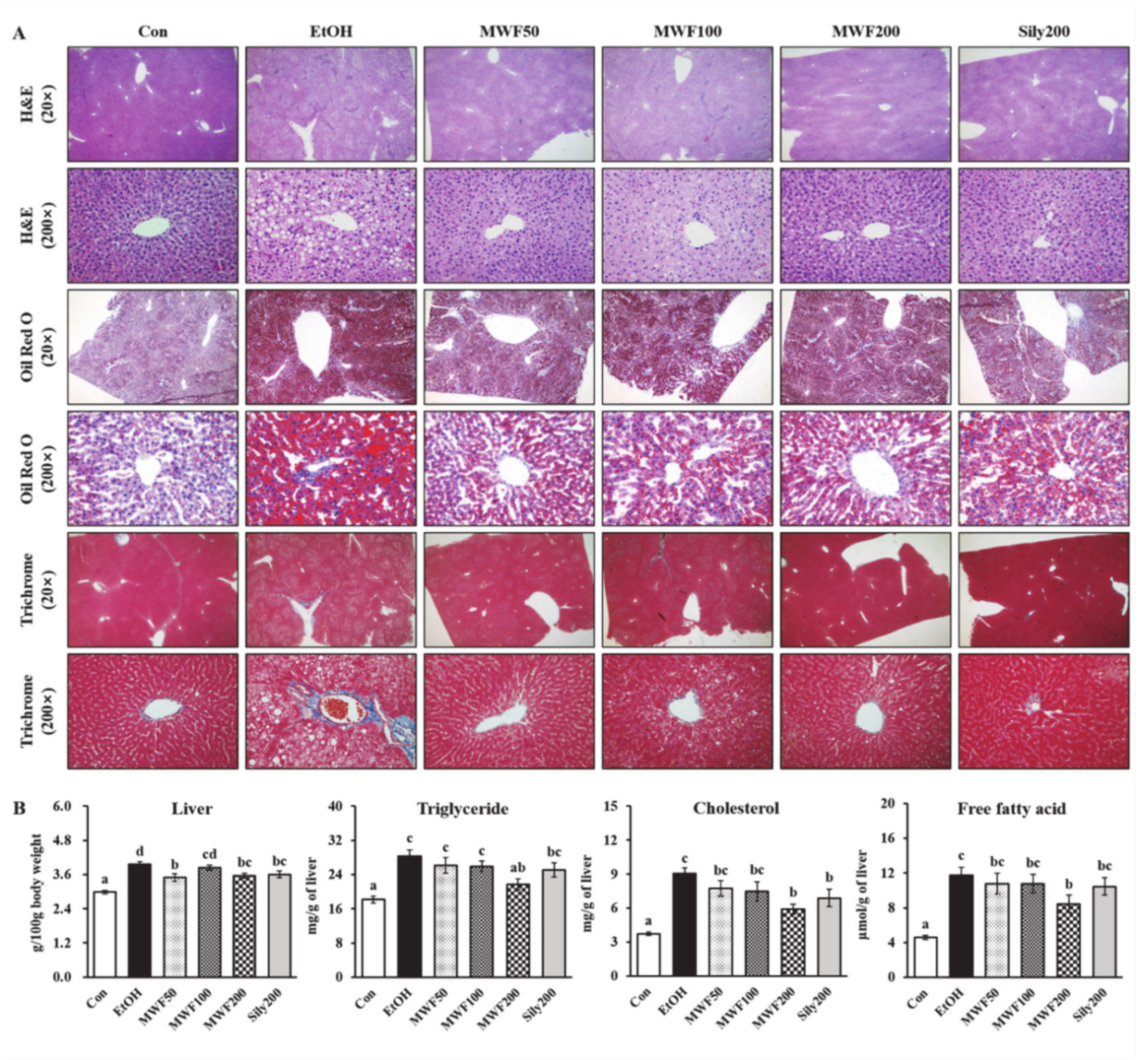
2.5. Hepatic Histological Analysis
2.6. RNA Isolation and Quantitative Real-Time PCR Analysis
2.7. Western Blot Analysis
2.8. Hepatic Enzymatic Activities and Glutathione Level
2.9. Microbial Community Analysis in Colon
2.10. Statistical Analysis
3. Results
3.1. MWF Ameliorated Alcoholic Hepatosteatosis
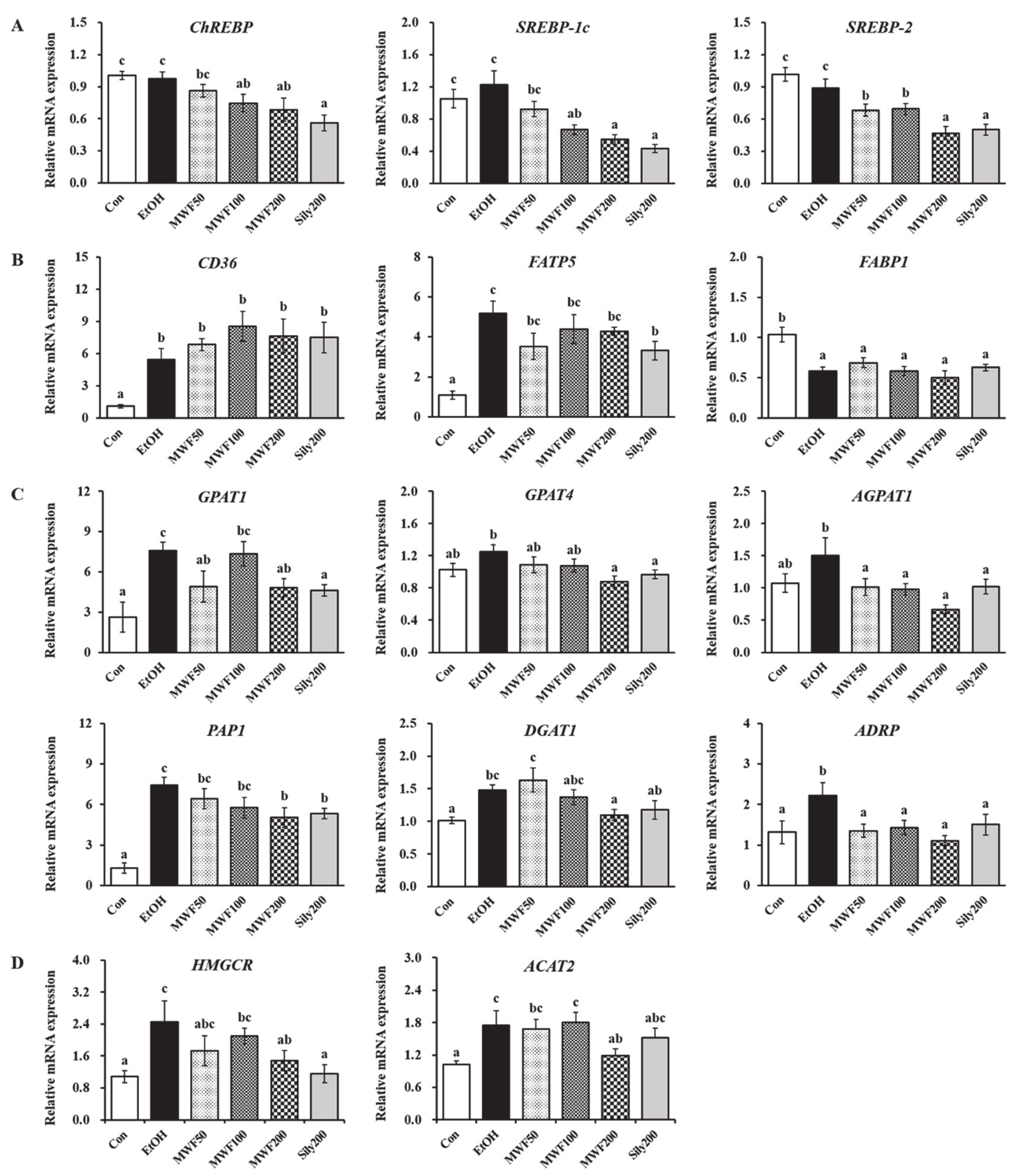
3.2. MWF Down-Regulated Lipogenesis-Related Gene Expression
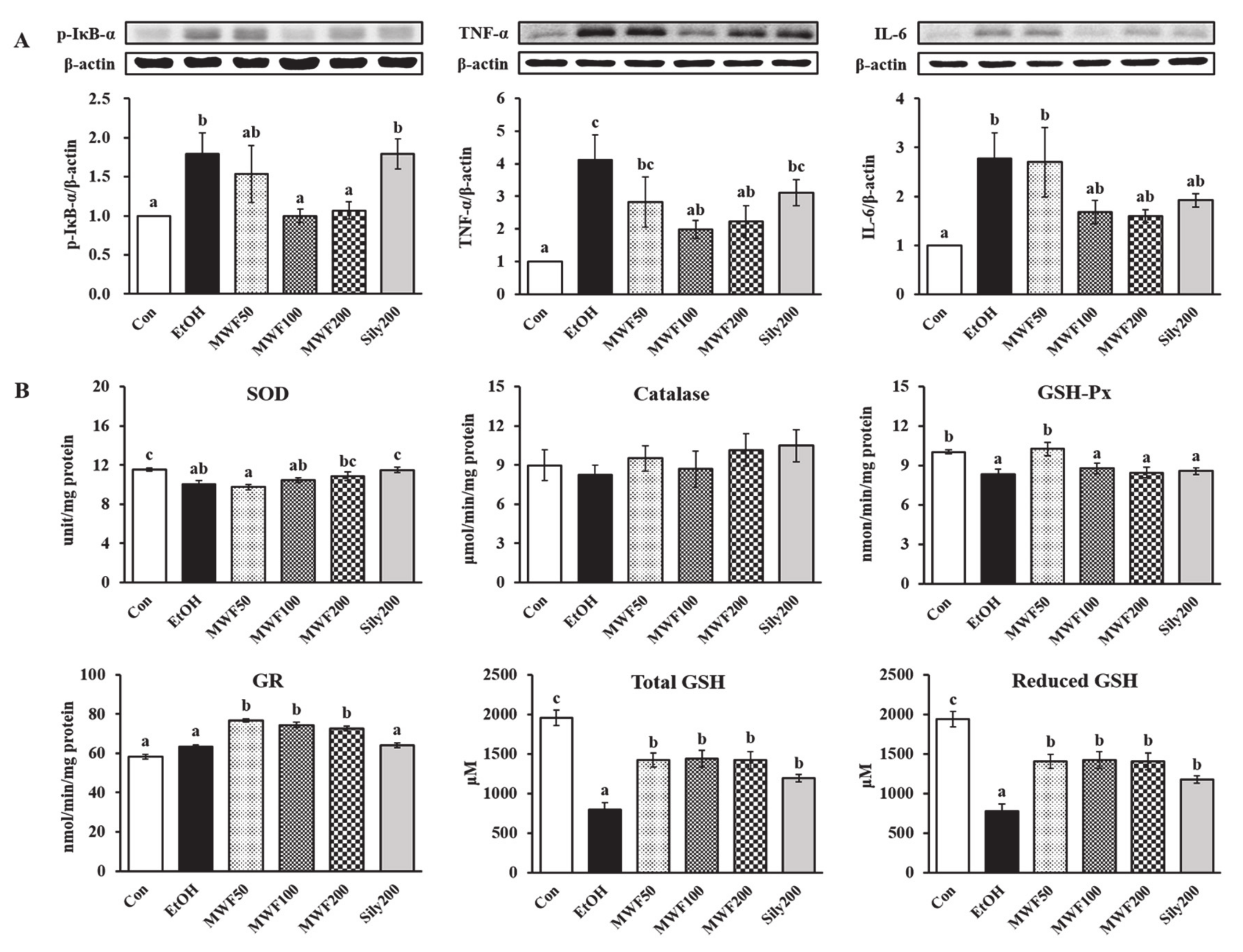
3.3. MWF Inhibited Alcohol-Induced Inflammatory Response
3.4. MWF Reversed Alcohol-Induced Antioxidant Defense System Dysfunction
3.5. MWF Altered the Bacterial Community Composition
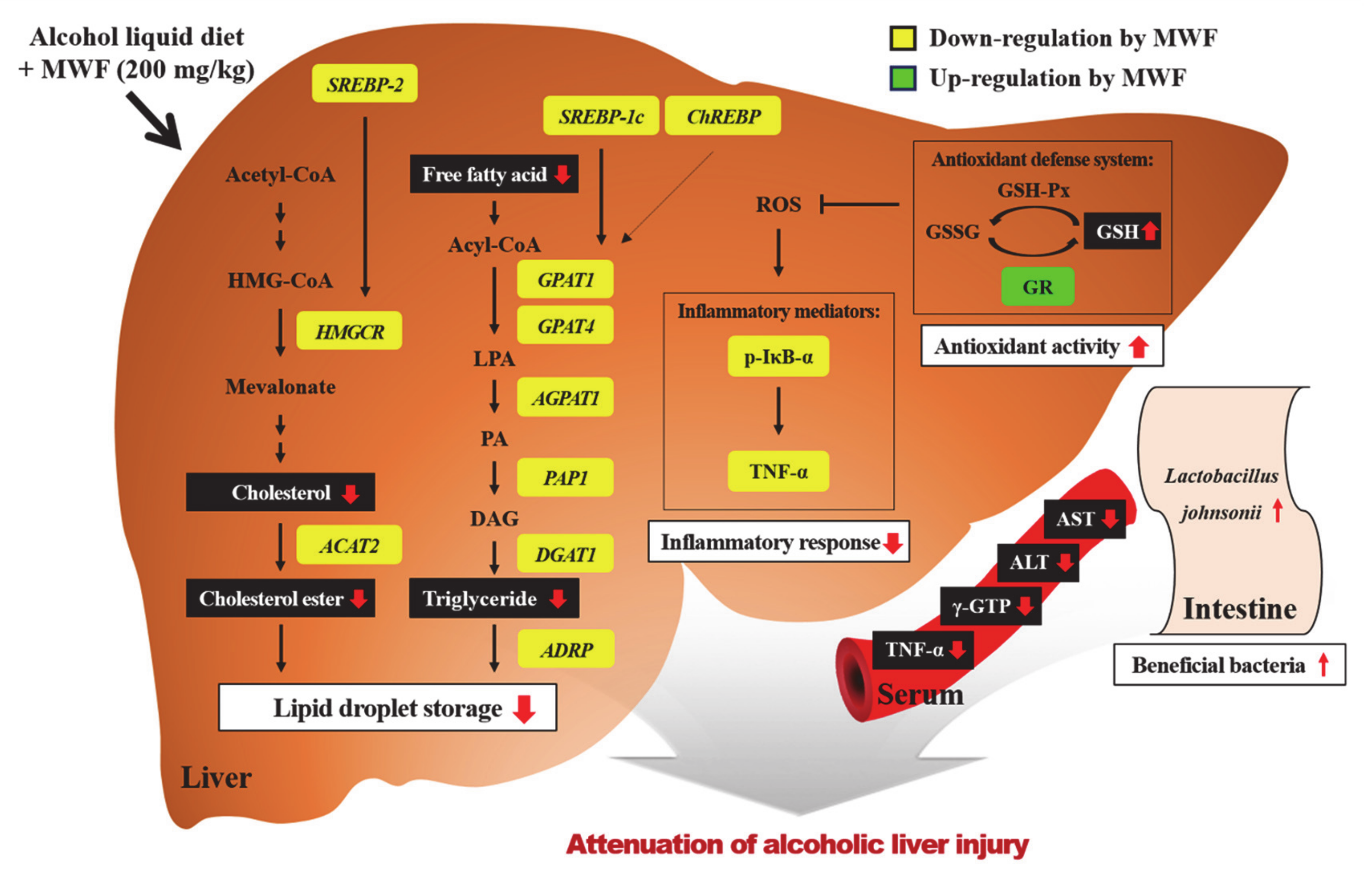
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zakhari, S. Overview: How Is Alcohol Metabolized by the Body? Alcohol Res. Health 2006, 29, 245–254. [Google Scholar]
- Assiri, M.A.; Ali, H.R.; Marentette, J.O.; Yun, Y.; Liu, J.; Hirschey, M.D.; Saba, L.M.; Harris, P.S.; Fritz, K.S. Investigating RNA Expression Profiles Altered by Nicotinamide Mononucleotide Therapy in a Chronic Model of Alcoholic Liver Disease. Hum. Genom. 2019, 13, 65. [Google Scholar] [CrossRef]
- Huang, H.; Lin, Z.; Zeng, Y.; Lin, X.; Zhang, Y. Probiotic and Glutamine Treatments Attenuate Alcoholic Liver Disease in a Rat Model. Exp. Ther. Med. 2019, 18, 4733–4739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasineni, K.; Casey, C.A. Molecular Mechanism of Alcoholic Fatty Liver. Indian J. Pharmacol. 2012, 44, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, Y.; Li, Q.; Xu, M.; Bai, J.; Wu, S. Resveratrol Improves Alcoholic Fatty Liver Disease by Downregulating HIF-1α Expression and Mitochondrial ROS Production. PLoS ONE 2017, 12, e0183426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, L.Z.; Chandimali, N.; Han, Y.H.; Lee, D.H.; Kim, J.S.; Kim, S.U.; Kim, T.D.; Jeong, D.K.; Sun, H.N.; Lee, D.S. Pathogenesis, Early Diagnosis, and Therapeutic Management of Alcoholic Liver Disease. Int. J. Mol. Sci. 2019, 20, E2712. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Ong, M.; Qu, X. Optimal Management for Alcoholic Liver Disease: Conventional Medications, Natural Therapy or Combination? World J. Gastroenterol. 2016, 22, 8–23. [Google Scholar] [CrossRef]
- Park, J.G.; Tak, W.Y.; Park, S.Y.; Kweon, Y.O.; Jang, S.Y.; Lee, Y.R.; Bae, S.H.; Jang, J.Y.; Kim, D.Y.; Lee, J.S.; et al. Effects of Branched-Chain Amino Acids (BCAAs) on the Progression of Advanced Liver Disease: A Korean Nationwide, Multicenter, Retrospective, Observational, Cohort Study. Medicine (Baltimore) 2017, 96, e6580. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, E.H. Therapeutic Effects of Amino Acids in Liver Diseases: Current Studies and Future Perspectives. J. Cancer Prev. 2019, 24, 72–78. [Google Scholar] [CrossRef]
- Tedesco, L.; Corsetti, G.; Ruocco, C.; Ragni, M.; Rossi, F.; Carruba, M.O.; Valerio, A.; Nisoli, E. A Specific Amino Acid Formula Prevents Alcoholic Liver Disease in Rodents. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G566–G582. [Google Scholar] [CrossRef]
- Corsetti, G.; Stacchiotti, A.; Tedesco, L.; D’Antona, G.; Pasini, E.; Dioguardi, F.; Nisoli, E.; Rezzani, R. Essential Amino Acid Supplementation Decreases Liver Damage Induced by Chronic Ethanol Consumption in Rats. Int. J. Immunopathol. Pharmacol. 2011, 24, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Kim, H.W.; Choi, Y.S. Edible Insects as a Protein Source: A Review of Public Perception, Processing Technology, and Research Trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, B.B.; Chang, M.H.; Lee, J.H.; Heo, W.; Kim, J.K.; Pan, J.H.; Kim, Y.J.; Kim, J.H. The Edible Insect Gryllus bimaculatus Protects against Gut-Derived Inflammatory Responses and Liver Damage in Mice after Acute Alcohol Exposure. Nutrients 2019, 11, 857. [Google Scholar] [CrossRef] [Green Version]
- Ido, A.; Hashizume, A.; Ohta, T.; Takahashi, T.; Miura, C.; Miura, T. Replacement of Fish Meal by Defatted Yellow Mealworm (Tenebrio molitor) Larvae in Diet Improves Growth Performance and Disease Resistance in Red Seabream (Pargus major). Animals 2019, 9, E100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of Food Composition Data for Edible Insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Potential and Challenges of Insects as an Innovative Source for Food and Feed Production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Feng, S. Tenebrio molitor L., Entomophagy and Processing into Ready to Use Therapeutic Ingredients: A Review. J. Nutr. Health Food Eng. 2018, 8, 280–285. [Google Scholar] [CrossRef]
- Youn, K.J.; Yun, E.Y.; Lee, J.H.; Kim, J.Y.; Hwang, J.S.; Jeong, W.S.; Jun, M.R. Oleic Acid and Linoleic Acid from Tenebrio molitor Larvae Inhibit BACE1 Activity in vitro: Molecular Docking Studies. J. Med. Food 2014, 17, 284–289. [Google Scholar] [CrossRef]
- Seo, M.C.; Goo, T.W.; Chung, M.Y.; Baek, M.H.; Hwang, J.S.; Kim, M.A.; Yun, E.Y. Tenebrio molitor Larvae Inhibit Adipogenesis through AMPK and MAPKs Signaling in 3T3-L1 Adipocytes and Obesity in High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2017, 18, E518. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.A.; Ding, Q.; Lu, H.; Tan, H.; Sun, N.; Wang, K.; He, R.; Luo, L.; Ma, H.; Li, Z. Caspase 3-Mediated Cytotoxicity of Mealworm Larvae (Tenebrio molitor) Oil Extract against Human Hepatocellular Carcinoma and Colorectal Adenocarcinoma. J. Ethnopharmacol. 2020, 250, 112438. [Google Scholar] [CrossRef]
- Kim, G.H.; Baek, H.K.; Lee, J.S.; Kim, S.J.; Yi, S.S. Chronic Oral Administration of Tenebrio molitor Extract Exhibits Inhibitory Effect on Glucocorticoid Receptor Overexpression in the Hippocampus of Ovariectomy-Induced Estrogen Deficient Mice. J. Food Sci. 2019, 84, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Baraniak, B.; Karaś, M. Antioxidant and Anti-Inflammatory Activities of Hydrolysates and Peptide Fractions Obtained by Enzymatic Hydrolysis of Selected Heat-Treated Edible Insects. Nutrients 2017, 9, E970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, R.Y.; Ham, J.R.; Ryu, H.S.; Park, K.W.; Kang, K.Y.; Lee, M.K. The Effects of Defatted Tenebrio molitor Larva Ferment Extract on CCl4-Induced Liver Damage in Mice. J. Korean Soc. Food Sci. Nutr. 2019, 48, 501–508. [Google Scholar] [CrossRef]
- Xiong, F.; Guan, Y.S. Cautiously Using Natural Medicine to Treat Liver Problems. World J. Gastroenterol. 2017, 23, 3388–3395. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Woo, M.J.; Ham, J.R.; Lee, M.K. Anti-Steatotic and Anti-Inflammatory Effects of Hovenia dulcis Thunb. Extracts in Chronic Alcohol-Fed Rats. Biomed. Pharmacother. 2017, 90, 393–401. [Google Scholar] [CrossRef]
- Han, S.R.; Yun, E.Y.; Kim, J.Y.; Hwang, J.S.; Jeong, E.J.; Moon, K.S. Evaluation of Genotoxicity and 28-Day Oral Dose Toxicity on Freeze-Dried Powder of Tenebrio molitor Larvae (Yellow Mealworm). Toxicol. Res. 2014, 30, 121–130. [Google Scholar] [CrossRef]
- Han, S.R.; Lee, B.S.; Jung, K.J.; Yu, H.J.; Yun, E.Y.; Hwang, J.S.; Moon, K.S. Safety Assessment of Freeze-Dried Powdered Tenebrio Molitor Larvae (Yellow Mealworm) as Novel Food Source: Evaluation of 90-Day Toxicity in Sprague-Dawley Rats. Regul. Toxicol. Pharmacol. 2016, 77, 206–212. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Seo, K.I.; Choi, M.S.; Jung, U.J.; Kim, H.J.; Yeo, J.Y.; Jeon, S.M.; Lee, M.K. Effect of Curcumin Supplementation on Blood Glucose, Plasma Insulin, and Glucose Homeostasis Related Enzyme Activities in Diabetic db/db Mice. Mol. Nutr. Food Res. 2008, 52, 995. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Chan, Y.L.; Tsai, Y.S.; Chen, S.A.; Wang, C.J.; Chen, K.F.; Chung, I.F. Airway Microbial Diversity Is Inversely Associated with Mite-Sensitized Rhinitis and Asthma in Early Childhood. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.H.; Ahn, H.Y.; Kim, Y.W.; Sim, S.Y.; Seo, K.I.; Cho, Y.S. Hepatoprotective Effect of Bacillus subtilis-Fermented Silkworm (Bombyx mori L.) Extract on an Alcoholic Fatty Liver in Rats. J. Life. Sci. 2018, 28, 697–707. [Google Scholar]
- Lee, J.H.; Kim, N.K.; Lee, D.Y.; Lee, C.H. Protective Effect of Selected Amino Acids and Food Extracts on Ethanol Toxicity Decrement in Rat Liver. Korean J. Food Sci. Technol. 1999, 31, 802–808. [Google Scholar]
- Yu, J.H.; Song, S.J.; Kim, A.R.; Choi, Y.J.; Seok, J.W.; Kim, H.J.; Lee, Y.J.; Lee, K.S.; Kim, J.W. Suppression of PPARγ-Mediated Monoacylglycerol O-Acyltransferase 1 Expression Ameliorates Alcoholic Hepatic Steatosis. Sci. Rep. 2016, 6, 29352. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; So, J.S.; Park, J.G.; Lee, A.H. Transcriptional Control of Hepatic Lipid Metabolism by SREBP and ChREBP. Semin. Liver Dis. 2013, 33, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.Q.; Kim, M.G.; Kim, J.H.; Kong, G.; Kang, K.S.; Kim, H.L.; Yoon, B.I.; Lee, M.O.; Lee, B.H. Differential Gene Expression and Lipid Metabolism in Fatty Liver Induced by Acute Ethanol Treatment in Mice. Toxicol. Appl. Pharmacol. 2007, 223, 225–233. [Google Scholar] [CrossRef]
- Karasawa, K.; Tanigawa, K.; Harada, A.; Yamashita, A. Transcriptional Regulation of Acyl-CoA: Glycerol-sn-3-Phosphate Acyltransferases. Int. J. Mol. Sci. 2019, 20, E964. [Google Scholar] [CrossRef] [Green Version]
- Mak, K.M.; Ren, C.; Ponomarenko, A.; Cao, Q.; Lieber, C.S. Adipose Differentiation-related Protein Is a Reliable Lipid Droplet Marker in Alcoholic Fatty Liver of Rats. Alcohol Clin. Exp. Res. 2008, 32, 683–689. [Google Scholar] [CrossRef]
- You, M.; Arteel, G.E. Effect of Ethanol on Lipid Metabolism. J. Hepatol. 2019, 70, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Berk, P.D.; Zhou, S.; Bradbury, M.W. Increased Hepatocellular Uptake of Long Chain Fatty Acids Occurs by Different Mechanisms in Fatty Livers Due to Obesity or Excess Ethanol Use, Contributing to Development of Steatohepatitis in Both Settings. Trans. Am. Clin. Climatol. Assoc. 2005, 116, 335–344. [Google Scholar] [PubMed]
- Zhong, W.; Zhao, Y.; Tang, Y.; Wei, X.; Shi, X.; Sun, W.; Sun, X.; Yin, X.; Sun, X.; Kim, S. Chronic Alcohol Exposure Stimulates Adipose Tissue Lipolysis in Mice: Role of Reverse Triglyceride Transport in the Pathogenesis of Alcoholic Steatosis. Am. J. Pathol. 2012, 180, 998–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohl, J.; Ring, A.; Herrmann, T.; Stremmel, W. New Concepts of Cellular Fatty Acid Uptake: Role of Fatty Acid Transport Proteins and of Caveolae. Proc. Nutr. Soc. 2004, 63, 259–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.J.; Park, J.W.; Merrill Jr, A.H.; Storch, J.; Pewzner-Jung, Y.; Futerman, A.H. Hepatic Fatty Acid Uptake Is Regulated by the Sphingolipid Acyl Chain Length. Biochim. Biophys. Acta 2014, 1841, 1754–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doege, H.; Baillie, R.A.; Ortegon, A.M.; Tsang, B.; Wu, Q.; Punreddy, S.; Hirsch, D.; Watson, N.; Gimeno, R.E.; Stahl, A. Targeted Deletion of FATP5 Reveals Multiple Functions in Liver Metabolism: Alterations in Hepatic Lipid Homeostasis. Gastroenterology 2006, 130, 1245–1258. [Google Scholar] [CrossRef] [Green Version]
- Szabo, G.; Mandrekar, P. Focus on: Alcohol and the Liver. Alcohol Res. Health 2010, 33, 87–96. [Google Scholar]
- McClain, C.J.; Barve, S.; Deaciuc, I.; Kugelmas, M.; Hill, D. Cytokines in Alcoholic Liver Disease. Semin. Liver Dis. 1999, 19, 205–219. [Google Scholar] [CrossRef]
- Lin, Y.L.; Tai, S.Y.; Chen, J.W.; Chou, C.H.; Fu, S.G.; Chen, Y.C. Ameliorative Effects of Pepsin-Digested Chicken Liver Hydrolysates on Development of Alcoholic Fatty Livers in Mice. Food Funct. 2017, 8, 1763–1774. [Google Scholar] [CrossRef]
- Wu, J.; Xue, X.; Wu, Z.; Zhao, H.L.; Cao, H.M.; Sun, D.Q.; Wang, R.M.; Sun, J.; Liu, Y.; Guo, R.C. Anti-Tumor Effect of Paeonol via Regulating NF-κB, AKT and MAPKs Activation: A Quick Review. Biomed. Prev. Nutr. 2014, 4, 9–14. [Google Scholar] [CrossRef]
- Sun, N.; Xu, T.; Liu, Y.; Ye, C.; Jiang, Z.; Du, F.; Wang, Y. Preparation of Two Oligopeptides from Corn Protein and Their Protective Effect on Acute Alcohol Intoxication in Mice. Biomed. Res. 2018, 29, 1284–1289. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.T.; Lin, Y.L.; Lin, Y.X.; Wang, S.Y.; Wu, Y.H.S.; Chou, C.H.; Fu, S.G.; Chen, Y.C. Protective Effects of Antioxidant Egg-Chalaza Hydrolysates Against Chronic Alcohol Consumption-Induced Liver Steatosis in Mice. J. Sci. Food Agric. 2019, 99, 2300–2310. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Y.; Yang, X.; Zhao, Y. Supplementation of Okra Seed Oil Ameliorates Ethanol-Induced Liver Injury and Modulates Gut Microbiota Dysbiosis in Mice. Food Funct. 2019, 10, 6385–6398. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deleve, L.D.; Kaplowitz, N. Importance and Regulation of Hepatic Glutathione. Semin. Liver Dis. 1990, 10, 251–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, R.B.; Reddy, K.S.; Husain, K.; Schlorff, E.C.; Rybak, L.P.; Somani, S.M. Dose Response of Ethanol on Antioxidant Defense System of Liver, Lung, and Kidney in Rat. Pathophysiology 2000, 7, 25–32. [Google Scholar] [CrossRef]
- Gould, R.L.; Pazdro, R. Impact of Supplementary Amino Acids, Micronutrients, and Overall Diet on Glutathione Homeostasis. Nutrients 2019, 11, E1056. [Google Scholar] [CrossRef] [Green Version]
- Je, J.Y.; Cha, J.Y.; Cho, Y.S.; Ahn, H.Y.; Lee, J.H.; Cho, Y.S.; Ahn, C.B. Hepatoprotective Effect of Peptic Hydrolysate from Salmon Pectoral Fin Protein Byproducts on Ethanol-Induced Oxidative Stress in Sprague–Dawley Rats. Food Res. Int. 2013, 51, 648–653. [Google Scholar] [CrossRef]
- Kołota, A.; Głąbska, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. Influence of Alcohol Consumption on Body Mass Gain and Liver Antioxidant Defense in Adolescent Growing Male Rats. Int. J. Environ. Res. Public Health 2019, 16, 2320. [Google Scholar] [CrossRef] [Green Version]
- Yan, A.W.; Fouts, D.E.; Brandl, J.; Stärkel, P.; Torralba, M.; Schott, E.; Tsukamoto, H.; Nelson, K.E.; Brenner, D.A.; Schnabl, B. Enteric Dysbiosis Associated with a Mouse Model of Alcoholic Liver Disease. Hepatology 2011, 53, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Bull-Otterson, L.; Feng, W.; Kirpich, I.; Wang, Y.; Qin, X.; Liu, Y.; Gobejishvili, L.; Joshi-Barve, S.; Ayvaz, T.; Petrosino, J.; et al. Metagenomic Analyses of Alcohol Induced Pathogenic Alterations in the Intestinal Microbiome and the Effect of Lactobacillus rhamnosus GG Treatment. PLoS ONE 2013, 8, e53028. [Google Scholar] [CrossRef]
- Leclercq, S.; de Timary, P.; Delzenne, N.M.; Stärkel, P. The Link between Inflammation, Bugs, the Intestine and the Brain in Alcohol Dependence. Transl. Psychiatry 2017, 7, e1048. [Google Scholar] [CrossRef] [PubMed]

| Gene | Full Name | Forward/Reverse(5′–3′) |
|---|---|---|
| ACAT2 | Acetyl-CoA acetyltransferase 2 | CGGTTTGATTCGAGCACCAC/ACTTTGATGGCCCCTGTTCC |
| ADRP | Adipose differentiation-related protein | CCTATCATCAGGCTCTCGGC/GTGCACATTCTTCCTGGCGA |
| AGPAT1 | 1-Acylglycerol-3-phosphate O-acyltransferase 1 | AGGACATCCCCAAATCCTGTCG/GCCACAGCTCCATTCTGGTCA |
| CD36 | Cluster of differentiation 36 | TCCTCGGATGGCTAGCTGATT/TGCTTTCTATGTGGCCTGGTT |
| ChREBP | Carbohydrate-response element-binding protein | TATGCCGGGACAAGATTCGG/AGGTTTCCGGTGCTCATCTG |
| DGAT1 | Diacylglycerol O-acyltransferase 1 | CAGCAGTGGATGGTCCCTAC/ACCGCCAGCTTTAAGAGACG |
| FABP1 | Fatty acid-binding protein 1 | CTTCTCCGGCAAGTACCAAGT/CATGCACGATTTCTGACACCC |
| FATP5 | Fatty acid transport protein 5 | CTGAGGGCTGCTCAATCACA/GTGGCTTCCTTCAGCTTTGC |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | AGTGCCAGCCTCGTCTCATA/ATGAAGGGGTCGTTGATGGC |
| GPAT1 | Glycerol-3-phosphate acyltransferase 1 | ACCACATCAAGGATACAGCTC/CCTCATTCGTGTGTTTACATCGG |
| GPAT4 | Glycerol-3-phosphate acyltransferase 4 | TGTGGGACGGTGGATTGAAG/GCTCCGGTCCTCATGGTTAC |
| HMGCR | 3-Hydroxy-3-methylglutaryl-CoA reductase | CCTCCATTGAGATCCGGAGG/GATGGGAGGCCACAAAGAGG |
| PAP1 | Phosphatidate phosphatase 1 | TCACTACCCAGTACCAGGGC/TGAGTCCAATCCTTTCCCAG |
| SREBP-1c | Sterol regulatory element-binding protein 1c | GACGAGCTACCCTTCGGTG/GGGGCATCAAATAGGCCAGG |
| SREBP2 | Sterol regulatory element-binding protein 2 | CGAACTGGGCGATGGATGAGA/TCTCCCACTTGATTGCTGACA |
| Con | EtOH | MWF50 | MWF100 | MWF200 | Sily200 | |
|---|---|---|---|---|---|---|
| TNF-α (pg/mL) | 54.74 ± 5.42 a | 74.66 ± 6.05 b | 53.87 ± 4.85 a | 42.52 ± 6.07 a | 45.55 ± 2.61 a | 46.06 ± 9.14 a |
| IL-6 (pg/mL) | 3.03 ± 0.30 | 3.03 ± 0.50 | 2.90 ± 0.39 | 2.19 ± 0.30 | 1.87 ± 0.12 | 2.36 ± 0.27 |
| γ-GTP (U/L) | 21.06 ± 4.31 a | 50.44 ± 6.84 c | 29.55 ± 3.92 ab | 35.61 ± 3.03 b | 38.48 ± 3.46 b | 37.41 ± 2.92 b |
| AST (U/L) | 65.10 ± 1.47 a | 259.83 ± 42.84 c | 162.12 ± 17.94 b | 189.45 ± 16.37 bc | 172.88 ± 32.75 b | 184.38 ± 32.70 bc |
| ALT (U/L) | 28.50 ± 2.26 a | 173.94 ± 40.95 c | 90.12 ± 15.46 ab | 118.50 ± 13.49 bc | 95.22 ± 18.29 ab | 131.33 ± 36.38 bc |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, R.-Y.; Ham, J.R.; Ryu, H.-S.; Lee, S.S.; Miguel, M.A.; Paik, M.-J.; Ji, M.; Park, K.-W.; Kang, K.-Y.; Lee, H.-I.; et al. Defatted Tenebrio molitor Larva Fermentation Extract Modifies Steatosis, Inflammation and Intestinal Microflora in Chronic Alcohol-Fed Rats. Nutrients 2020, 12, 1426. https://doi.org/10.3390/nu12051426
Choi R-Y, Ham JR, Ryu H-S, Lee SS, Miguel MA, Paik M-J, Ji M, Park K-W, Kang K-Y, Lee H-I, et al. Defatted Tenebrio molitor Larva Fermentation Extract Modifies Steatosis, Inflammation and Intestinal Microflora in Chronic Alcohol-Fed Rats. Nutrients. 2020; 12(5):1426. https://doi.org/10.3390/nu12051426
Chicago/Turabian StyleChoi, Ra-Yeong, Ju Ri Ham, Hyo-Seon Ryu, Sang Suk Lee, Michelle A. Miguel, Man-Jeong Paik, Moongi Ji, Kyung-Wuk Park, Kyung-Yun Kang, Hae-In Lee, and et al. 2020. "Defatted Tenebrio molitor Larva Fermentation Extract Modifies Steatosis, Inflammation and Intestinal Microflora in Chronic Alcohol-Fed Rats" Nutrients 12, no. 5: 1426. https://doi.org/10.3390/nu12051426
APA StyleChoi, R.-Y., Ham, J. R., Ryu, H.-S., Lee, S. S., Miguel, M. A., Paik, M.-J., Ji, M., Park, K.-W., Kang, K.-Y., Lee, H.-I., & Lee, M.-K. (2020). Defatted Tenebrio molitor Larva Fermentation Extract Modifies Steatosis, Inflammation and Intestinal Microflora in Chronic Alcohol-Fed Rats. Nutrients, 12(5), 1426. https://doi.org/10.3390/nu12051426