Association between Obesity and Omega-3 Status in Healthy Young Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.3.1. Anthropometry
2.3.2. Blood Collection and Biochemical Analysis
2.3.3. Food Frequency Questionnaire (FFQ)
2.3.4. Additional Data Collection
2.4. Statistical Analyses
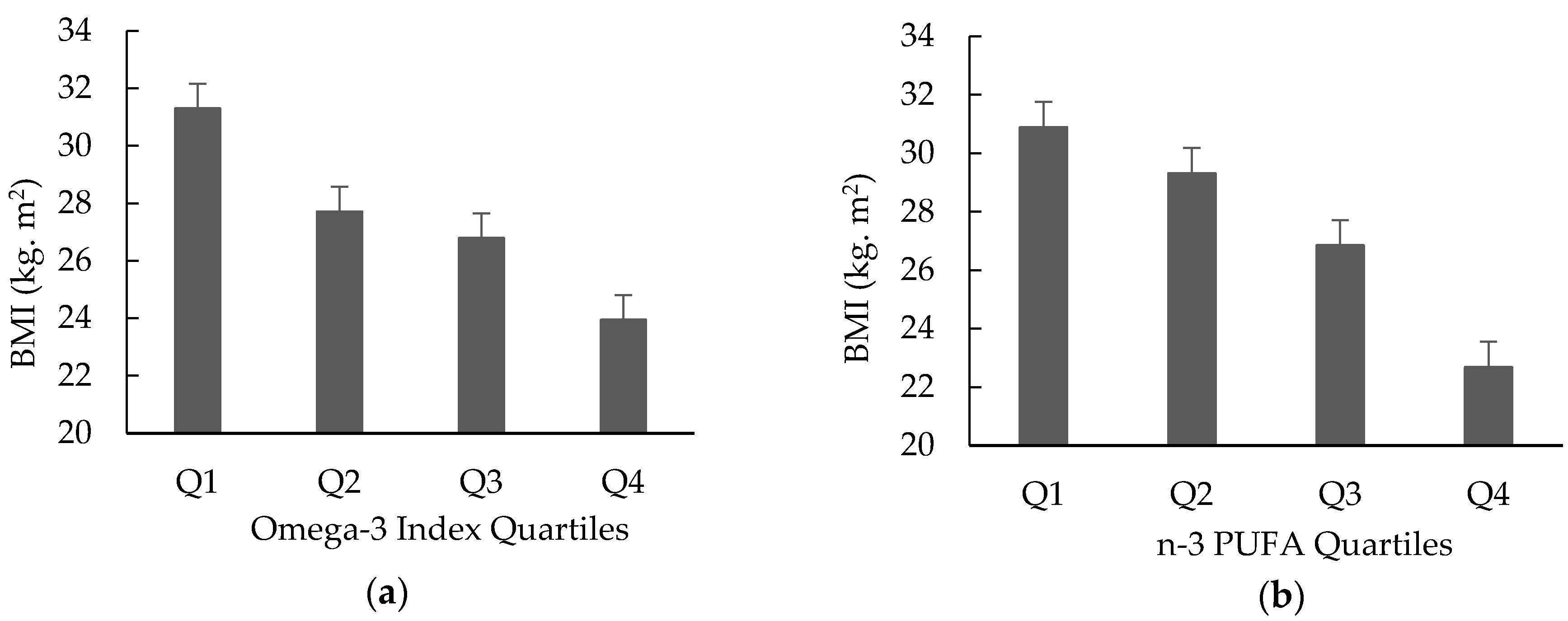
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Australian Bureau of Statistics. Overweight and Obesity. Available online: https://www.abs.gov.au/ausstats/[email protected]/Lookup/by%20Subject/4364.0.55.001~2017-18~Main%20Features~Overweight%20and%20obesity~90 (accessed on 29 March 2019).
- Blüher, M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Caterson, I.; Seidell, J.C.; James, W.P.T. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 2004, 7, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. (Bethesda) 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Micallef, M.; Munro, I.; Phang, M.; Garg, M. Plasma n-3 polyunsaturated fatty acids are negatively associated with obesity. Br. J. Nutr. 2009, 102, 1370–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mingay, E.; Veysey, M.; Lucock, M.; Niblett, S.; King, K.; Patterson, A.; Garg, M. Sex-dependent association between omega-3 index and body weight status in older Australians. J. Nutr. Intermed. Metab. 2016, 5, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Cazzola, R.; Rondanelli, M.; Russo-Volpe, S.; Ferrari, E.; Cestaro, B. Decreased membrane fluidity and altered susceptibility to peroxidation and lipid composition in overweight and obese female erythrocytes. J. Lipid Res. 2004, 45, 1846–1851. [Google Scholar] [CrossRef] [Green Version]
- Burdge, G.C.; Wootton, S.A. Conversion of α-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Jeromson, S.; Gallagher, I.; Galloway, S.; Hamilton, D. Omega-3 Fatty Acids and Skeletal Muscle Health. Mar. Drugs 2015, 13, 6977–7004. [Google Scholar] [CrossRef]
- Luchtman, D.W.; Song, C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: Findings from animal and clinical studies. Neuropharmacology 2013, 64, 550–565. [Google Scholar] [CrossRef]
- Calder, P.C. Very long chain omega-3 (n-3) fatty acids and human health. Eur. J. Lipid Sci. Technol. 2014, 116, 1280–1300. [Google Scholar] [CrossRef]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic Acid: Membrane Properties of a Unique Fatty Acid; Elsevier Ireland Ltd.: Shannon, Ireland, 2003; Volume 126, pp. 1–27. [Google Scholar]
- Sun, Q.; Ma, J.; Campos, H.; Hankinson, S.E.; Hu, F.B. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women.(Original Research Communications)(Author abstract). Am. J. Clin. Nutr. 2007, 86, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Schulze, M.B.; Manson, J.E.; Meigs, J.B.; Albert, C.M.; Rifai, N.; Willett, W.C.; Hu, F.B. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J. Nutr. 2004, 134, 1806–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Schacky, C. n-3 PUFA in CVD: Influence of cytokine polymorphism. Proc. Nutr. Soc. 2007, 66, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Davidson, M.H. Mechanisms for the Hypotriglyceridemic Effect of Marine Omega-3 Fatty Acids. Am. J. Cardiol. 2006, 98, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, M.; Tan, L.; Wang, C.; Ma, J.; Li, N.; Li, Y.; Xu, G.; Li, J. Docosahexaenoic acid changes lipid composition and interleukin-2 receptor signaling in membrane rafts. J. Lipid Res. 2005, 46, 1904–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leaf, X.A.; Kang, E.J.; Xiao, E.Y.-F.; Billman, E.G. Clinical Prevention of Sudden Cardiac Death by n-3 Polyunsaturated Fatty Acids and Mechanism of Prevention of Arrhythmias by n-3 Fish Oils. Circ. J. Am. Heart Assoc. 2003, 107, 2646–2652. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Fernández, L.; Laiglesia, L.M.; Huerta, A.E.; Martínez, J.A.; Moreno-Aliaga, M.J. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 2015, 121, 24–41. [Google Scholar] [CrossRef]
- Munro, I.A.; Garg, M.L. Prior supplementation with long chain omega-3 polyunsaturated fatty acids promotes weight loss in obese adults: A double-blinded randomised controlled trial. Food Funct. 2013, 4, 650–658. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Australian Bureau of Statistics. Fat. Available online: http://www.abs.gov.au/ausstats/[email protected]/Lookup/by%20Subject/4364.0.55.007~2011-12~Main%20Features~Fat~707 (accessed on 23 April 2019).
- Jacka, F.N.; Pasco, J.A.; Williams, L.J.; Meyer, B.J.; Digger, R.; Berk, M. Dietary intake of fish and PUFA, and clinical depressive and anxiety disorders in women. Br. J. Nutr. 2013, 109, 2059–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, B. Are we consuming enough long chain omega-3 polyunsaturated fatty acids for optimal health? Prostaglandins Leukot. Essent. Fat. Acids (Plefa) 2011, 85, 275–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, B.J. Australians are not Meeting the Recommended Intakes for Omega-3 Long Chain Polyunsaturated Fatty Acids: Results of an Analysis from the 2011–2012 National Nutrition and Physical Activity Survey. Nutrients 2016, 8, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, K.; Crawford, D.; Ireland, P.; Hodge, A. Patterns and demographic predictors of 5-year weight change in a multi-ethnic cohort of men and women in Australia. Public Health Nutr. 2003, 6, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Mishra, G.D.; Loxton, D.; Anderson, A.; Hockey, R.; Powers, J.; Brown, W.J.; Dobson, A.J.; Duffy, L.; Graves, A.; Harris, M.; et al. Health and Wellbeing of Women Aged 18–23 in 2013 and 1996: Findings from the Australian Longitudinal Study on Women’s Health; Australian Government Department of Health: Canberra, Australia, 2015.
- Sivayoganathan, D.; Maruthini, D.; Glanville, J.M.; Balen, A.H. Full investigation of patients with polycystic ovary syndrome (PCOS) presenting to four different clinical specialties reveals significant differences and undiagnosed morbidity. Hum. Fertil. 2011, 14, 261–265. [Google Scholar] [CrossRef]
- Childs, C.E.; Romeu-Nadal, M.; Burdge, G.C.; Calder, P.C. Gender differences in the n-3 fatty acid content of tissues. Proc. Nutr. Soc. 2008, 67, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Giltay, E.J.; Gooren, L.J.; Toorians, A.W.; Katan, M.B.; Zock, P.L. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am. J. Clin. Nutr. 2004, 80, 1167–1174. [Google Scholar] [CrossRef] [Green Version]
- Bakewell, L.; Burdge, G.C.; Calder, P.C. Polyunsaturated fatty acid concentrations in young men and women consuming their habitual diets. Br. J. Nutr. 2006, 96, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.J.; Pang, E.; Roberts, D. Persistent changes in the fatty acid composition of erythrocyte membranes after moderate intake of n−3 polyunsaturated fatty acids: Study design implications. Am. J. Clin. Nutr. 1991, 54, 668–673. [Google Scholar] [CrossRef]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Deslypere, J.; Van Birgelen, A.; Penders, M.; Zegwaard, M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: An 18-month controlled study. J. Lipid Res. 1997, 38, 2012–2022. [Google Scholar] [PubMed]
- Burrows, T.; Collins, C.; Garg, M. Omega-3 index, obesity and insulin resistance in children. Int. J. Pediatric Obes. 2011, 6, e532–e539. [Google Scholar] [CrossRef]
- Cook, R.L.; O’Dwyer, N.J.; Donges, C.E.; Parker, H.M.; Cheng, H.L.; Steinbeck, K.S.; Cox, E.P.; Franklin, J.L.; Garg, M.L.; Rooney, K.B. Relationship between obesity and cognitive function in young women: The food, mood and mind study. J. Obes. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, I.; Parker, H.M.; Rangan, A.; Prvan, T.; Cook, R.L.; Donges, C.E.; Steinbeck, K.S.; O’Dwyer, N.J.; Cheng, H.L.; Franklin, J.L. Association between haem and non-haem iron intake and serum ferritin in healthy young women. Nutrients 2018, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- World Health Organisation. Mean Body Mass Index (BMI). Available online: https://www.who.int/gho/ncd/risk_factors/bmi_text/en/ (accessed on 16 November 2019).
- Delbridge, E.; Proietto, J. State of the science: VLED (Very Low Energy Diet) for obesity. Asia Pac. J. Clin. Nutr. 2006, 15, 49. [Google Scholar]
- Schofield, W.N.; Schofield, C.; James, W.P.T. Basal Metabolic Rate: Review and Prediction, Together with an Annotated Bibliography of Source Material; Libbey: London, UK, 1985. [Google Scholar]
- Morley, J.J.; Kushner, I. SERUM C-REACTIVE PROTEIN LEVELS IN DISEASE. Ann. N. Y. Acad. Sci. 1982, 389, 406–418. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Ferguson, J.J.; Veysey, M.; Lucock, M.; Niblett, S.; King, K.; MacDonald-Wicks, L.; Garg, M.L. Association between omega-3 index and blood lipids in older Australians. J. Nutr. Biochem. 2016, 27, 233–240. [Google Scholar] [CrossRef]
- Harris, W.S. The omega-3 index as a risk factor for coronary heart disease. Am. J. Clin. Nutr. 2008, 87, 1997S–2002S. [Google Scholar] [CrossRef]
- Cancer Council Victoria. Dietary Questionnaires. Available online: http://www.cancervic.org.au/aboutour/research/epidemiology/nutritional_assessment_services (accessed on 11 September 2017).
- Ambrosini, G.; Mackerras, D.; De Klerk, N.; Musk, A. Comparison of an Australian food-frequency questionnaire with diet records: Implications for nutrition surveillance. Public Health Nutr. 2003, 6, 415–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Australian Department of Health. The Australian Guide to Helathy Eating. Available online: http://www.health.gov.au/internet/publications/publishing.nsf/Content/nhsc-guidelines~aus-guide-healthy-eating (accessed on 24 November 2019).
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Australian Department of Health. Recommendations to Reduce Chronic Disease Risk; Australian Department of Health: Canberra, Australia, 2017.
- Scaglioni, S.; Verduci, E.; Salvioni, M.; Bruzzese, M.G.; Radaelli, G.; Zetterström, R.; Riva, E.; Agostoni, C. Plasma long-chain fatty acids and the degree of obesity in Italian children. Acta Paediatr. 2006, 95, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Mårild, S.; Brandberg, J.; Lönn, L.; Friberg, P.; Strandvik, B. Serum Phospholipid Fatty Acids, Adipose Tissue, and Metabolic Markers in Obese Adolescents*. Obesity 2006, 14, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Gil-Campos, M.; Carmen Ramírez-Tortosa, M.; Larqué, E.; Linde, J.; Aguilera, C.M.; Cañete, R.; Gil, A. Metabolic Syndrome Affects Fatty Acid Composition of Plasma Lipids in Obese Prepubertal Children. Lipids 2008, 43, 723–732. [Google Scholar] [CrossRef]
- Couet, C.; Delarue, J.; Ritz, P.; Antoine, J.; Lamisse, F. Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int. J. Obes. 1997, 21, 637–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singla, P.; Bardoloi, A.; Parkash, A.A. Metabolic effects of obesity: A review. World J. Diabetes 2010, 1, 76. [Google Scholar] [CrossRef]
- Alsharari, Z.D.; Risérus, U.; Leander, K.; Sjögren, P.; Carlsson, A.C.; Vikström, M.; Laguzzi, F.; Gigante, B.; Cederholm, T.; De Faire, U. Serum fatty acids, desaturase activities and abdominal obesity–a population-based study of 60-year old men and women. PLoS ONE 2017, 12, e0170684. [Google Scholar] [CrossRef]
- Cazzola, R.; Rondanelli, M.; Trotti, R.; Cestaro, B. Effects of weight loss on erythrocyte membrane composition and fluidity in overweight and moderately obese women. J. Nutr. Biochem. 2011, 22, 388–392. [Google Scholar] [CrossRef]
- Haugaard, S.B.; Madsbad, S.; Høy, C.E.; Vaag, A. Dietary intervention increases n-3 long-chain polyunsaturated fatty acids in skeletal muscle membrane phospholipids of obese subjects. Implications for insulin sensitivity. Clin. Endocrinol. 2006, 64, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Bose, K.; Chaudhuri, D.; Baran, A. Association of food patterns, central obesity measures and metabolic risk factors for coronary heart disease (CHD) in middle aged Bengalee Hindu men, Calcutta, India. Asia Pac. J. Clin. Nutr. 2003, 12, 166–171. [Google Scholar] [PubMed]
- Bray, G.A.; Clearfield, M.B.; Fintel, D.J.; Nelinson, D.S. Overweight and obesity: The pathogenesis of cardiometabolic risk. Clin. Cornerstone 2009, 9, 30–42. [Google Scholar] [CrossRef]
- Parra, D.; Ramel, A.; Bandarra, N.; Kiely, M.; Martínez, J.A.; Thorsdottir, I. A diet rich in long chain omega-3 fatty acids modulates satiety in overweight and obese volunteers during weight loss. Appetite 2008, 51, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Udani, J.K.; Ritz, B.W. High potency fish oil supplement improves omega-3 fatty acid status in healthy adults: An open-label study using a web-based, virtual platform. Nutr. J. 2013, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Hibbeln, J.R. Seafood consumption, the DHA content of mothers’ milk and prevalence rates of postpartum depression: A cross-national, ecological analysis. J. Affect. Disord. 2002, 69, 15–29. [Google Scholar] [CrossRef]
- Holman, R.T.; Johnson, S.B.; Ogburn, P.L. Deficiency of essential fatty acids and membrane fluidity during pregnancy and lactation. Proc. Natl. Acad. Sci. USA 1991, 88, 4835–4839. [Google Scholar] [CrossRef] [Green Version]
- Kristal, A.R.; Peters, U.; Potter, J.D. Is it time to abandon the food frequency questionnaire? Cancer Epidemiol. Biomark. Prev. 2005, 14, 2826–2828. [Google Scholar] [CrossRef] [Green Version]
- Andersen, L.F.; Tomten, H.; Haggarty, P.; Løvø, A.; Hustvedt, B. Validation of energy intake estimated from a food frequency questionnaire: A doubly labelled water study. Eur. J. Clin. Nutr. 2003, 57, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Kaskoun, M.C.; Johnson, R.K.; Goran, M.I. Comparison of energy intake by semiquantitative food-frequency questionnaire with total energy expenditure by the doubly labeled water method in young children. Am. J. Clin. Nutr. 1994, 60, 43–47. [Google Scholar] [CrossRef]
- Sam, S.; Dunaif, A. Polycystic ovary syndrome: Syndrome XX? Trends Endocrinol. Metab. 2003, 14, 365–370. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total Group (n = 244) | HW (n = 138) | OB (n = 106) | p-Value * |
|---|---|---|---|---|
| Age (years) | 25.7 ± 5.1 | 24.8 ± 4.6 | 26.8 ± 5.4 | 0.002 |
| Location (n, %) | ||||
| Urban | 165 | 96 | 69 | 0.46 |
| Rural | 79 | 42 | 37 | |
| Years of education | 16.2 ± 2.2 | 16.6 ± 2.2 | 15.8 ± 2.1 | 0.009 |
| BMI (kg/m2) | 27.4 ± 7.3 | 21.8 ± 1.7 | 34.8 ± 4.6 | <0.001 |
| Waist circumference (cm) | 82.4 ± 16.9 | 69.5 ± 4.4 | 99.1 ± 11.5 | <0.001 |
| Biochemistry 1 | ||||
| O3I (%) | 6.4 ± 1.6 | 6.7 ± 1.5 | 5.9 ± 1.5 | <0.001 |
| n-3 PUFA (%) | 11.2 ± 2.9 | 12.3 ± 3.0 | 9.8 ± 2.1 | <0.001 |
| ALA (%) | 0.31 ± 0.18 | 0.33 ± 0.18 | 0.28 ± 0.18 | 0.070 |
| EPA (%) | 0.94 ± 0.40 | 0.93 ± 0.44 | 0.96 ± 0.34 | 0.61 |
| DPA (%) | 4.6 ± 2.1 | 5.3 ± 2.3 | 3.6 ± 1.2 | <0.001 |
| DHA (%) | 5.4 ± 1.4 | 5.8 ± 1.3 | 4.9 ± 1.4 | <0.001 |
| CRP (mg/L) | 2.3 ± 2.4 | 1.2 ± 1.4 | 3.7 ± 2.6 | <0.001 |
| Intake | ||||
| ALA (mg/day) | 1018 ± 513 | 958 ± 458 | 1095 ± 569 | 0.04 |
| EPA (mg/day) | 129 ± 147 | 130 ± 156 | 128 ± 135 | 0.91 |
| DPA (mg/day) | 48 ± 41 | 47 ± 43 | 48 ± 37 | 0.78 |
| DHA (mg/day) | 278 ± 288 | 277 ± 305 | 279 ± 265 | 0.95 |
| Energy-Adjusted Intake | HW (n = 138) | OB (n = 106) | ||
|---|---|---|---|---|
| O3I | n-3 PUFA | O3I | n-3 PUFA | |
| ALA (mg/day) | r = −0.15 | r = −0.08 | r = −0.17 | r = −0.11 |
| p = 0.086 | p = 0.345 | p = 0.085 | p = 0.282 | |
| EPA (mg/day) | r = 0.42 | r = 0.27 | r = 0.37 | r = 0.19 |
| p < 0.001 | p = 0.001 | p < 0.001 | p = 0.048 | |
| DPA (mg/day) | r = 0.44 | r = 0.27 | r = 0.44 | r = 0.24 |
| p < 0.001 | p = 0.001 | p < 0.001 | p = 0.014 | |
| DHA (mg/day) | r = 0.42 | r = 0.26 | r = 0.37 | r = 0.19 |
| p < 0.001 | p = 0.002 | p < 0.001 | p = 0.049 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, I.E.; Parker, H.M.; Cook, R.L.; O’Dwyer, N.J.; Garg, M.L.; Steinbeck, K.S.; Cheng, H.L.; Donges, C.; Franklin, J.L.; O’Connor, H.T. Association between Obesity and Omega-3 Status in Healthy Young Women. Nutrients 2020, 12, 1480. https://doi.org/10.3390/nu12051480
Young IE, Parker HM, Cook RL, O’Dwyer NJ, Garg ML, Steinbeck KS, Cheng HL, Donges C, Franklin JL, O’Connor HT. Association between Obesity and Omega-3 Status in Healthy Young Women. Nutrients. 2020; 12(5):1480. https://doi.org/10.3390/nu12051480
Chicago/Turabian StyleYoung, Isabel E., Helen M. Parker, Rebecca L. Cook, Nicholas J. O’Dwyer, Manohar L. Garg, Kate S. Steinbeck, Hoi Lun Cheng, Cheyne Donges, Janet L. Franklin, and Helen T. O’Connor. 2020. "Association between Obesity and Omega-3 Status in Healthy Young Women" Nutrients 12, no. 5: 1480. https://doi.org/10.3390/nu12051480





