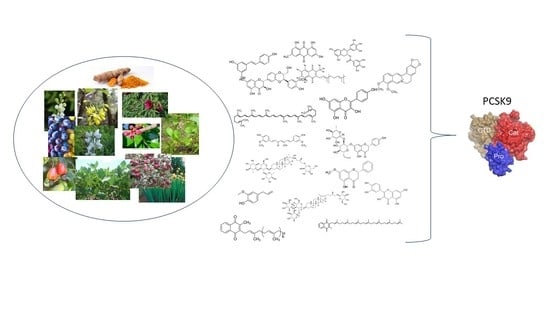
Naturally Occurring PCSK9 Inhibitors
Abstract
1. Introduction
2. Berberine
2.1. In Vitro Studies
2.2. In Vivo Studies
2.3. Cilinical Studies
3. Sterol/Stanols and Vegetable Proteins
3.1. Lupin
3.2. Soy Proteins
4. Polyphenols
4.1. Quercetin
4.2. Epigallocatechin Gallate
4.3. Resveratrol
4.4. Other Polyphenols
4.5. Eugenol
5. Nutrients
5.1. Curcumin
5.2. Welsh Onion
5.3. Cashew Nuts (Anacardium Occidentale L., Anacardiaceae)
5.4. Kenaf
5.5. Vitamin MK7
5.6. Lycopene
5.7. Omega 3
6. Other Inhibitors
6.1. Probiotics
6.2. Dioscorea
6.3. Emodin
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferri, N.; Ruscica, M. Proprotein convertase subtilisin/kexin type 9 (PCSK9) and metabolic syndrome: Insights on insulin resistance, inflammation, and atherogenic dyslipidemia. Endocrinology 2016, 54, 588–601. [Google Scholar] [CrossRef]
- Seidah, N.G.; Benjannet, S.; Wickham, L.; Marcinkiewicz, J.; Jasmin, S.B.; Stifani, S.; Basak, A.; Prat, A.; Chrétien, M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): Liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. USA 2003, 100, 928–933. [Google Scholar] [CrossRef]
- Tavori, H.; Christian, D.C.; Minnier, J.; Plubell, D.; Shapiro, M.D.; Yeang, C.; Giunzioni, I.; Croyal, M.; Duell, P.B.; Lambert, G.; et al. PCSK9 Association with Lipoprotein(a). Circ. Res. 2016. [Google Scholar] [CrossRef]
- Ruscica, M.; Simonelli, S.; Botta, M.; Ossoli, A.; Lupo, M.G.; Magni, P.; Corsini, A.; Arca, M.; Pisciotta, L.; Veglia, F.; et al. Plasma PCSK9 levels and lipoprotein distribution are preserved in carriers of genetic HDL disorders. Biochim. Biophys. Acta (BBA) Mol. Cell Boil. Lipids 2018, 1863, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Macchi, C.; Banach, M.; Corsini, A.; Sirtori, C.R.; Ferri, N.; Ruscica, M. Changes in circulating pro-protein convertase subtilisin/kexin type 9 levels—Experimental and clinical approaches with lipid-lowering agents. Eur. J. Prev. Cardiol. 2019, 26, 930–949. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Shah, N.A.; Warrington, J.A.; Anderson, N.N.; Park, S.W.; Brown, M.S.; Goldstein, J.L. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA 2003, 100, 12027–12032. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, G.; Chamberland, A.; Wassef, H.; Davignon, J.; Seidah, N.G.; Bernier, L.; Prat, A. Statins UpregulatePCSK9, the Gene Encoding the Proprotein Convertase Neural Apoptosis-Regulated Convertase-1 Implicated in Familial Hypercholesterolemia. Arter. Thromb. Vasc. Boil. 2004, 24, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Ricci, C.; Macchi, C.; Magni, P.; Cristofani, R.; Liu, J.; Corsini, A.; Ferri, N. Suppressor of Cytokine Signaling-3 (SOCS-3) Induces Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Expression in Hepatic HepG2 Cell Line*. J. Boil. Chem. 2015, 291, 3508–3519. [Google Scholar] [CrossRef]
- Li, H.; Dong, B.; Park, S.W.; Lee, H.-S.; Chen, W.; Liu, J. Hepatocyte Nuclear Factor 1α Plays a Critical Role in PCSK9 Gene Transcription and Regulation by the Natural Hypocholesterolemic Compound Berberine*. J. Boil. Chem. 2009, 284, 28885–28895. [Google Scholar] [CrossRef]
- Cohen, J.; Pertsemlidis, A.; Kotowski, I.K.; Graham, R.; Garcia, C.K.; Hobbs, H.H. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 2005, 37, 161–165. [Google Scholar] [CrossRef]
- Abifadel, M.; Varret, M.; Rabès, J.-P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Corsini, A.; Macchi, C.; Magni, P.; Ruscica, M. Proprotein convertase subtilisin kexin type 9 and high-density lipoprotein metabolism: Experimental animal models and clinical evidence. Transl. Res. 2016, 173, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, N.; Zhao, L.; Lu, F. Berberine in the Treatment of Type 2 Diabetes Mellitus: A Systemic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Zhao, Y.; Dong, F.; Yan, Z.; Zheng, W.; Fan, J.; Sun, G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 2015, 161, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ye, X.; Hua, Y.; Song, Y. Berberine alleviates adipose tissue fibrosis by inducing AMP-activated kinase signaling in high-fat diet-induced obese mice. Biomed. Pharmacother. 2018, 105, 121–129. [Google Scholar] [CrossRef]
- Andola, H.C.; Gaira, K.S.; Rawal, R.S.; Rawat, M.S.; Bhatt, I.D. Habitat-dependent variations in berberine content of Berberis asiatica Roxb. ex. DC. in Kumaon, Western Himalaya. Chem. Biodivers. 2010, 7, 415–420. [Google Scholar] [CrossRef]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Cameron, J.; Ranheim, T.; Kulseth, M.A.; Leren, T.P.; Berge, K.E. Berberine decreases PCSK9 expression in HepG2 cells. Atherosclerosis 2008, 201, 266–273. [Google Scholar] [CrossRef]
- Abidi, P.; Chen, W.; Kraemer, F.B.; Li, H.; Liu, J. The medicinal plant goldenseal is a natural LDL-lowering agent with multiple bioactive components and new action mechanisms. J. Lipid Res. 2006, 47, 2134–2147. [Google Scholar] [CrossRef]
- Kong, W.-J.; Zhang, H.; Song, D.-Q.; Xue, R.; Zhao, W.; Wei, J.; Wang, Y.-M.; Shan, N.; Zhou, Z.-X.; Yang, P.; et al. Berberine reduces insulin resistance through protein kinase C–dependent up-regulation of insulin receptor expression. Metabolism 2009, 58, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Li, H.; Singh, A.B.; Cao, A.; Liu, J. Inhibition ofPCSK9Transcription by Berberine Involves Down-regulation of Hepatic HNF1α Protein Expression through the Ubiquitin-Proteasome Degradation Pathway. J. Boil. Chem. 2014, 290, 4047–4058. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hao, H.; Xie, H.-G.; Lai, L.; Wang, Q.; Liu, C.; Wang, G. Extensive Intestinal First-Pass Elimination and Predominant Hepatic Distribution of Berberine Explain Its Low Plasma Levels in Rats. Drug Metab. Dispos. 2010, 38, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Ding, L.; Chen, Y.; Gong, B.; He, J.; Xu, G. Determination of berberine in human plasma by liquid chromatography–electrospray ionization–mass spectrometry. J. Pharm. Biomed. Anal. 2007, 44, 931–937. [Google Scholar] [CrossRef]
- Shitan, N.; Tanaka, M.; Terai, K.; Ueda, K.; Yazaki, K. Human MDR1 and MRP1 Recognize Berberine as Their Transport Substrate. Biosci. Biotechnol. Biochem. 2007, 71, 242–245. [Google Scholar] [CrossRef]
- Cao, S.; Xu, P.; Yan, J.; Liu, H.; Liu, L.; Cheng, L.; Qiu, F.; Kang, N. Berberrubine and its analog, hydroxypropyl-berberrubine, regulate LDLR and PCSK9 expression via the ERK signal pathway to exert cholesterol-lowering effects in human hepatoma HepG2 cells. J. Cell. Biochem. 2018, 120, 1340–1349. [Google Scholar] [CrossRef]
- Horton, J.D.; A Cuthbert, J.; Spady, D.K. Dietary fatty acids regulate hepatic low density lipoprotein (LDL) transport by altering LDL receptor protein and mRNA levels. J. Clin. Investig. 1993, 92, 743–749. [Google Scholar] [CrossRef]
- Xiao, H.-B.; Sun, Z.-L.; Zhang, H.-B.; Zhang, D.-S. Berberine inhibits dyslipidemia in C57BL/6 mice with lipopolysaccharide induced inflammation. Pharmacol. Rep. 2012, 64, 889–895. [Google Scholar] [CrossRef]
- Jia, Y.-J.; Xu, R.-X.; Sun, J.; Tang, Y.; Li, J.-J. Enhanced circulating PCSK9 concentration by berberine through SREBP-2 pathway in high fat diet-fed rats. J. Transl. Med. 2014, 12, 103. [Google Scholar] [CrossRef]
- Liu, D.-L.; Xu, L.-J.; Dong, H.; Chen, G.; Huang, Z.-Y.; Zou, X.; Wang, K.-F.; Luo, Y.-H.; Lu, F. Inhibition of proprotein convertase subtilisin/kexin type 9: A novel mechanism of berberine and 8-hydroxy dihydroberberine against hyperlipidemia. Chin. J. Integr. Med. 2014, 21, 132–138. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, Y.; Zhao, L.; Lu, F. The Effects of Berberine on Blood Lipids: A Systemic Review and Meta-Analysis of Randomized Controlled Trials. Planta Med. 2013, 79, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Adorni, M.P.; Ferri, N.; Marchianò, S.; Trimarco, V.; Rozza, F.; Izzo, R.; Bernini, F.; Zimetti, F. Effect of a novel nutraceutical combination on serum lipoprotein functional profile and circulating PCSK9. Ther. Clin. Risk Manag. 2017, 13, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Simental-Mendía, L.E.; Guerrero-Romero, F.; Golledge, J.; Watts, G.F. Effect of statin therapy on plasma proprotein convertase subtilisin kexin 9 (PCSK9) concentrations: A systematic review and meta-analysis of clinical trials. Diabetes Obes. Metab. 2015, 17, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Lupo, M.G.; Macchi, C.; Marchianò, S.; Cristofani, R.; Greco, M.F.; Dall’Acqua, S.; Chen, H.; Sirtori, C.R.; Corsini, A.; Ruscica, M.; et al. Differential effects of red yeast rice, Berberis aristata and Morus alba extracts on PCSK9 and LDL uptake. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1245–1253. [Google Scholar] [CrossRef]
- Spigoni, V.; Aldigeri, R.; Antonini, M.; Micheli, M.M.; Fantuzzi, F.; Fratter, A.; Pellizzato, M.; Derlindati, E.; Zavaroni, I.; Bonadonna, R.C.; et al. Effects of a New Nutraceutical Formulation (Berberine, Red Yeast Rice and Chitosan) on Non-HDL Cholesterol Levels in Individuals with Dyslipidemia: Results from a Randomized, Double Blind, Placebo-Controlled Study. Int. J. Mol. Sci. 2017, 18, 1498. [Google Scholar] [CrossRef]
- Formisano, E.; Pasta, A.; Cremonini, A.L.; Favari, E.; Ronca, A.; Carbone, F.; Semino, T.; Di Pierro, F.; Sukkar, G.S.; Pisciotta, L. Efficacy of Nutraceutical Combination of Monacolin K, Berberine, and Silymarin on Lipid Profile and PCSK9 Plasma Level in a Cohort of Hypercholesterolemic Patients. J. Med. Food 2019. [Google Scholar] [CrossRef]
- Pisciotta, L.; Bellocchio, A.; Bertolini, S. Nutraceutical pill containing berberine versus ezetimibe on plasma lipid pattern in hypercholesterolemic subjects and its additive effect in patients with familial hypercholesterolemia on stable cholesterol-lowering treatment. Lipids Heal. Dis. 2012, 11, 123. [Google Scholar] [CrossRef]
- Dubuc, G.; Tremblay, M.; Paré, G.; Jacques, H.; Hamelin, J.; Benjannet, S.; Boulet, L.; Genest, J.; Bernier, L.; Seidah, N.G.; et al. A new method for measurement of total plasma PCSK9: Clinical applications. J. Lipid Res. 2009, 51, 140–149. [Google Scholar] [CrossRef]
- Awan, Z.; Seidah, N.G.; MacFadyen, J.G.; Benjannet, S.; I Chasman, D.; Ridker, P.M.; Genest, J. Rosuvastatin, Proprotein Convertase Subtilisin/Kexin Type 9 Concentrations, and LDL Cholesterol Response: The JUPITER Trial. Clin. Chem. 2012, 58, 183–189. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Tan, Z.-R.; Klaassen, C.D.; Zhou, H.-H. Repeated administration of berberine inhibits cytochromes P450 in humans. Eur. J. Clin. Pharmacol. 2011, 68, 213–217. [Google Scholar] [CrossRef]
- Wu, C.; Xi, C.; Tong, J.; Zhao, J.; Jiang, H.; Wang, J.; Wang, Y.; Liu, H. Design, synthesis, and biological evaluation of novel tetrahydroprotoberberine derivatives (THPBs) as proprotein convertase subtilisin/kexin type 9 (PCSK9) modulators for the treatment of hyperlipidemia. Acta Pharm. Sin. B 2019, 9, 1216–1230. [Google Scholar] [CrossRef] [PubMed]
- Ochin, C.; Garelnabi, M. Berberine Encapsulated PLGA-PEG Nanoparticles Modulate PCSK-9 in HepG2 Cells. Cardiovasc. Hematol. Disord. Targets 2018, 18, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-H.; Feng, C.-L.; Zhang, W.-X.; Luo, Z.-G.; Zhang, H.-J.; Zhang, T.-T.; Ma, C.; Zhan, Y.; Li, R.; Wu, S.; et al. Liver-target nanotechnology facilitates berberine to ameliorate cardio-metabolic diseases. Nat. Commun. 2019, 10, 1981. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Banach, M.; Pirro, M.; Katsiki, N.; Sahebkar, A. Regulation of PCSK9 by nutraceuticals. Pharmacol. Res. 2017, 120, 157–169. [Google Scholar] [CrossRef]
- Simonen, P.; Stenman, U.-H.; Gylling, H. Serum proprotein convertase subtilisin/kexin type 9 concentration is not increased by plant stanol ester consumption in normo- to moderately hypercholesterolaemic non-obese subjects. The BLOOD FLOW intervention study. Clin. Sci. 2015, 129, 439–446. [Google Scholar] [CrossRef]
- De Smet, E.; Mensink, R.P.; Konings, M.; Brufau, G.; Groen, A.K.; Havinga, R.; Schonewille, M.; Kerksiek, A.; Lütjohann, D.; Plat, J. Acute intake of plant stanol esters induces changes in lipid and lipoprotein metabolism-related gene expression in the liver and intestines of mice. Lipids 2015, 50, 529–541. [Google Scholar] [CrossRef]
- Boachie, R.; Yao, S.; Udenigwe, C.C. Molecular mechanisms of cholesterol-lowering peptides derived from food proteins. Curr. Opin. Food Sci. 2018, 20, 58–63. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Scigliuolo, G.M.; D’Amato, A.; Arnoldi, A. Lupin Peptides Lower Low-Density Lipoprotein (LDL) Cholesterol through an Up-regulation of the LDL Receptor/Sterol Regulatory Element Binding Protein 2 (SREBP2) Pathway at HepG2 Cell Line. J. Agric. Food Chem. 2014, 62, 7151–7159. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Vistoli, G. Two Peptides from Soy beta-Conglycinin Induce a Hypocholesterolemic Effect in HepG2 Cells by a Statin-Like Mechanism: Comparative in Vitro and in Silico Modeling Studies. J. Agric. Food Chem. 2015, 63, 7945–7951. [Google Scholar] [CrossRef]
- Zanoni, C.; Aiello, G.; Arnoldi, A.; Lammi, C. Hempseed Peptides Exert Hypocholesterolemic Effects with a Statin-Like Mechanism. J. Agric. Food Chem. 2017, 65, 8829–8838. [Google Scholar] [CrossRef]
- Lin, S.-H.; Chang, D.-K.; Chou, M.-J.; Huang, K.-J.; Shiuan, D. Peptide inhibitors of human HMG-CoA reductase as potential hypocholesterolemia agents. Biochem. Biophys. Res. Commun. 2015, 456, 104–109. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Lovati, M.R.; Manzoni, C.; Castiglioni, S.; Duranti, M.; Magni, C.; Morandi, S.; D’Agostina, A.; Arnoldi, A. Proteins of White Lupin Seed, a Naturally Isoflavone-Poor Legume, Reduce Cholesterolemia in Rats and Increase LDL Receptor Activity in HepG2 Cells. J. Nutr. 2004, 134, 18–23. [Google Scholar] [CrossRef]
- Marchesi, M.; Parolini, C.; Diani, E.; Rigamonti, E.; Cornelli, L.; Arnoldi, A.; Sirtori, C.R.; Chiesa, G. Hypolipidaemic and anti-atherosclerotic effects of lupin proteins in a rabbit model. Br. J. Nutr. 2008, 100, 707–710. [Google Scholar] [CrossRef]
- Bähr, M.; Fechner, A.; Krämer, J.; Kiehntopf, M.; Jahreis, G. Lupin protein positively affects plasma LDL cholesterol and LDL:HDL cholesterol ratio in hypercholesterolemic adults after four weeks of supplementation: A randomized, controlled crossover study. Nutr. J. 2013, 12, 107. [Google Scholar] [CrossRef]
- Bähr, M.; Fechner, A.; Kiehntopf, M.; Jahreis, G. Consuming a mixed diet enriched with lupin protein beneficially affects plasma lipids in hypercholesterolemic subjects: A randomized controlled trial. Clin. Nutr. 2015, 34, 7–14. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Triolo, M.; Bosisio, R.; Bondioli, A.; Calabresi, L.; De Vergori, V.; Gomaraschi, M.; Mombelli, G.; Pazzucconi, F.; Zacherl, C.; et al. Hypocholesterolaemic effects of lupin protein and pea protein/fibre combinations in moderately hypercholesterolaemic individuals. Br. J. Nutr. 2011, 107, 1176–1183. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Calabresi, L.; Arnoldi, A. Lupin protein exerts cholesterol-lowering effects targeting PCSK9: From clinical evidences to elucidation of the in vitro molecular mechanism using HepG2 cells. J. Funct. Foods 2016, 23, 230–240. [Google Scholar] [CrossRef]
- Pavanello, C.; Lammi, C.; Ruscica, M.; Bosisio, R.; Mombelli, G.; Zanoni, C.; Calabresi, L.; Sirtori, C.R.; Magni, P.; Arnoldi, A. Effects of a lupin protein concentrate on lipids, blood pressure and insulin resistance in moderately dyslipidaemic patients: A randomised controlled trial. J. Funct. Foods 2017, 37, 8–15. [Google Scholar] [CrossRef]
- Lammi, C.; Bollati, C.; Lecca, D.; Abbracchio, M.P.; Arnoldi, A. Lupin Peptide T9 (GQEQSHQDEGVIVR) Modulates the Mutant PCSK9D374Y Pathway: In vitro Characterization of its Dual Hypocholesterolemic Behavior. Nutrients 2019, 11, 1665. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Aiello, G.; Arnoldi, A.; Grazioso, G. Lupin Peptides Modulate the Protein-Protein Interaction of PCSK9 with the Low Density Lipoprotein Receptor in HepG2 Cells. Sci. Rep. 2016, 6, 29931. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Pavanello, C.; Calabresi, L.; Ruscica, M. Nutraceutical approaches to metabolic syndrome. Ann. Med. 2017, 49, 678–697. [Google Scholar] [CrossRef]
- Banach, M.; Patti, A.M.; Giglio, R.V.; Cicero, A.F.G.; Atanasov, A.G.; Bajraktari, G.; Bruckert, É; Descamps, O.; Djuric, D.M.; Ezhov, M.; et al. The Role of Nutraceuticals in Statin Intolerant Patients. J. Am. Coll. Cardiol. 2018, 72, 96–118. [Google Scholar] [CrossRef]
- Ruscica, M.; Pavanello, C.; Gandini, S.; Gomaraschi, M.; Vitali, C.; Macchi, C.; Morlotti, B.; Aiello, G.; Bosisio, R.; Calabresi, L.; et al. Effect of soy on metabolic syndrome and cardiovascular risk factors: A randomized controlled trial. Eur. J. Nutr. 2016, 57, 499–511. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Potì, F.; Santi, D.; Spaggiari, G.; Zimetti, F.; Zanotti, I. Polyphenol Health Effects on Cardiovascular and Neurodegenerative Disorders: A Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 351. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A. Polyphenols Effect on Circulating Lipids and Lipoproteins: From Biochemistry to Clinical Evidence. Curr. Pharm. Des. 2018, 24, 178–190. [Google Scholar] [CrossRef]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2012, 18, 1818–1892. [Google Scholar] [CrossRef]
- Moon, J.; Lee, S.-M.; Do, H.J.; Cho, Y.; Chung, J.H.; Shin, M.-J. Quercetin Up-regulates LDL Receptor Expression in HepG2 Cells. Phytother. Res. 2012, 26, 1688–1694. [Google Scholar] [CrossRef]
- Mbikay, M.; Sirois, F.; Simões, S.; Mayne, J.; Chrétien, M. Quercetin-3-glucoside increases low-density lipoprotein receptor (LDLR) expression, attenuates proprotein convertase subtilisin/kexin 9 (PCSK9) secretion, and stimulates LDL uptake by Huh7 human hepatocytes in culture. FEBS Open Bio 2014, 4, 755–762. [Google Scholar] [CrossRef]
- Nishikido, T.; Ray, K.K. Non-antibody Approaches to Proprotein Convertase Subtilisin Kexin 9 Inhibition: siRNA, Antisense Oligonucleotides, Adnectins, Vaccination, and New Attempts at Small-Molecule Inhibitors Based on New Discoveries. Front. Cardiovasc. Med. 2019, 5. [Google Scholar] [CrossRef]
- Li, S.; Cao, H.; Shen, D.; Jia, Q.; Chen, C.; Xing, S.L. Quercetin protects against ox-LDL-induced injury via regulation of ABCAl, LXR-α and PCSK9 in RAW264.7 macrophages. Mol. Med. Rep. 2018, 18, 799–806. [Google Scholar] [CrossRef]
- Adorni, M.P.; Cipollari, E.; Favari, E.; Zanotti, I.; Zimetti, F.; Corsini, A.; Ricci, C.; Bernini, F.; Ferri, N. Inhibitory effect of PCSK9 on Abca1 protein expression and cholesterol efflux in macrophages. Atherosclerosis 2017, 256, 1–6. [Google Scholar] [CrossRef]
- Ricci, C.; Ruscica, M.; Camera, M.; Rossetti, L.; Macchi, C.; Colciago, A.; Zanotti, I.; Lupo, M.G.; Adorni, M.P.; Cicero, A.F.G.; et al. PCSK9 induces a pro-inflammatory response in macrophages. Sci. Rep. 2018, 8, 2267. [Google Scholar] [CrossRef]
- Mbikay, M.; Mayne, J.; Sirois, F.; Fedoryak, O.; Raymond, A.; Noad, J.; Chrétien, M. Mice Fed a High-Cholesterol Diet Supplemented with Quercetin-3-Glucoside Show Attenuated Hyperlipidemia and Hyperinsulinemia Associated with Differential Regulation of PCSK9 and LDLR in their Liver and Pancreas. Mol. Nutr. Food Res. 2018, 62, e1700729. [Google Scholar] [CrossRef]
- Jia, Q.; Cao, H.; Shen, D.; Li, S.; Yan, L.; Chen, C.; Xing, S.; Dou, F. Quercetin protects against atherosclerosis by regulating the expression of PCSK9, CD36, PPARgamma, LXRalpha and ABCA1. Int. J. Mol. Med. 2019, 44, 893–902. [Google Scholar]
- Tabrizi, R.; Tamtaji, O.R.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Dadgostar, E.; Asemi, Z. The effects of quercetin supplementation on lipid profiles and inflammatory markers among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 1–14. [Google Scholar] [CrossRef]
- Terao, J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 2017, 139, 15–23. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, A.M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef]
- Cooray, H.C.; Janvilisri, T.; Van Veen, H.W.; Hladky, S.B.; A Barrand, M. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem. Biophys. Res. Commun. 2004, 317, 269–275. [Google Scholar] [CrossRef]
- Hsiu, S.-L.; Hou, Y.-C.; Wang, Y.-H.; Tsao, C.-W.; Su, S.-F.; Chao, P.-D.L. Quercetin significantly decreased cyclosporin oral bioavailability in pigs and rats. Life Sci. 2002, 72, 227–235. [Google Scholar] [CrossRef]
- Scambia, G.; Ranelletti, F.O.; Panici, P.B.; De Vincenzo, R.; Bonanno, G.; Ferrandina, G.; Piantelli, M.; Bussa, S.; Rumi, C.; Cianfriglia, M.; et al. Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: P-glycoprotein as a possible target. Cancer Chemother. Pharmacol. 1994, 34, 459–464. [Google Scholar] [CrossRef]
- Santangelo, R.; Silvestrini, A.; Mancuso, C. Ginsenosides, catechins, quercetin and gut microbiota: Current evidence of challenging interactions. Food Chem. Toxicol. 2019, 123, 42–49. [Google Scholar] [CrossRef]
- Dabeek, W.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome(R), a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef]
- Li, L.; Stillemark-Billton, P.; Beck, C.; Boström, P.; Andersson, L.; Rutberg, M.; Ericsson, J.; Magnusson, B.; Marchesan, D.; Ljungberg, A.; et al. Epigallocatechin gallate increases the formation of cytosolic lipid droplets and decreases the secretion of apoB-100 VLDL. J. Lipid Res. 2005, 47, 67–77. [Google Scholar] [CrossRef]
- Zanka, K.; Kawaguchi, Y.; Okada, Y.; Nagaoka, S. Epigallocatechin Gallate Induces Upregulation of LDL Receptor via the 67 kDa Laminin Receptor-Independent Pathway in HepG2 Cells. Mol. Nutr. Food Res. 2020, 64, e1901036. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S. Epigallocatechin gallate suppresses hepatic cholesterol synthesis by targeting SREBP-2 through SIRT1/FOXO1 signaling pathway. Mol. Cell Biochem. 2018, 448, 175–185. [Google Scholar] [CrossRef]
- Kitamura, K.; Okada, Y.; Okada, K.; Kawaguchi, Y.; Nagaoka, S. Epigallocatechin gallate induces an up-regulation of LDL receptor accompanied by a reduction of PCSK9 via the annexin A2-independent pathway in HepG2 cells. Mol. Nutr. Food Res. 2017, 61, 1600836. [Google Scholar] [CrossRef]
- Momose, Y.; Maeda-Yamamoto, M.; Nabetani, H. Systematic review of green tea epigallocatechin gallate in reducing low-density lipoprotein cholesterol levels of humans. Int. J. Food Sci. Nutr. 2016, 67, 606–613. [Google Scholar] [CrossRef]
- Huang, L.-H.; Liu, C.-Y.; Wang, L.-Y.; Huang, C.-J.; Hsu, C.-H. Effects of green tea extract on overweight and obese women with high levels of low density-lipoprotein-cholesterol (LDL-C): A randomised, double-blind, and cross-over placebo-controlled clinical trial. BMC Complement. Altern. Med. 2018, 18, 294. [Google Scholar] [CrossRef]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 1025–1032. [Google Scholar]
- Del Rio, D.; Calani, L.; Cordero, C.E.I.; Salvatore, S.; Pellegrini, N.; Brighenti, F. Bioavailability and catabolism of green tea flavan-3-ols in humans. Nutrients 2010, 26, 1110–1116. [Google Scholar] [CrossRef]
- Sang, S.; Lambert, J.D.; Ho, C.-T.; Yang, C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef]
- Scholl, C.; Lepper, A.; Lehr, T.; Hanke, N.; Schneider, K.L.; Brockmöller, J.; Seufferlein, T.; Stingl, J. Population nutrikinetics of green tea extract. PLoS ONE 2018, 13, e0193074. [Google Scholar] [CrossRef]
- Yashiro, T.; Nanmoku, M.; Shimizu, M.; Inoue, J.; Sato, R. Resveratrol increases the expression and activity of the low density lipoprotein receptor in hepatocytes by the proteolytic activation of the sterol regulatory element-binding proteins. Atherosclerosis 2012, 220, 369–374. [Google Scholar] [CrossRef]
- Jing, Y.; Hu, T.; Lin, C.; Xiong, Q.; Liu, F.; Yuan, J.; Zhao, X.; Wang, R. Resveratrol downregulates PCSK9 expression and attenuates steatosis through estrogen receptor α-mediated pathway in L02 cells. Eur. J. Pharmacol. 2019, 855, 216–226. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, J.; Li, J.; Chen, C.; Huang, H.; Liu, P.-Q.; Huang, H. Polydatin ameliorates lipid and glucose metabolism in type 2 diabetes mellitus by downregulating proprotein convertase subtilisin/kexin type 9 (PCSK9). Cardiovasc. Diabetol. 2016, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shen, C.; Huang, Y.X.; Li, Y.N.; Liu, X.F.; Liu, X.M.; Liu, J.H. A New Strategy for Rapidly Screening Natural Inhibitors Targeting the PCSK9/LDLR Interaction In Vitro. Molecules 2018, 23, 2397. [Google Scholar] [CrossRef] [PubMed]
- Haghighatdoost, F.; Hariri, M. Effect of resveratrol on lipid profile: An updated systematic review and meta-analysis on randomized clinical trials. Pharmacol. Res. 2018, 129, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-F.; Li, J.; Tang, J.; Li, D. Effects of resveratrol supplementation on risk factors of non-communicable diseases: A meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2017, 58, 3016–3029. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites—Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, W.; Chen, S.; Liu, C. Silibinin A decreases statin-induced PCSK9 expression in human hepatoblastoma HepG2 cells. Mol. Med. Rep. 2019, 20, 1383–1392. [Google Scholar] [CrossRef]
- Barzaghi, N.; Crema, F.; Gatti, G.; Pifferi, G.; Perucca, E. Pharmacokinetic studies on IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects. Eur. J. Drug Metab. Pharmacokinet. 1990, 15, 333–338. [Google Scholar] [CrossRef]
- Valentová, K.; Havlik, J.; Kosina, P.; Papoušková, B.; Jaimes, J.D.; Káňová, K.; Petrásková, L.; Ulrichová, J.; Kren, V. Biotransformation of Silymarin Flavonolignans by Human Fecal Microbiota. Metabolism 2020, 10, 29. [Google Scholar] [CrossRef]
- Sui, G.-G.; Xiao, H.-B.; Lu, X.-Y.; Sun, Z.-L. Naringin Activates AMPK Resulting in Altered Expression of SREBPs, PCSK9, and LDLR To Reduce Body Weight in Obese C57BL/6J Mice. J. Agric. Food Chem. 2018, 66, 8983–8990. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Su, W.; Zheng, Y.; He, Y.; He, Y.; Rao, H.; Peng, W.; Yao, H. Pharmacokinetics, Tissue Distribution, Metabolism, and Excretion of Naringin in Aged Rats. Front. Pharmacol. 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.-Y.; Chen, P.-Y.; Chen, S.-F.; Wu, M.-J.; Chang, H.-Y.; Yen, J.-H. Pinostrobin Inhibits Proprotein Convertase Subtilisin/Kexin-type 9 (PCSK9) Gene Expression through the Modulation of FoxO3a Protein in HepG2 Cells. J. Agric. Food Chem. 2018, 66, 6083–6093. [Google Scholar] [CrossRef] [PubMed]
- Sayre, C.; Alrushaid, S.; Martinez, S.E.; Anderson, H.D.; Davies, N.M.; Sayre, C.L.; Alrushaid, S.; Martinez, S.E.; Anderson, H.D.; Pharmacy, N.M.D.O.; et al. Pre-Clinical Pharmacokinetic and Pharmacodynamic Characterization of Selected Chiral Flavonoids: Pinocembrin and Pinostrobin. J. Pharm. Pharm. Sci. 2015, 18, 368. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.K.; Kim, G.W.; Jeong, K.J.; Kim, D.Y.; Chung, S.H. Eugenol Ameliorates Hepatic Steatosis and Fibrosis by Down-Regulating SREBP1 Gene Expression via AMPK-mTOR-p70S6K Signaling Pathway. Boil. Pharm. Bull. 2014, 37, 1341–1351. [Google Scholar] [CrossRef]
- Elbahy, D.A.; Madkour, H.I.; Abdel-Raheem, M.H. Evaluation of antihyperlipidemic activity of eugenol in triton induced hyperlipidemia in rats. Int. J. Res. Stud. Biosci. 2015, 3, 19–26. [Google Scholar]
- Zia, S.; Batool, S.; Shahid, R. Could PCSK9 be a new therapeutic target of Eugenol? In vitro and in silico evaluation of hypothesis. Med. Hypotheses 2020, 136, 109513. [Google Scholar] [CrossRef]
- Guénette, S.A.; Ross, A.; Marier, J.-F.; Beaudry, F.; Vachon, P. Pharmacokinetics of eugenol and its effects on thermal hypersensitivity in rats. Eur. J. Pharmacol. 2007, 562, 60–67. [Google Scholar] [CrossRef]
- Dou, X.; Fan, C.; Wo, L.; Wo, X.; Yan, J.; Qian, Y. Curcumin Up-Regulates LDL Receptor Expression via the Sterol Regulatory Element Pathway in HepG2 Cells. Planta Med. 2008, 74, 1374–1379. [Google Scholar] [CrossRef]
- Peschel, D.; Koerting, R.; Nass, N. Curcumin induces changes in expression of genes involved in cholesterol homeostasis. J. Nutr. Biochem. 2007, 18, 113–119. [Google Scholar] [CrossRef]
- Kang, Q.; Chen, A. Curcumin inhibits srebp-2 expression in activated hepatic stellate cells in vitro by reducing the activity of specificity protein-1. Endocrinology 2009, 150, 5384–5394. [Google Scholar] [CrossRef]
- Tai, M.-H.; Chen, P.-K.; Chen, P.-Y.; Wu, M.-J.; Ho, C.-T.; Yen, J.-H. Curcumin enhances cell-surface LDLR level and promotes LDL uptake through downregulation of PCSK9 gene expression in HepG2 cells. Mol. Nutr. Food Res. 2014, 58, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Nozue, T. Lipid Lowering Therapy and Circulating PCSK9 Concentration. J. Atheroscler. Thromb. 2017, 24, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lu, D.; Zou, Y.; Zhou, C.; Liu, H.; Tu, C.; Li, F.; Zhang, S. Curcumin Protects Against Intestinal Origin Endotoxemia in Rat Liver Cirrhosis by Targeting PCSK9. J. Food Sci. 2017, 82, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Ahmadi, Y.; Teymouri, M.; Johnston, T.P.; Sahebkar, A. Curcumin as a potential candidate for treating hyperlipidemia: A review of cellular and metabolic mechanisms. J. Cell. Physiol. 2017, 233, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Pirro, M.; Gotto, A.M.; Banach, M.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Lipid-modifying activity of curcuminoids: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2017, 59, 1178–1187. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; A Walters, M. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Stohs, S.; Chen, O.; Ray, S.; Ji, J.; Bucci, L.; Preuss, H. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef]
- Nasery, M.M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Kim, S.H.; Kim, D.-S.; Park, S.H.; Yoo, B.W.; Kim, H.K. Nutritional composition and anti-obesity effects of cereal bar containing Allium fistulosum (welsh onion) extract. J. Funct. Foods 2014, 6, 428–437. [Google Scholar] [CrossRef]
- Choi, H.-K.; Hwang, J.; Nam, T.G.; Kim, S.H.; Min, D.-K.; Park, S.W.; Chung, M.-Y. Welsh onion extract inhibits PCSK9 expression contributing to the maintenance of the LDLR level under lipid depletion conditions of HepG2 cells. Food Funct. 2017, 8, 4582–4591. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Sung, Y.-Y.; Yoon, T.; Kim, S.J.; Yang, W.-K. Anti-obesity activity of Allium fistulosum L. extract by down-regulation of the expression of lipogenic genes in high-fat diet-induced obese mice. Mol. Med. Rep. 2011, 4, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; A Ference, B.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Hear. J. 2019, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.J.; A Novotny, J. Consumption of cashew nuts does not influence blood lipids or other markers of cardiovascular disease in humans: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Mukuddem-Petersen, J.; (Oosthuizen), W.S.; Jerling, J.C.; Hanekom, S.M.; White, Z. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: A controlled feeding trial. Br. J. Nutr. 2007, 97, 1144–1153. [Google Scholar] [CrossRef]
- Mah, E.; A Schulz, J.; Kaden, V.N.; Lawless, A.L.; Rotor, J.; Mantilla, L.B.; Liska, D.J. Cashew consumption reduces total and LDL cholesterol: A randomized, crossover, controlled-feeding trial. Am. J. Clin. Nutr. 2017, 105, 1070–1078. [Google Scholar] [CrossRef]
- Mohan, V.; Gayathri, R.; Jaacks, L.M.; Lakshmipriya, N.; Anjana, R.M.; Spiegelman, D.; Jeevan, R.G.; Balasubramaniam, K.K.; Shobana, S.; Jayanthan, M.; et al. Cashew Nut Consumption Increases HDL Cholesterol and Reduces Systolic Blood Pressure in Asian Indians with Type 2 Diabetes: A 12-Week Randomized Controlled Trial. J. Nutr. 2018, 148, 63–69. [Google Scholar] [CrossRef]
- Chan, K.W.; Ismail, M.; Imam, M.U.; Ooi, D.J.; Khong, N.; Maznah, I.; Esa, N.M.; Der-Jiun, O. Dietary supplementation of defatted kenaf (Hibiscus cannabinus L.) seed meal and its phenolics–saponins rich extract effectively attenuates diet-induced hypercholesterolemia in rats. Food Funct. 2018, 9, 925–936. [Google Scholar] [CrossRef]
- Yeh, Y.H.; Lee, Y.T.; Hsieh, H.S.; Hwang, D.F. Dietary caffeic acid, ferulic acid and coumaric acid supplements on cholesterol metabolism and antioxidant activity in rats. J. Food Drug Anal. 2009, 17, 123–132. [Google Scholar]
- Hsu, C.-L.; Yen, G.-C. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br. J. Nutr. 2007, 98, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Chiu, A.; Barone, M.K.; Avino, D.; Wang, F.; Coleman, C.I.; Phung, O.J. Green Tea Catechins Decrease Total and Low-Density Lipoprotein Cholesterol: A Systematic Review and Meta-Analysis. J. Am. Diet. Assoc. 2011, 111, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D. Challenges associated with elucidating the mechanisms of the hypocholesterolaemic activity of saponins. J. Funct. Foods 2016, 23, 52–65. [Google Scholar] [CrossRef]
- Van Ballegooijen, A.J.; Beulens, J.W. The Role of Vitamin K Status in Cardiovascular Health: Evidence from Observational and Clinical Studies. Curr. Nutr. Rep. 2017, 6, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Schurgers, L.J.; Uenishi, K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutr. J. 2012, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Fu, X.; Booth, S.L. Vitamin K Nutrition, Metabolism, and Requirements: Current Concepts and Future Research12. Adv. Nutr. 2012, 3, 182–195. [Google Scholar] [CrossRef]
- Nagasawa, Y.; Fujii, M.; Kajimoto, Y.; Imai, E.; Hori, M. Vitamin K2 and serum cholesterol in patients on continuous ambulatory peritoneal dialysis. Lancet 1998, 351, 724. [Google Scholar] [CrossRef]
- Lupo, M.G.; Biancorosso, N.; Brilli, E.; Tarantino, G.; Adorni, M.P.; Vivian, G.; Salvalaio, M.; Dall’Acqua, S.; Sut, S.; Neutel, C.; et al. Cholesterol-Lowering Action of a Novel Nutraceutical Combination in Uremic Rats: Insights into the Molecular Mechanism in a Hepatoma Cell Line. Nutrients 2020, 12, 436. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.; Tew, K. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.; Young, A. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Pinho, O.; Monteiro, P.R.R. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018, 245, 1148–1153. [Google Scholar] [CrossRef]
- Alvi, S.S.; Ansari, I.A.; Khan, I.; Iqbal, J.; Khan, M.S. Potential role of lycopene in targeting proprotein convertase subtilisin/kexin type-9 to combat hypercholesterolemia. Free Radic. Boil. Med. 2017, 108, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Jiang, L.; Peng, J.; Ren, Z.; Wei, D.; Wu, C.; Pan, L.; Jiang, Z.; Liu, L. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-kappaB activation in THP-1-derived macrophages. Int. J. Mol. Med. 2012, 30, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Alvi, S.S.; Ansari, I.A.; Ahmad, M.K.; Iqbal, J.; Khan, M.S. Lycopene amends LPS induced oxidative stress and hypertriglyceridemia via modulating PCSK-9 expression and Apo-CIII mediated lipoprotein lipase activity. Biomed. Pharmacother. 2017, 96, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An update on the health effects of tomato lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Moia, V.M.; Portilho, F.L.; Pádua, T.A.; Corrêa, L.B.; Ricci-Junior, E.; Rosas, E.C.; Alencar, L.M.R.; Sinfronio, F.S.M.; Sampson, A.; Iram, S.H.; et al. Lycopene used as Anti-inflammatory Nanodrug for the Treatment of Rheumathoid Arthritis: Animal assay, Pharmacokinetics, ABC Transporter and Tissue Deposition. Coll. Surf. B Biointerf. 2020, 188, 110814. [Google Scholar] [CrossRef]
- Kiefer, C.; Hessel, S.; Lampert, J.M.; Vogt, K.; Lederer, M.O.; Breithaupt, D.E.; Von Lintig, J. Identification and Characterization of a Mammalian Enzyme Catalyzing the Asymmetric Oxidative Cleavage of Provitamin A. J. Boil. Chem. 2001, 276, 14110–14116. [Google Scholar] [CrossRef]
- Wang, X.-D. Lycopene metabolism and its biological significance. Am. J. Clin. Nutr. 2012, 96, 1214S–1222S. [Google Scholar] [CrossRef]
- Innes, J.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef]
- Mason, R.P.; Libby, P.; Bhatt, D.L. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arter. Thromb. Vasc. Boil. 2020, 40, 1135–1147. [Google Scholar] [CrossRef]
- Scorletti, E.; Byrne, C.D. Omega-3 fatty acids and non-alcoholic fatty liver disease: Evidence of efficacy and mechanism of action. Mol. Asp. Med. 2018, 64, 135–146. [Google Scholar] [CrossRef]
- Pizzini, A.; Lunger, L.; Demetz, E.; Hilbe, R.; Weiss, G.; Ebenbichler, C.; Tancevski, I. The Role of Omega-3 Fatty Acids in Reverse Cholesterol Transport: A Review. Nutrients 2017, 9, 1099. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, H.; Tian, Y.; Li, Q.; He, L.; Li, N.; Liu, Z. Fish oil alleviated high-fat diet–induced non-alcoholic fatty liver disease via regulating hepatic lipids metabolism and metaflammation: A transcriptomic study. Lipids Heal. Dis. 2016, 15, 20. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Yang, Z.-H.; Vaisman, B.; Thacker, S.; Yu, Z.-X.; Sampson, M.; Serhan, C.N.; Remaley, A.T. Addition of aspirin to a fish oil-rich diet decreases inflammation and atherosclerosis in ApoE-null mice. J. Nutr. Biochem. 2016, 35, 58–65. [Google Scholar] [CrossRef]
- Pu, S.; Rodriguez-Perez, C.; Ramprasath, V.R.; Segura-Carretero, A.; Jones, P.J.H. Dietary high oleic canola oil supplemented with docosahexaenoic acid attenuates plasma proprotein convertase subtilisin kexin type 9 (PCSK9) levels in participants with cardiovascular disease risk: A randomized control trial. Vasc. Pharmacol. 2016, 87, 60–65. [Google Scholar] [CrossRef]
- Graversen, C.B.; Lundbye-Christensen, S.; Thomsen, B.; Christensen, J.H.; Schmidt, E.B. Marine n-3 polyunsaturated fatty acids lower plasma proprotein convertase subtilisin kexin type 9 levels in pre- and postmenopausal women: A randomised study. Vasc. Pharmacol. 2016, 76, 37–41. [Google Scholar] [CrossRef]
- Bradberry, J.C.; Hilleman, D.E. Overview of Omega-3 Fatty Acid Therapies. PTJ Formul. Manag. 2013, 38, 681–691. [Google Scholar]
- Allaire, J.; Couture, P.; Leclerc, M.; Charest, A.; Marin, J.; Lépine, M.-C.; Talbot, D.; Tchernof, A.; Lamarche, B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: The Comparing EPA to DHA (ComparED) Study. Am. J. Clin. Nutr. 2016, 104, 280–287. [Google Scholar] [CrossRef]
- Allaire, J.; Vors, C.; Tremblay, A.J.; Marin, J.; Charest, A.; Tchernof, A.; Couture, P.; Lamarche, B. High-Dose DHA Has More Profound Effects on LDL-Related Features Than High-Dose EPA: The ComparED Study. J. Clin. Endocrinol. Metab. 2018, 103, 2909–2917. [Google Scholar] [CrossRef]
- Allaire, J.; Vors, C.; Harris, W.S.; Jackson, K.H.; Tchernof, A.; Couture, P.; Lamarche, B. Comparing the serum TAG response to high-dose supplementation of either DHA or EPA among individuals with increased cardiovascular risk: The ComparED study. Br. J. Nutr. 2019, 121, 1223–1234. [Google Scholar] [CrossRef]
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012. [Google Scholar] [CrossRef]
- Kathiresan, S.; Willer, C.J.; Peloso, G.M.; Demissie, S.; Musunuru, K.; E Schadt, E.; Kaplan, L.; Bennett, D.; Li, Y.; Tanaka, T.; et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 2008, 41, 56–65. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, T.; Zheng, Y.; Wang, T.; Heianza, Y.; Sun, D.; Campos, H.; Qi, L. PCSK9 variant, long-chain n-3 PUFAs, and risk of nonfatal myocardial infarction in Costa Rican Hispanics. Am. J. Clin. Nutr. 2017, 105, 1198–1203. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R. Strategies to improve bioavailability of omega-3 fatty acids from ethyl ester concentrates. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 116–123. [Google Scholar] [CrossRef]
- Cuenoud, B.; Rochat, I.; Gosoniu, M.; Dupuis, L.; Berk, E.; Jaudszus, A.; Mainz, J.; Hafen, G.; Beaumont, M.; Cruz-Hernandez, C. Monoacylglycerol Form of Omega-3s Improves Its Bioavailability in Humans Compared to Other Forms. Nutrients 2020, 12, 1014. [Google Scholar] [CrossRef]
- Mann, G.V.; Spoerry, A. Studies of a surfactant and cholesteremia in the Maasai. Am. J. Clin. Nutr. 1974, 27, 464–469. [Google Scholar] [CrossRef]
- Ruscica, M.; Pavanello, C.; Gandini, S.; Macchi, C.; Botta, M.; Dall’Orto, D.; Del Puppo, M.; Bertolotti, M.; Bosisio, R.; Mombelli, G.; et al. Nutraceutical approach for the management of cardiovascular risk—A combination containing the probiotic Bifidobacterium longum BB536 and red yeast rice extract: Results from a randomized, double-blind, placebo-controlled study. Nutr. J. 2019, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.L. Comparative analysis of lipid-lowering effect of Diao Xinxuekang and Xin nao shu tong. Shandong Med. J. 1997, 37. [Google Scholar]
- Qu, L.; Li, D.; Gao, X.; Li, Y.; Wu, J.; Zou, W. Di’ao Xinxuekang Capsule, a Chinese Medicinal Product, Decreases Serum Lipids Levels in High-Fat Diet-Fed ApoE(-/-) Mice by Downregulating PCSK9. Front. Pharmacol. 2018, 9, 1170. [Google Scholar] [CrossRef] [PubMed]
- Gai, Y.; Li, Y.; Xu, Z.; Chen, J. Pseudoprotodioscin inhibits SREBPs and microRNA 33a/b levels and reduces the gene expression regarding the synthesis of cholesterol and triglycerides. Fitoterapia 2019, 139, 104393. [Google Scholar] [CrossRef]
- Guo, L.; Dial, S.; Shi, L.; Branham, W.; Liu, J.; Fang, J.-L.; Green, B.; Deng, H.; Kaput, J.; Ning, B. Similarities and differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug Metab. Dispos. 2010, 39, 528–538. [Google Scholar] [CrossRef]
- Westerink, W.M.; Schoonen, W.G. Phase II enzyme levels in HepG2 cells and cryopreserved primary human hepatocytes and their induction in HepG2 cells. Toxicol. Vitr. 2007, 21, 1592–1602. [Google Scholar] [CrossRef]
- Westerink, W.M.A.; Schoonen, W.G. Cytochrome P450 enzyme levels in HepG2 cells and cryopreserved primary human hepatocytes and their induction in HepG2 cells. Toxicol. Vitr. 2007, 21, 1581–1591. [Google Scholar] [CrossRef]
- Shang, X.; Yuan, Z.-B. Determination of six effective components in Rheum by cyclodextrin modified micellar electrokinetic chromatography. Yao Xue Xue Bao Acta Pharm. Sin. 2002, 37, 798–801. [Google Scholar]
- Li, J.; Ding, L.; Song, B.; Xiao, X.; Qi, M.; Yang, Q.; Yang, Q.; Tang, X.; Wang, Z.; Yang, L. Emodin improves lipid and glucose metabolism in high fat diet-induced obese mice through regulating SREBP pathway. Eur. J. Pharmacol. 2016, 770, 99–109. [Google Scholar] [CrossRef]
- Wang, J.; Ji, J.; Song, Z.; Zhang, W.; He, X.; Li, F.; Zhang, C.-F.; Guo, C.-R.; Wang, C.; Yuan, C. Hypocholesterolemic effect of emodin by simultaneous determination of in vitro and in vivo bile salts binding. Fitoterapia 2016, 110, 116–122. [Google Scholar] [CrossRef]
- Su, Z.-L.; Hang, P.-Z.; Hu, J.; Zheng, Y.-Y.; Sun, H.-Q.; Guo, J.; Liu, K.-Y.; Du, Z.-M. Aloe-emodin exerts cholesterol-lowering effects by inhibiting proprotein convertase subtilisin/kexin type 9 in hyperlipidemic rats. Acta Pharmacol. Sin. 2020, 1–8. [Google Scholar] [CrossRef]
- Maeng, H.-J.; Yoo, H.-J.; Kim, I.-W.; Song, I.-S.; Chung, S.-J.; Shim, C.-K. P-Glycoprotein–Mediated Transport of Berberine across Caco-2 Cell Monolayers. J. Pharm. Sci. 2002, 91, 2614–2621. [Google Scholar] [CrossRef]
- Dooren, M.M.G.-V.; Ronden, J.E.; Soute, B.A.; Vermeer, C. Bioavailability of phylloquinone and menaquinones after oral and colorectal administration in vitamin K-deficient rats. Biochem. Pharmacol. 1995, 50, 797–801. [Google Scholar] [CrossRef]
- Cohn, W.; Tenter, U.; Aebischer, C.; Schierle, J.; Schalch, W. Comparative multiple dose plasma kinetics of lycopene administered in tomato juice, tomato soup or lycopene tablets. Eur. J. Nutr. 2004, 43, 304–312. [Google Scholar] [CrossRef]
- Lin, S.-P.; Chu, P.-M.; Tsai, S.-Y.; Wu, M.-H.; Hou, Y.-C. Pharmacokinetics and tissue distribution of resveratrol, emodin and their metabolites after intake of Polygonum cuspidatum in rats. J. Ethnopharmacol. 2012, 144, 671–676. [Google Scholar] [CrossRef]
- Yu, J.; Guo, X.; Zhang, Q.; Peng, Y.; Zheng, J. Metabolite profile analysis and pharmacokinetic study of emodin, baicalin and geniposide in rats. Xenobiotica 2017, 48, 927–937. [Google Scholar] [CrossRef]
- Macchi, C.; Sirtori, C.; Corsini, A.; Santos, R.; Watts, G.F.; Ruscica, M. A new dawn for managing dyslipidemias: The era of rna-based therapies. Pharmacol. Res. 2019, 150, 104413. [Google Scholar] [CrossRef]
- Poli, A.; Barbagallo, C.M.; Cicero, A.F.; Corsini, A.; Manzato, E.; Trimarco, B.; Bernini, F.; Visioli, F.; Bianchi, A.; Canzone, G.; et al. Nutraceuticals and functional foods for the control of plasma cholesterol levels. An intersociety position paper. Pharmacol. Res. 2018, 134, 51–60. [Google Scholar] [CrossRef]
- Fogacci, F.; Banach, M.; Mikhailidis, D.P.; Bruckert, E.; Toth, P.P.; Watts, G.F.; Reiner, Z.; Mancini, J.; Rizzo, M.; Mitchenko, O.; et al. Safety of red yeast rice supplementation: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 143, 1–16. [Google Scholar] [CrossRef]
- Ruscica, M.; Gomaraschi, M.; Mombelli, G.; Macchi, C.; Bosisio, R.; Pazzucconi, F.; Pavanello, C.; Calabresi, L.; Arnoldi, A.; Sirtori, C.R.; et al. Nutraceutical approach to moderate cardiometabolic risk: Results of a randomized, double-blind and crossover study with Armolipid Plus. J. Clin. Lipidol. 2014, 8, 61–68. [Google Scholar] [CrossRef]

| Natural Compound | Chemical Structure | Bioavail. | Tmax (h) | Half-Life Time (h) | Metabolism | Mechanism of Action | Transport. | Level of Evidence on PCSK9 | Counteracts Statins | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Berberine |  | 0.37% | 9.8 | 28.6 | Demethylation and glucuronide | Inhibits SREBP; HNF1α | P-gp and MRP1 | In vitro, in vivo and clinical | Yes | [23,24,28,30,33,35,36,37,188] |
| Sterol/Stanols and Vegetable Proteins | ||||||||||
| Lupin peptide | LILPKHSDAD | Poor (predicted) | Not known | Not known | Proteases | Inhibits interaction PCSK9-LDLR; Reduces HNF1α | No (predicted) | In vitro. Clinic (negative) | Not known | [61,64] |
| Polyphenols | ||||||||||
| Quercetin |  | 0.31% | 2.5 ÷ 3 | 2.1 | Liver: Sulfate and methyl glucuronide; Gut microbiota: free aglycone; 3,4-dihydroxyphenylacetic acid, 3-(3-hydroxyphenyl) propionic acid, 3,4-dihydroxybenzoic acid and 4-hydroxybenzoic acid | Inhibits secretion (sortilin) and SREBP | P-gp sand BCRP | In vitro and in vivo | Not known | [72,73] [78,80,81] |
| Epigallocatechin gallate |  | 0.1% | 1 ÷ 2 | 3.4 | Liver: Methyl, sulfate, and glucuronide; Gut microbiota: phenylvalerolactones and phenylvaleric acids | Inhibits secretion and SREBP | MRP2 and OATP1B1 | In vitro | Yes | [85,91,92,95,96,97,98] |
| Resveratrol |  | <1% | 3 | 9.2 | Liver: Sulfate and glucuronide; Gut microbiota: dihydroresveratrol | Inhibits SREBP1c and interaction PCSK9-LDLR | Not known | In vivo and in vivo | Not known | [100,101,102,105,106,107] |
| Curcumin |  | <1% | Not known | Not known | Liver: reduction and glucuronide, sulfate, and glutathione; Gut microbiota: tetrahydrocurcumin, demethylcurcumin, bisdemethylcurcumin etc. | Inhibits; HNF1α | Not known | In vitro and in vivo | Yes | [122,123,124,127,128,129] |
| Silibinin A |  | <1% | 1.4 | Not known | Sulfate and glucuronide | Inhibits transcription | P-gp inhibitor | In vitro | Yes | [108,109,110] |
| Naringin |  | <1% | 0.5 | 9.5 | Liver: Hydrolysis and then glucuronide, sulfate, methylation; Gut microbiota: Phenolic derivatives | Inhibits SREBP | P-gp and OATP1A5 inhibitor | In vivo | Not known | [111,112] |
| Pinostrobin |  | 1.8% (S); 13.8% (R) | 6.0 | 38.1 | Glucuronide | Inhibits transcription and catalytic activity | Not known | In vitro | Not known | [113,114] |
| Eugenol |  | <1% | 2.1 | 14.0 | Phenol, glucuronide and sulphate | Direct interaction with PCSK9 and inhibits SREBP | Not known | In vitro | Not known | [115,117,118] |
| Nutrients | ||||||||||
| Kaempferol |  | 2.5% | Not known | Not known | Glucuronide and sulphate | Inhibits transcription | Not known | In vitro | Yes | [86,132] |
| p-Coumaric acid |  | 24% | 0.17 | 0.25 | Glucuronide and sulphate | Inhibits transcription | Not known | In vitro | Yes | [132] |
| Vitamin K7 |  | 2% | 6.0 | 60 | Not known | Not known | Not known | In vitro and in vivo | Not known | [145,148,189] |
| Lycopene |  | 33.9% | 24 | 235 | Phase I, oxidation | Inhibits transcription and interaction PCSK9-LDLR | Not Known | In vitro and in vivo | Not Known | [151,152,154,190] |
| Other Inhibitors | ||||||||||
| Protodioscin |  | 0.2% | 20 | 20 | Oxidation, deglycosylation and glucuronide | Inhibits transcription | Not known | In vitro and in vivo | Not known | [179,180] |
| Emodin |  | low | 0.13 | 8.6 | Glucuronide and sulphate | Inhibits SREBP; HNF1α | Not known | In vitro | Not known | [191,192] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adorni, M.P.; Zimetti, F.; Lupo, M.G.; Ruscica, M.; Ferri, N. Naturally Occurring PCSK9 Inhibitors. Nutrients 2020, 12, 1440. https://doi.org/10.3390/nu12051440
Adorni MP, Zimetti F, Lupo MG, Ruscica M, Ferri N. Naturally Occurring PCSK9 Inhibitors. Nutrients. 2020; 12(5):1440. https://doi.org/10.3390/nu12051440
Chicago/Turabian StyleAdorni, Maria Pia, Francesca Zimetti, Maria Giovanna Lupo, Massimiliano Ruscica, and Nicola Ferri. 2020. "Naturally Occurring PCSK9 Inhibitors" Nutrients 12, no. 5: 1440. https://doi.org/10.3390/nu12051440
APA StyleAdorni, M. P., Zimetti, F., Lupo, M. G., Ruscica, M., & Ferri, N. (2020). Naturally Occurring PCSK9 Inhibitors. Nutrients, 12(5), 1440. https://doi.org/10.3390/nu12051440