Vitamin D-Binding Protein in Pregnancy and Reproductive Health
Abstract
:1. Introduction
2. Overview of Vitamin D
2.1. Vitamin D Sources and Metabolism
2.2. Epidemiology, Definitions, and Causes of Vitamin D Deficiency
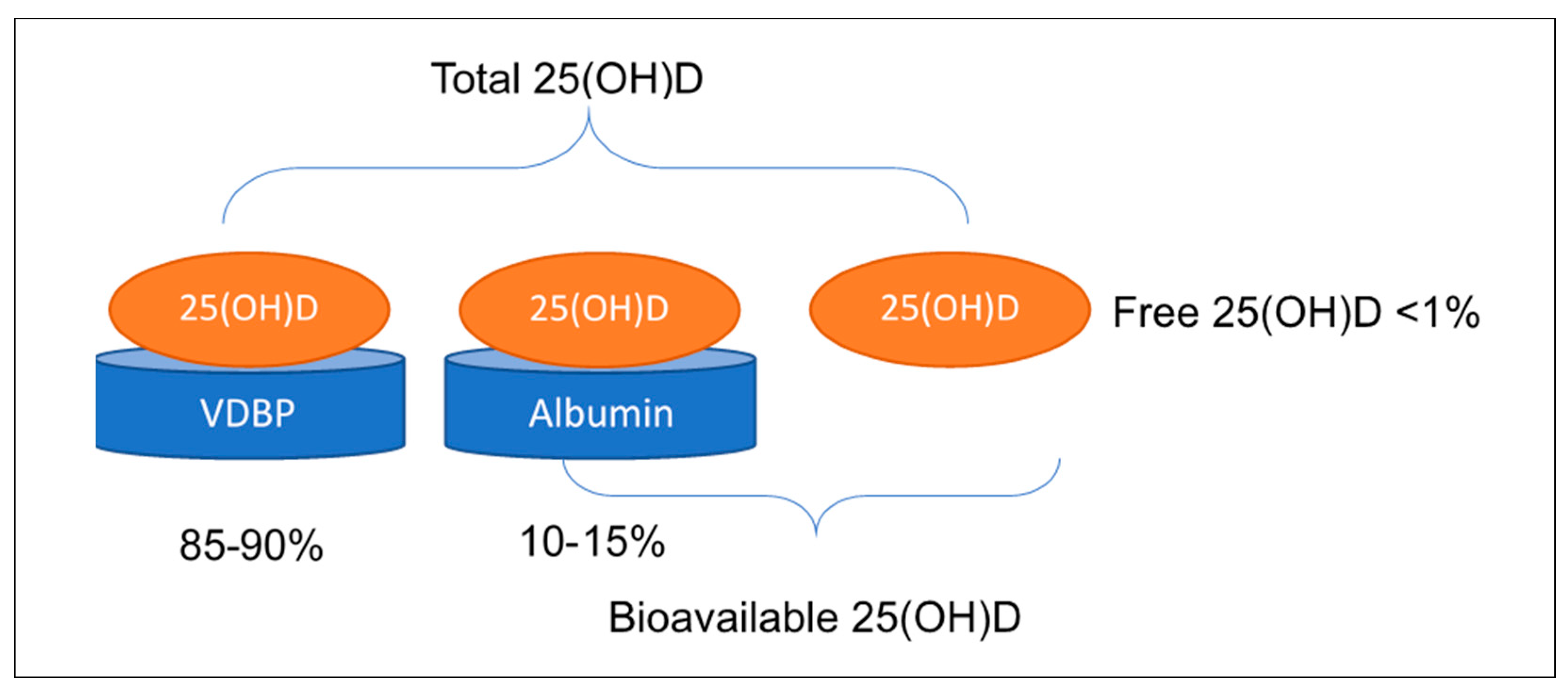
2.3. Controversy Regarding the Measurement of Vitamin D and Its Metabolites to Determine Vitamin D Status
+ ((binding constant DBP) × DBP))
2.4. Guidelines for Treatment
3. Vitamin D Binding Protein
VDBP in Pregnancy and Lactation
4. VDBP and Fertility-Related Outcomes
4.1. VDBP in Fertility and Assisted Reproduction
4.2. VDBP and Polycystic Ovary Syndrome
4.3. VDBP and Pregnancy Loss or Miscarriage
5. VDBP and Pregnancy Outcomes
5.1. VDBP and Preeclampsia
5.2. VDBP and Gestational Diabetes Mellitus
5.3. VDBP and Preterm Birth
6. VDBP and Offspring Outcomes
6.1. VDBP and Neonatal Vitamin D Status
6.2. VDBP and Fetal Growth and Birthweight
6.3. VDBP and Infant Health
7. Critical Appraisal: Limitations and Future Directions
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holick, M.F.; Chen, T. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, S1080–S1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Ma, J.; Zhang, X.; Fan, Y.; Wang, L. Protective role of the vitamin D receptor. Cell. Immunol. 2012, 279, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Bellia, A.; Garcovich, C.; D’Adamo, M.; Lombardo, M.; Tesauro, M.; Donadel, G.; Gentileschi, P.; Lauro, D.; Federici, M.; Lauro, R.; et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern. Emerg. Med. 2011, 8, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Luan, J.; Cooper, A.; Boucher, B.; Wareham, N.J. Baseline Serum 25-Hydroxy Vitamin D Is Predictive of Future Glycemic Status and Insulin Resistance. Diabetes 2008, 57, 2619–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquina, C.; Mousa, A.; Scragg, R.; De Courten, B. Vitamin D and cardiometabolic disorders: A review of current evidence, genetic determinants and pathomechanisms. Obes. Rev. 2018, 20, 262–277. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef] [Green Version]
- Mousa, A.; Naderpoor, N.; Teede, H.J.; Scragg, R.; De Courten, B. Vitamin D supplementation for improvement of chronic low-grade inflammation in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2018, 76, 380–394. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.; Mousa, A.; Ebeling, P.R.; Scott, D.; De Courten, B. Effects of vitamin D supplementation on inflammatory markers in heart failure: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2018, 8, 1169. [Google Scholar] [CrossRef] [Green Version]
- Martineau, A.R.; Cates, C.J.; Urashima, M.; Jensen, M.; Griffiths, A.P.; Nurmatov, U.; Sheikh, A.; Griffiths, C.J. Vitamin D for the management of asthma. Cochrane Database Syst. Rev. 2016, 2016, CD011511. [Google Scholar] [CrossRef]
- Di Marco, N.; Kaufman, J.; Rodda, C.P. Shedding Light on Vitamin D Status and its complexities during pregnancy, infancy and childhood: An Australian perspective. Int. J. Environ. Res. Public Health 2019, 16, 538. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, H.; Zheng, M.; Wu, Y.; Zeng, T.; Fu, J.; Zeng, D. Maternal vitamin D deficiency increases the risk of adverse neonatal outcomes in the Chinese population: A prospective cohort study. PLoS ONE 2018, 13, e0195700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. What diseases are causally linked to vitamin D deficiency? Arch. Dis. Child. 2016, 101, 185–189. [Google Scholar] [CrossRef]
- Mousa, A.; Abell, S.; Scragg, R.; De Courten, B. Vitamin D in reproductive health and pregnancy. Semin. Reprod. Med. 2016, 34, 1–13. [Google Scholar] [CrossRef]
- Karras, S.N.; Koufakis, T.; Fakhoury, H.A.; Kotsa, K. Deconvoluting the biological roles of vitamin D-binding protein during pregnancy: A both clinical and theoretical challenge. Front. Endocrinol. 2018, 9, 259. [Google Scholar] [CrossRef]
- Liong, S.; Di Quinzio, M.; Fleming, G.; Permezel, M.; Rice, G.; Georgiou, H.M. New biomarkers for the prediction of spontaneous preterm labour in symptomatic pregnant women: A comparison with fetal fibronectin. BJOG Int. J. Obstet. Gynaecol. 2014, 122, 370–379. [Google Scholar] [CrossRef]
- Liong, S.; Di Quinzio, M.; Heng, Y.J.; Fleming, G.; Permezel, M.; Rice, G.E.; Georgiou, H.M. Proteomic analysis of human cervicovaginal fluid collected before preterm premature rupture of the fetal membranes. Reproduction 2013, 145, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Song, Y.; Rawal, S.; Wu, J.; Hinkle, S.N.; Tsai, M.Y.; Zhang, C.-L. Vitamin D status during pregnancy and the risk of gestational diabetes mellitus: A longitudinal study in a multiracial cohort. Diabetes Obes. Metab. 2019, 21, 1895–1905. [Google Scholar] [CrossRef]
- Norman, A.W. 1α,25(OH)2 Vitamin D3, vitamin D nuclear receptor (VDR) and plasma vitamin D-binding protein (dbp) structures and ligand shape preferences for genomic and rapid biological responses. In Principles of Bone Biology; Elsevier BV: Amsterdam, The Netherlands, 2008; pp. 749–778. [Google Scholar]
- Holick, M.F.; Binkley, N.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.; Hashmi, O.; Dutton, D.; Mavrodaris, A.; Stranges, S.; Kandala, N.-B.; Clarke, A.; Franco, O.H. Levels of vitamin D and cardiometabolic disorders: Systematic review and meta-analysis. Maturitas 2010, 65, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Streeten, E.A.; Levine, M.A. Chapter 98-vitamin D metabolism or action. In Emery and Rimoin’s Principles and Practice of Medical Genetics; Rimoin, D., Pyeritz, R., Korf, B., Eds.; Academic Press: Oxford, UK, 2013; pp. 1–28. [Google Scholar]
- Jones, G. Chapter 83—Vitamin D and analogues. In Principles of Bone Biology; Bilezikian, J.P., Raisz, L.G., Martin, T.J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 1777–1799. [Google Scholar]
- Nykjaer, A.; Dragun, D.; Walther, D.; Vorum, H.; Jacobsen, C.; Herz, J.; Melsen, F.; Christensen, E.I.; Willnow, T.E. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999, 96, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Hassan-Smith, Z.K.; Jenkinson, C.; Smith, D.J.; Hernández, I.; Morgan, S.A.; Crabtree, N.J.; Gittoes, N.J.; Keevil, B.G.; Stewart, P.M.; Hewison, M. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 exert distinct effects on human skeletal muscle function and gene expression. PLoS ONE 2017, 12, e0170665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Towards a trial-based definition of vitamin D deficiency. Lancet Diabetes Endocrinol. 2016, 4, 376–377. [Google Scholar] [CrossRef] [Green Version]
- Peris, P.; Filella, X.; Monegal, A.; Guañabens, N.; Foj, L.; Bonet, M.; Boquet, D.; Casado, E.; Cerdá, D.; Erra, A.; et al. Comparison of total, free and bioavailable 25-OH vitamin D determinations to evaluate its biological activity in healthy adults: The LabOscat study. Osteoporos. Int. 2017, 260, 245–2464. [Google Scholar] [CrossRef]
- Rosen, C.J.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; Kovacs, C.S.; et al. IOM Committee Members Respond to Endocrine Society Vitamin D Guideline. J. Clin. Endocrinol. Metab. 2012, 97, 1146–1152. [Google Scholar] [CrossRef]
- Catharine Ross, A.Y.; Heather, B.; Del, V. Dietary Reference Intakes for Calcium and Vitamin D, in Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; National Academies Press (US) Institute of Medicine: Washington, DC, USA, 2011. [Google Scholar]
- Nowson, C.; McGrath, J.J.; Ebeling, P.R.; Haikerwal, A.; Daly, R.M.; Sanders, K.M.; Seibel, M.; Mason, R.S. Vitamin D and health in adults in Australia and New Zealand: A position statement. Med. J. Aust. 2012, 196, 686–687. [Google Scholar] [CrossRef]
- Mousa, A.; Misso, M.; Teede, H.J.; Scragg, R.; De Courten, B. Effect of vitamin D supplementation on inflammation: Protocol for a systematic review. BMJ Open 2016, 6, e010804. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Vitamin D deficiency in 2010: Health benefits of vitamin D and sunlight: A d-bate. Nat. Rev. Endocrinol. 2011, 7, 73–75. [Google Scholar] [CrossRef]
- Prentice, A. Vitamin D deficiency: A global perspective. Nutr. Rev. 2008, 66, S153–S164. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.M.; Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Sikaris, K.; Zimmet, P.; Ebeling, P.R.; Shaw, J.E. Prevalence of vitamin D deficiency and its determinants in Australian adults aged 25 years and older: A national, population-based study. Clin. Endocrinol. 2012, 77, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.K.; Hill, C.; Shanahan, E.M.; Taylor, A.W.; Appleton, S.L.; Grant, J.F.; Shi, Z.; Grande, E.D.; Price, K.; Adams, R.J. Vitamin D levels in an Australian population. BMC Public Health 2014, 14, 1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souberbielle, J.-C. Epidemiology of vitamin-D deficiency. Gériatrie Psychol. Neuropsychiatr. Viellissement 2016, 14, 7–15. [Google Scholar] [CrossRef]
- Parva, N.R.; Tadepalli, S.; Singh, P.; Qian, A.; Joshi, R.; Kandala, H.; Nookala, V.K.; Cheriyath, P. prevalence of vitamin D deficiency and associated risk factors in the US population (2011–2012). Cureus 2018, 10, e2741. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, M.K.; Romundstad, P.R.; Stafne, S.N.; Helvik, A.-S.; Stunes, A.K.; Mørkved, S.; Salvesen, K.; Thorsby, P.M.; Syversen, U. Alterations in the vitamin D endocrine system during pregnancy: A longitudinal study of 855 healthy Norwegian women. PLoS ONE 2018, 13, e0195041. [Google Scholar] [CrossRef] [Green Version]
- Van Schoor, N.; Lips, P. Worldwide vitamin D status. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 671–680. [Google Scholar] [CrossRef]
- Gröber, U.; Kisters, K. Influence of drugs on vitamin D and calcium metabolism. Derm. Endocrinol. 2012, 4, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Bodnar, L.; Simhan, H.N.; Powers, R.W.; Frank, M.P.; Cooperstein, E.; Roberts, J.M. High prevalence of vitamin D insufficiency in Black and White pregnant women residing in the northern United States and their neonates. J. Nutr. 2007, 137, 447–452. [Google Scholar] [CrossRef]
- Harris, S.S. Vitamin D and African Americans. J. Nutr. 2006, 136, 1126–1129. [Google Scholar] [CrossRef] [Green Version]
- Best, C.; Pressman, E.K.; Queenan, R.A.; Cooper, E.; O’Brien, K.O. Longitudinal changes in serum vitamin D binding protein and free 25-hydroxyvitamin D in a multiracial cohort of pregnant adolescents. J. Steroid Biochem. Mol. Boil. 2019, 186, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.B.; Lai, J.; Lizaola, B.; Kane, L.; Marková, S.; Weyland, P.; Terrault, N.A.; Stotland, N.; Bikle, D. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J. Clin. Endocrinol. Metab. 2014, 99, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Bouillon, R.; Thadhani, R.; Schoenmakers, I. Vitamin D metabolites in captivity? Should we measure free or total 25(OH)D to assess vitamin D status? J. Steroid Biochem. Mol. Boil. 2017, 173, 105–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouillon, R.; Van Baelen, H. Transport of vitamin D: Significance of free and total concentrations of the vitamin D metabolites. Calcif. Tissue Int. 1981, 33, 451–453. [Google Scholar] [CrossRef]
- Kim, H.-J.; Ji, M.; Song, J.; Moon, H.-W.; Hur, M.; Yun, Y.-M. Clinical utility of measurement of vitamin D-binding protein and calculation of bioavailable vitamin d in assessment of vitamin D status. Ann. Lab. Med. 2017, 37, 34–38. [Google Scholar] [CrossRef]
- Heijboer, A.C.; Blankenstein, M.; Kema, I.P.; Buijs, M.M. Accuracy of 6 routine 25-hydroxyvitamin D assays: Influence of vitamin D binding protein concentration. Clin. Chem. 2012, 58, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Karras, S.N.; Kotsa, K.; Angeloudi, E.; Zebekakis, P.; Naughton, D. The road not so travelled: Should measurement of vitamin D epimers during pregnancy affect our clinical decisions? Nutrients 2017, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Reddy, G.S.; Norman, A.W.; Willis, W.; Goltzman, D.; Guyda, H.; Solomon, S.; Philips, D.R.; Bishop, J.E.; Mayer, E. Regulation of vitamin D metabolism in normal human pregnancy. J. Clin. Endocrinol. Metab. 1983, 56, 363–370. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Lucey, A.; Horgan, R.; Kenny, L.C.; Kiely, M. Impact of pregnancy on vitamin D status: A longitudinal study. Br. J. Nutr. 2014, 112, 1081–1087. [Google Scholar] [CrossRef] [Green Version]
- Vargas, S.; Bouillon, R.; Van Baelen, H.; Raisz, L.G. Effects of vitamin D-binding protein on bone resorption stimulated by 1,25 dihydroxyvitamin D3. Calcif. Tissue Int. 1990, 47, 164–168. [Google Scholar] [CrossRef]
- Denburg, M.; Hoofnagle, A.N.; Sayed, S.; Gupta, J.; De Boer, I.H.; Appel, L.J.; Durazo-Arvizu, R.; Whitehead, K.; Feldman, H.I.; Leonard, M.B.; et al. Comparison of two ELISA methods and mass spectrometry for measurement of vitamin D-binding protein: Implications for the assessment of bioavailable vitamin D concentrations across genotypes. J. Bone Miner. Res. 2016, 31, 1128–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikle, D.; Malmstroem, S.; Schwartz, J. Current Controversies. Endocrinol. Metab. Clin. North Am. 2017, 46, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Gee, E.; Halloran, B.; Kowalski, M.A.; Ryzen, E.; Haddad, J.G. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 1986, 63, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Gee, E.; Halloran, B.; Haddad, J.G. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J. Clin. Investig. 1984, 74, 1966–1971. [Google Scholar] [CrossRef] [Green Version]
- Heureux, N.; Lindhout, E.; Swinkels, L. A direct assay for measuring free 25-hydroxyvitamin D. J. AOAC Int. 2017, 100, 1318–1322. [Google Scholar] [CrossRef]
- Nowson, C.A.; McGrath, J.J.; Ebeling, P.R.; Haikerwal, A.; Daly, R.M.; Sanders, K.M.; Seibel, M.J.; Mason, R.S. Working group of the Australian and New Zealand Bone and Mineral Society, Endocrine Society of Australia and Osteoporosis Australia. Vitamin D and adult bone health in Australia and New Zealand: A position statement. Med. J. Aust. 2005, 182, 281–285. [Google Scholar] [CrossRef]
- Sangrador, M.R.; De Miguel, B.B.; Murillas, L.Q.; Vives, C.C.; Tuny, O.M. The contribution of diet and sun exposure to the nutritional status of vitamin D in elderly Spanish women: The five countries study (OPTIFORD Project). Nutr. Hosp. 2009, 23, 567–576. [Google Scholar]
- Liberati, A.; Altman, U.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Atkins, D.; the GRADE Working Group; Briss, P.A.; Eccles, M.P.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Hill, S.; Jaeschke, R.; Liberati, A. Systems for grading the quality of evidence and the strength of recommendations II: Pilot study of a new system. BMC Health Serv. Res. 2005, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Higgins, S.G. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- McKinney, K.; Breitkopf, C.R.; Berenson, A.B. Association of race, body fat and season with vitamin D status among young women: A cross-sectional study. Clin. Endocrinol. 2008, 69, 535–541. [Google Scholar] [CrossRef]
- Barrett, C.B. Measuring Food Insecurity. Science 2010, 327, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Doneray, H.; Yesilcibik, R.S.; Laloglu, E.; Ingec, M.; Orbak, Z. Serum vitamin D and vitamin D-binding protein levels in mother-neonate pairs during the lactation period. Ital. J. Pediatr. 2018, 44, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, S.G.; Retallack, R.W.; Kent, J.C.; Worth, G.K.; Gutteridge, D.H. Serum free 1,25-dihydroxyvitamin D and the free 1,25-dihydroxyvitamin D index during a longitudinal study of human pregnancy and lactation. Clin. Endocrinol. 1990, 32, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Cohen, W.R.; Silva, P.; Epstein, F.H. Elevated 1,25-dihydroxyvitamin D plasma levels in normal human pregnancy and lactation. J. Clin. Investig. 1979, 63, 342–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madej, J.P.; Nowacki, W.; Boratyński, J.; Borkowski, J.; Włodarczyk-Szydłowska, A.; Musiał, E. The relationship between concentrations of vitamin D-binding protein (DBP) in serum and colostrum of mares and in serum of their foals in the neonatal period. Pol. J. Veter Sci. 2009, 12, 499–507. [Google Scholar]
- Barrett, H.L.; McElduff, A. Vitamin D and pregnancy: An old problem revisited. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 527–539. [Google Scholar] [CrossRef]
- Brooke, O.G.; Brown, I.R.; Bone, C.D.; Carter, N.D.; Cleeve, H.J.; Maxwell, J.; Robinson, V.P.; Winder, S.M. Vitamin D supplements in pregnant Asian women: Effects on calcium status and fetal growth. BMJ 1980, 280, 751–754. [Google Scholar] [CrossRef] [Green Version]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Pludowski, P.; Jones, G. Vitamin D toxicity–A clinical perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, D.; Sweeney, K.P.; Lau, J.; Lichtenstein, A.H. Critical assessment of high-circulation print newspaper coverage of the Institute of Medicine report Dietary Reference Intakes for Calcium and Vitamin D. Public Health Nutr. 2013, 17, 1868–1876. [Google Scholar] [CrossRef] [Green Version]
- Hollis, B.W.; Wagner, C.L. New insights into the vitamin D requirements during pregnancy. Bone Res. 2017, 5, 17030. [Google Scholar] [CrossRef]
- Friedman, W.F.; Roberts, W.C. Vitamin D and the supravalvar aortic stenosis syndrome. The transplacental effects of vitamin D on the aorta of the rabbit. Circulation 1966, 34, 77–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latorre, G. Effect of overdose of vitamin D2 on pregnancy in the rat. Fertil. Steril. 1961, 12, 343–345. [Google Scholar] [CrossRef]
- Speeckaert, M.M.; Speeckaert, R.; van Geel, N.; Delanghe, J.R. Chapter one—Vitamin D binding protein: A multifunctional protein of clinical importance. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 63, pp. 1–57. [Google Scholar]
- Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Behind the scenes of vitamin D binding protein: More than vitamin D binding. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 773–786. [Google Scholar] [CrossRef]
- Arnaud, J.; Constans, J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Qual. Life Res. 1993, 92, 183–188. [Google Scholar] [CrossRef]
- Nielson, C.M.; Jones, K.S.; Chun, R.F.; Jacobs, J.M.; Wang, Y.; Hewison, M.; Adams, J.S.; Swanson, C.M.; Lee, C.G.; Vanderschueren, D.; et al. Free 25-Hydroxyvitamin D: Impact of vitamin d binding protein assays on racial-genotypic associations. J. Clin. Endocrinol. Metab. 2016, 101, 2226–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, S.; Fu, L.; Juras, D.J.; Karmali, M.; Wong, B.Y.L.; Gozdzik, A.; Cole, D.E. Common variants of the vitamin D binding protein gene and adverse health outcomes. Crit. Rev. Clin. Lab. Sci. 2013, 50, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palterer, B.; Vitiello, G.; Carraresi, A.; Giudizi, M.G.; Cammelli, D.; Parronchi, P. Bench to bedside review of myositis autoantibodies. Clin. Mol. Allergy 2018, 16, 5. [Google Scholar] [CrossRef] [Green Version]
- Chun, S.; Shin, S.; Kim, M.Y.; Joung, H.; Chung, J. Effects of maternal genetic polymorphisms in vitamin D-binding protein and serum 25-hydroxyvitamin D concentration on infant birth weight. Nutrients 2017, 35, 36–42. [Google Scholar] [CrossRef]
- Bouillon, R.; Van Baelen, H.; De Moor, P. The measurement of the vitamin D-binding protein in human serum. J. Clin. Endocrinol. Metab. 1977, 45, 225–231. [Google Scholar] [CrossRef]
- Jones, K.S.; Assar, S.; Prentice, A.; Schoenmakers, I. Vitamin D expenditure is not altered in pregnancy and lactation despite changes in vitamin D metabolite concentrations. Sci. Rep. 2016, 6, 26795. [Google Scholar] [CrossRef] [Green Version]
- Van Hoof, H.; De Sevaux, R.; Van Baelen, H.; Swinkels, L.; Klipping, C.; Ross, H.; Sweep, F.C.G.J. Relationship between free and total 1,25-dihydroxyvitamin D in conditions of modified binding. Eur. J. Endocrinol. 2001, 144, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Brannon, P.M.; West, A.A.; Yan, J.; Jiang, X.; Perry, C.A.; Malysheva, O.V.; Mehta, S.; Caudill, M. Vitamin D metabolism varies among women in different reproductive states consuming the same intakes of vitamin D and related nutrients. J. Nutr. 2016, 146, 1537–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.; Gu, Y.; Zhao, S.; Sun, J.; Groome, L.J.; Wang, Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am. J. Physiol. Metab. 2012, 303, E928–E935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, P.H.; Lucas, R.M.; Walsh, J.P.; Zosky, G.R.; Whitehouse, A.J.O.; Zhu, K.; Allen, K.L.; Kusel, M.M.; Anderson, D.; Mountain, J.A. Vitamin D in fetal development: Findings from a birth cohort study. Pediatrics 2014, 135, e167–e173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.S.; Choi, M.Y.; Longtine, M.S.; Nelson, D.M. Vitamin D effects on pregnancy and the placenta. Placenta 2010, 31, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Hoogenboezem, T.; Degenhart, H.J.; Keizer-Schrama, S.M.; Bouillon, R.; Grose, W.F.; Hackeng, W.H.L.; Visser, H.K. Vitamin D metabolism in breast-fed infants and their mothers. Pediatr. Res. 1989, 25, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Borght, M.V.; Wyns, C.; Mélodie, V.B.; Christine, W. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Obeid, R.; Hahn, A.; Pludowski, P.; Trummer, C.; Lerchbaum, E.; Pérez-López, F.R.; Karras, S.N.; März, W. The role of vitamin D in fertility and during pregnancy and lactation: A review of clinical data. Int. J. Environ. Res. Public Health 2018, 15, 2241. [Google Scholar] [CrossRef] [Green Version]
- Thys-Jacobs, S.; Donovan, D.; Papadopoulos, A.; Sarrel, P.; Bilezikian, J.P. Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids 1999, 64, 430–435. [Google Scholar] [CrossRef]
- Jensen, M.B.; Bjerrum, P.J.; Jessen, T.E.; Nielsen, J.E.; Joensen, U.N.; Olesen, I.A.; Petersen, J.H.; Juul, A.; Dissing, S.; Jørgensen, N. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum. Reprod. 2011, 26, 1307–1317. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.-J.; Yu, N.; Yin, T.-L.; Liu, L.; Yang, J. Can Vitamin D supplementation be used as adjunctive treatment for oligozoospermia or asthenozoospermia accompanied with Vitamin D deficiency? Asian J. Androl. 2014, 17, 165–167. [Google Scholar] [CrossRef]
- Aleyasin, A.; Hosseini, M.A.; Mahdavi, A.; Safdarian, L.; Fallahi, P.; Mohajeri, M.R.; Abbasi, M.; Esfahani, F. Predictive value of the level of vitamin D in follicular fluid on the outcome of assisted reproductive technology. Eur. J. Obstet. Gynecol. Reprod. Boil. 2011, 159, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Paffoni, A.; Ferrari, S.; Viganò, P.; Pagliardini, L.; Papaleo, E.; Candiani, M.; Tirelli, A.; Fedele, L.; Somigliana, E. Vitamin D deficiency and infertility: Insights from in vitro fertilization Cycles. J. Clin. Endocrinol. Metab. 2014, 99, 2372. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Shapses, S.; Sun, W.; Wang, X. Vitamin D binding protein is lower in infertile patients compared to fertile controls: A case control study. Fertil. Res. Pract. 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estes, S.J.; Ye, B.; Qiu, W.; Cramer, D.; Hornstein, M.D.; Missmer, S.A. A proteomic analysis of IVF follicular fluid in women <or=32 years old. Fertil. Steril. 2009, 92, 1569–1578. [Google Scholar]
- Shahine, L.K.; Lamb, J.D.; Lathi, R.B.; Milki, A.A.; Langen, E.; Westphal, L.M. Poor Prognosis with in vitro fertilization in Indian women compared to Caucasian women despite similar embryo quality. PLoS ONE 2009, 4, e7599. [Google Scholar] [CrossRef]
- Awumey, E.M.K.; Mitra, D.A.; Hollis, B.W.; Kumar, R.; Bell, N.H. Vitamin D metabolism is altered in Asian Indians in the Southern United States: A clinical research center study. J. Clin. Endocrinol. Metab. 1998, 83, 169–173. [Google Scholar] [CrossRef]
- Özkan, S.; Jindal, S.; Greenseid, K.; Shu, J.; Zeitlian, G.; Hickmon, C.; Pal, L. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil. Steril. 2009, 94, 1314–1319. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Ross, L.; Pandian, R.; Ingles, S.; Paulson, R.; Bendikson, K. Supratherapeutic levels of bioavailable vitamin D are associated with poor IVF outcomes in asian women, but not in white women. Fertil. Steril. 2017, 108, e320–e321. [Google Scholar] [CrossRef]
- Naderpoor, N.; Shorakae, S.; Abell, S.K.; Mousa, A.; Joham, A.E.; Moran, L.J.; Stepto, N.; Spritzer, P.M.; Teede, H.J.; De Courten, B. Bioavailable and free 25-hydroxyvitamin D and vitamin D binding protein in polycystic ovary syndrome: Relationships with obesity and insulin resistance. J. Steroid Biochem. Mol. Boil. 2018, 177, 209–215. [Google Scholar] [CrossRef]
- Kuliczkowska-Plaksej, J.; Pasquali, R.; Milewicz, A.; Lwow, F.; Jedrzejuk, D.; Bolanowski, M. Serum vitamin D binding protein level associated with metabolic cardiovascular risk factors in women with the polycystic ovary syndrome. Horm. Metab. Res. 2018, 51, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Haldar, D.; Agrawal, N.; Patel, S.; Kambale, P.R.; Arora, K.; Sharma, A.; Tripathi, M.; Batra, A.; Kabi, B.C. Association of VDBP and CYP2R1 gene polymorphisms with vitamin D status in women with polycystic ovarian syndrome: A north Indian study. Eur. J. Nutr. 2016, 57, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Song, D.K.; Lee, H.; Hong, Y.S.; Sung, Y.-A. Vitamin D receptor and binding protein polymorphisms in women with polycystic ovary syndrome: A case control study. BMC Endocr. Disord. 2019, 19, 145–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jedrzejuk, D.; Kuliczkowska-Plaksej, J.; Milewicz, A.; Wilczewska, K.; Namiesnik, J.; Rutkowska, A. Bisphenol-A levels are negatively correlated with serum vitamin D-binding protein and sex hormone-binding globulin levels in women with polycystic ovary syndrome: A pilot study. Pol. Arch. Intern. Med. 2019, 129, 133–136. [Google Scholar] [PubMed] [Green Version]
- Cho, S.; Choi, Y.S.; Yim, S.Y.; Yang, H.I.; Jeon, Y.E.; Lee, K.E.; Kim, H.; Seo, S.K.; Lee, B.S. Urinary vitamin D-binding protein is elevated in patients with endometriosis. Hum. Reprod. 2011, 27, 515–522. [Google Scholar] [CrossRef] [Green Version]
- Ferrero, S.; Gillott, D.J.; Anserini, P.; Remorgida, V.; Price, K.M.; Ragni, N.; Grudzinskas, J.G. Vitamin D binding protein in endometriosis. J. Soc. Gynecol. Investig. 2005, 12, 272–277. [Google Scholar] [CrossRef]
- Cho, M.-C.; Kim, J.H.; Jung, M.H.; Cho, I.A.; Jo, H.C.; Shin, J.K.; Lee, S.A.; Choi, W.J.; Lee, J.H. Analysis of vitamin D-binding protein (VDBP) gene polymorphisms in Korean women with and without endometriosis. Clin. Exp. Reprod. Med. 2019, 46, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.C.; Jo, J.Y.; Lee, S.M.; Cho, I.A.; Shin, J.K.; Lee, S.A.; Lee, J.H.; Cho, M.-C.; Choi, W.J. Differences in 25-hydroxy vitamin D and vitamin D-binding protein concentrations according to the severity of endometriosis. Clin. Exp. Reprod. Med. 2019, 46, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Hou, H.; Zhang, J.; Chen, D.; Deng, F.; Morse, A.; Qiu, X.; He, P.; Lash, G. Altered decidual and placental catabolism of vitamin D may contribute to the aetiology of spontaneous miscarriage. Placenta 2020, 92, 1–8. [Google Scholar] [CrossRef]
- Gharesi-Fard, B.; Zolghadri, J.; Kamali-Sarvestani, E. Alteration in the expression of proteins in unexplained recurrent pregnancy loss compared with in the normal placenta. J. Reprod. Dev. 2014, 60, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Mekbeb, T. The association of serum proteins with preeclampsia. Ethiop. Med. J. 1990, 28, 9–14. [Google Scholar] [PubMed]
- Kolialexi, A.; Tsangaris, G.T.; Sifakis, S.; Gourgiotis, D.; Katsafadou, A.; Lykoudi, A.; Marmarinos, A.; Mavreli, D.; Pergialiotis, V.; Fexi, D.; et al. Plasma biomarkers for the identification of women at risk for early-onset preeclampsia. Expert Rev. Proteom. 2017, 14, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Gharesi-Fard, B.; Farzaneh, G.-S.; Leila, J.; Jaleh, Z.; Eskandar, K.-S. Presence of auto-antibody against two placental proteins, annexin A1 and vitamin D binding protein, in sera of women with pre-eclampsia. J. Reprod. Immunol. 2013, 99, 10–16. [Google Scholar] [CrossRef]
- Tannetta, D.; Redman, C.W.; Sargent, I.L. Investigation of the actin scavenging system in pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Boil. 2013, 172, 32–35. [Google Scholar] [CrossRef] [Green Version]
- Naidoo, Y.; Moodley, J.; Ramsuran, V.; Naicker, T. Polymorphisms within vitamin D binding protein gene within a preeclamptic South African population. Hypertens. Pregnancy 2019, 38, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.L.; Arnaud, P.; Galbraith, R.M. Evidence of increased Gc: Actin Complexes in pregnant serum: A possible result of trophoblast embolism. Am. J. Reprod. Immunol. 1983, 4, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Albejante, M.C.; Kunz, T.C.M.; Ferreira, M.F.C.; Júnior, J.H.Z.R.; De Almeida, R.J.; Bacigalupo, L.D.S.; Matheus, L.H.G.; Dalboni, M.A.; Camacho, C.P.; Dellê, H. Proteinuria is associated with urinary loss of cubilin and vitamin D-binding protein in patients with preeclampsia. Sci. Rep. 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, O.; Li, W.; Ma, L.; Ping, F.; Chen, L.; Nie, M. Variants in vitamin D binding protein gene are associated with gestational diabetes mellitus. Medicine 2015, 94, e1693. [Google Scholar] [CrossRef]
- Karras, S.N.; Polyzos, S.A.; Newton, D.A.; Wagner, C.L.; Hollis, B.W.; Ouweland, J.V.D.; Dursun, E.; Gezen-Ak, D.; Kotsa, K.; Annweiler, C.; et al. Adiponectin and vitamin D-binding protein are independently associated at birth in both mothers and neonates. Endocrine 2017, 59, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Kook, S.Y.; Park, K.H.; Jang, J.A.; Kim, Y.M.; Park, H.; Jeon, S.J. Vitamin D-binding protein in cervicovaginal fluid as a non-invasive predictor of intra-amniotic infection and impending preterm delivery in women with preterm labor or preterm premature rupture of membranes. PLoS ONE 2018, 13, e0198842. [Google Scholar] [CrossRef]
- D’Silva, A.M.; Hyett, J.A.; Coorssen, J.R. First trimester protein biomarkers for risk of spontaneous preterm birth: Identifying a critical need for more rigorous approaches to biomarker identification and validation. Fetal Diagn. Ther. 2020, 1–10. [Google Scholar] [CrossRef]
- Pereira, L.; Reddy, A.P.; Jacob, T.; Thomas, A.; Schneider, K.A.; Dasari, S.; Lapidus, J.A.; Lu, X.; Rodland, M.; Roberts, J.C.T.; et al. Identification of Novel protein biomarkers of preterm birth in human cervical−vaginal fluid. J. Proteome Res. 2007, 6, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, I.M.; Joner, G.; Jenum, P.A.; Eskild, A.; Brunborg, C.; Torjesen, P.A.; Stene, L.C. Vitamin D-binding protein and 25-hydroxyvitamin D during pregnancy in mothers whose children later developed type 1 diabetes. Diabetes Metab. Res. Rev. 2016, 32, 883–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liong, S.; Di Quinzio, M.; Fleming, G.; Permezel, M.; Georgiou, H.M. Is vitamin D binding protein a novel predictor of labour? PLoS ONE 2013, 8, e76490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouillon, R.; Van Baelen, H.; De Moor, P. 25-Hydroxyvitamin D and its binding protein in maternal and cord serum. J. Clin. Endocrinol. Metab. 1977, 45, 679–684. [Google Scholar] [CrossRef]
- Wookey, A.F.; Chollangi, T.; Yong, H.E.J.; Kalionis, B.; Brennecke, S.; Murthi, P.; Georgiou, H.M. Placental vitamin d-binding protein expression in human idiopathic fetal growth restriction. J. Pregnancy 2017, 2017, 5120267–5120275. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Hansen, R.L.; Hartiala, J.A.; Allayee, H.; Sconberg, J.L.; Schmidt, L.C.; Volk, H.E.; Tassone, F. Selected vitamin D metabolic gene variants and risk for autism spectrum disorder in the CHARGE Study. Early Hum. Dev. 2015, 91, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Tapia, G.; Mårild, K.; Dahl, S.R.; Lund-Blix, N.A.; Viken, M.K.; Lie, B.A.; Njølstad, P.R.; Joner, G.; Skrivarhaug, T.; Cohen, A.S.; et al. Maternal and newborn vitamin D–Binding Protein, Vitamin D levels, vitamin D receptor genotype, and childhood type 1 diabetes. Diabetes Care 2019, 42, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Ding, T.; Hardiman, P.J.; Petersen, I.; Wang, F.-F.; Qu, F.; Baio, G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: A systematic review and meta-analysis. Oncotarget 2017, 8, 96351–96358. [Google Scholar] [CrossRef] [Green Version]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.; Norman, R.; Davies, M. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2009, 25, 544–551. [Google Scholar] [CrossRef] [Green Version]
- Thomson, R.L.; Spedding, S.; Buckley, J.D. Vitamin D in the aetiology and management of polycystic ovary syndrome. Clin. Endocrinol. 2012, 77, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.; Doswell, A.; Krebs, K.; Cipolla, M. Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J. Clin. Endocrinol. Metab. 2014, 99, E1137–E1145. [Google Scholar] [CrossRef] [Green Version]
- Parasar, P.; Özcan, P.; Terry, K.L. Endometriosis: Epidemiology, diagnosis and clinical management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macer, M.L.; Taylor, H.S. Endometriosis and infertility. Obstet. Gynecol. Clin. N. Am. 2012, 39, 535–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, E.; Nisenblat, V.; Farquhar, C.; Fraser, I.; Bossuyt, P.M.; Johnson, N.; Hull, M.L.; Farquhar, C. Urinary biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2015, 2015, 012019. [Google Scholar] [CrossRef]
- Coccia, M.E.; Rizzello, F.; Cozzolino, M.; Turillazzi, V.; Capezzuoli, T. The effect of low-dose ovarian stimulation with HMG plus progesterone on pregnancy outcome in women with history of recurrent pregnancy loss and secondary infertility: A retrospective cohort study. Gynecol. Endocrinol. 2018, 34, 528–531. [Google Scholar] [CrossRef]
- Brown, M.A.; Lindheimer, M.D.; de Swiet, M.; Van Assche, A.; Moutquin, J.M. The classification and diagnosis of the hypertensive disorders of pregnancy: Statement from the international society for the study of hypertension in pregnancy (isshp). Hypertens. Pregnancy 2001, 20, IX–XIV. [Google Scholar] [CrossRef] [Green Version]
- Thornton, C.; Tooher, J.; Ogle, R.; Von Dadelszen, P.; Makris, A.; Hennessy, A. Benchmarking the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2016, 6, 279–284. [Google Scholar] [CrossRef]
- Thornton, C.; Dahlen, H.G.; Korda, A.; Hennessy, A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000–2008. Am. J. Obstet. Gynecol. 2013, 208, 476.e1–476.e5. [Google Scholar] [CrossRef]
- Gharesi-Fard, B.; Zolghadri, J.; Kamali-Sarvestani, E. Proteome differences in the first- and third-trimester human placentas. Reprod. Sci. 2014, 22, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Powe, C.E.; Seely, E.W.; Rana, S.; Bhan, I.; Ecker, J.; Karumanchi, S.A.; Thadhani, R. First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension 2010, 56, 758–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nankervis, A.; Price, S.; Conn, J. Gestational diabetes mellitus: A pragmatic approach to diagnosis and management. Aust. J. Gen. Pract. 2018, 47, 445–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burris, H.H.; Camargo, C.A. Vitamin D and Gestational Diabetes Mellitus. Curr. Diabetes Rep. 2013, 14, 451. [Google Scholar] [CrossRef] [Green Version]
- Mousa, A.; Abell, S.K.; Shorakae, S.; Harrison, C.L.; Naderpoor, N.; Hiam, D.; Moreno-Asso, A.; Stepto, N.; Teede, H.J.; De Courten, B. Relationship between vitamin D and gestational diabetes in overweight or obese pregnant women may be mediated by adiponectin. Mol. Nutr. Food Res. 2017, 61, 1700488. [Google Scholar] [CrossRef]
- Shi, A.; Wen, J.; Liu, G.; Liu, H.; Fu, Z.; Zhou, J.; Zhu, Y.; Liu, Y.; Guo, X.; Xu, J. Genetic variants in vitamin D signaling pathways and risk of gestational diabetes mellitus. Oncotarget 2016, 7, 67788–67795. [Google Scholar] [CrossRef] [Green Version]
- Roth, D.E.; Mahmud, A.; Raqib, R.; Black, R.E.; Baqui, A.H. Pharmacokinetics of a single oral dose of vitamin D3 (70,000 IU) in pregnant and non-pregnant women. Nutr. J. 2012, 11, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAree, T.; Jacobs, B.; Manickavasagar, T.; Sivalokanathan, S.; Brennan, L.; Bassett, P.A.; Rainbow, S.; Blair, M. Vitamin D deficiency in pregnancy-still a public health issue. Matern. Child. Nutr. 2012, 9, 23–30. [Google Scholar] [CrossRef]
- Karlsson, T.; Osmancevic, A.; Jansson, N.; Hulthén, L.; Holmäng, A.; Larsson, I. Increased vitamin D-binding protein and decreased free 25(OH)D in obese women of reproductive age. Eur. J. Nutr. 2013, 53, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Nyomba, B.L.; Bouillon, R.; De Moor, P. Evidence for an interaction of insulin and sex steroids in the regulation of vitamin D metabolism in the rat. J. Endocrinol. 1987, 115, 295–301. [Google Scholar] [CrossRef]
- Verhaeghe, J.; Bouillon, R.; Nyomba, B.L.; Lissens, W.; Van Assche, F.A. Vitamin D and bone mineral homeostasis during pregnancy in the diabetic BB rat. Endocrinology 1986, 118, 1019–1025. [Google Scholar] [CrossRef]
- Ashraf, A.P.; Huisingh, C.; Alvarez, J.A.; Wang, X.; Gower, B.A. Insulin resistance indices are inversely associated with vitamin D binding protein concentrations. J. Clin. Endocrinol. Metab. 2013, 99, 178–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Li, Y.; Li, L.; Yu, F.; Cui, L.; Ba, Y.; Li, W.; Wang, C. Association of the vitamin D binding protein polymorphisms with the risk of type 2 diabetes mellitus: A meta-analysis. BMJ Open 2014, 4, 005617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, J.-A.; Munoz, F.M.; Gonik, B.; Frau, L.; Cutland, C.L.; Mallett-Moore, T.; Kissou, A.; Wittke, F.; Das, M.; Nunes, T.; et al. Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine 2016, 34, 6047–6056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caughey, A.B.; Robinson, J.N.; Norwitz, E.R. Contemporary diagnosis and management of preterm premature rupture of membranes. Rev. Obstet. Gynecol. 2008, 1, 11–22. [Google Scholar] [PubMed]
- Gordijn, S.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Romo, A.; Carceller, R.; Tobajas, J. Intrauterine growth retardation (IUGR): Epidemiology and etiology. Pediatr. Endocrinol. Rev. 2009, 6, 332–336. [Google Scholar]
- Cosmi, E.; Fanelli, T.; Visentin, S.; Trevisanuto, D.; Zanardo, V. Consequences in infants that were intrauterine growth restricted. J. Pregnancy 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M.J. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad. Med. J. 2015, 92, 63–69. [Google Scholar] [CrossRef]
- Blanton, D.; Han, Z.; Bierschenk, L.; Linga-Reddy, M.P.; Wang, H.; Clare-Salzler, M.; Haller, M.; Schatz, D.; Myhr, C.; She, J.-X.; et al. Reduced serum vitamin D–binding protein levels are associated with type 1 diabetes. Diabetes 2011, 60, 2566–2570. [Google Scholar] [CrossRef] [Green Version]
- Ongagna, J.; Pinget, M.; Belcourt, A. Vitamin D-binding protein gene polymorphism association with IA-2 autoantibodies in type 1 diabetes. Clin. Biochem. 2005, 38, 415–419. [Google Scholar] [CrossRef]
- Ongagna, J.C.; Kaltenbacher, M.C.; Sapin, R.; Pinget, M.; Belcourt, A. The HLA-DQB alleles and amino acid variants of the vitamin D-binding protein in diabetic patients in Alsace. Clin. Biochem. 2001, 34, 59–63. [Google Scholar] [CrossRef]
- Johnson, C.P.; Myers, S.M. Identification and evaluation of children with autism spectrum disorders. Pediatrics 2007, 120, 1183–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannell, J. Vitamin D and autism, what’s new? Rev. Endocr. Metab. Disord. 2017, 18, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Saad, K.; Abdel-Rahman, A.A.; Elserogy, Y.M.; Al-Atram, A.A.; Cannell, J.J.; Bjørklund, G.; Abdel-Reheim, M.K.; Othman, H.A.K.; Houfey, A.A.E.; El-Aziz, N.H.R.A.; et al. Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutr. Neurosci. 2015, 19, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, G.; Henley, K.; Green, J. Autism: Will vitamin D supplementation during pregnancy and early childhood reduce the recurrence rate of autism in newborn siblings? Med. Hypotheses 2016, 88, 74–78. [Google Scholar] [CrossRef]
- Grant, C.C.; Stewart, A.W.; Scragg, R.; Milne, T.; Rowden, J.; Ekeroma, A.; Wall, C.; Mitchell, E.A.; Crengle, S.; Trenholme, A.; et al. Vitamin D during pregnancy and infancy and infant serum 25-hydroxyvitamin D concentration. Pediatrics 2013, 133, e143–e153. [Google Scholar] [CrossRef] [Green Version]
- Farrell, C.-J.L.; Martin, S.; McWhinney, B.; Straub, I.; Williams, P.; Herrmann, M. State-of-the-art vitamin D assays: A comparison of automated immunoassays with liquid chromatography–tandem mass spectrometry methods. Clin. Chem. 2012, 58, 531–542. [Google Scholar] [CrossRef]
- Thomas, S.; Ross, L.; Ingles, S.; Pandian, R.; Paulson, R.; Bendikson, K. Polyclonal antibody assays are better measurements of vitamin D status in asian women than monoclonal. Fertil. Steril. 2017, 107, e29. [Google Scholar] [CrossRef]
- Aloia, J.F.; Mikhail, M.; Dhaliwal, R.; Shieh, A.; Usera, G.; Stolberg, A.; Ragolia, L.; Islam, S. Free 25(OH)D and the vitamin D paradox in African Americans. J. Clin. Endocrinol. Metab. 2015, 100, 3356–3363. [Google Scholar] [CrossRef]
- Hong, K.; Florkowski, C.M.; Doogue, M.; Elder, P.A.; Lewis, J. A monoclonal antibody sandwich ELISA for vitamin D-binding protein (VDBP) is unaffected by Gc-globulin phenotype peptides and actin and demonstrates reduced levels in sepsis and non-sepsis intensive care patients. Clin. Chim. Acta 2018, 484, 7–13. [Google Scholar] [CrossRef]
| Status | Definition |
|---|---|
| Vitamin D adequacy | ≥ 50 nmol/L * |
| Mild vitamin D deficiency | 30–49 nmol/L |
| Moderate vitamin D deficiency | 12.5–29 nmol/L |
| Severe vitamin D deficiency | <12.5 nmol/L |
| Organisation (Country) | Recommended 25(OH)D Level (nmol/L) | Daily Recommended Supplementation Dose (IU) |
|---|---|---|
| World Health Organization | >50 | 200 |
| Institute of Medicine (US) | ≥30 | 600–1000 |
| Endocrine Society (US) | ≥75 | 1500–2000 |
| ACOG (US) | ≥50 | 600 |
| NICE (UK) | >30 | 400–800 |
| National Institutes of Health (US) | >50 | 600 |
| RANZCOG (Australia/NZ) | >50 | 400–2000 |
| Condition | Author, Year [Reference] | Participants | Key Findings | Implications | Limitations |
|---|---|---|---|---|---|
| Infertility | Franasiak, 2017 [100] | N = 68 women; 39 infertile premenopausal, 29 fertile premenopausal controls | Lower VDBP in the infertile group compared to fertile group | VDBP as a potential biomarker for screening/ diagnosis of infertility | Pilot study; small sample size; limited ethnic diversity |
| In vitro fertilisation (IVF) | Estes, 2009 [101] | N = 20 women; 10 with successful IVF (livebirth), 10 without a successful IVF (no pregnancy) | VDBP reduced in unsuccessful IVF candidates | VDBP as a biomarker for good versus bad responders in IVF | Small sample size, single-centre, use of 2D-PAGE methodology to measure proteins considered less accurate than other methods |
| Polycystic ovary syndrome (PCOS) | Naderpoor, 2018 [106] | N = 149 pre-menopausal women; 90 with PCOS, 59 controls | Lower VDBP in women with PCOS than controls. Similar total, free and bioavailable 25(OH)D levels | VDBP as a potential mechanistic biomarker for the development of PCOS | Small retrospective study; VDBP gene polymorphisms were not studied; androgen levels were not investigated; smaller control group |
| Kuliczkowska-Plaksej, 2019 [107] | N = 267 women aged 20-35 years; 167 with PCOS, 100 controls | Lower serum VDBP levels in obese women with PCOS | Involvement of VDBP in the clinical and biochemical picture of PCOS | Lack of ethnic variation, small sample size | |
| Haldar, 2018 [108] | N = 100 women; 50 diagnosed with PCOS, 50 controls | GC alleles rs7041 and rs2060793 in vitamin D-deficient women increase the risk of PCOS | Better understanding of PCOS, potential use of VDBP genotypes as biomarkers of PCOS | Small sample size, single ethnicity study, lack of investigation into other enzymes and proteins in the vitamin D system | |
| Song, 2019 [109] | N = 1359 women; 432 women with PCOS; 927 controls | Distribution of genotypes and allele frequencies of the VDBP rs4588, rs7041, and rs22822679 polymorphisms did not differ between PCOS and controls | VDBP polymorphisms do not differ between women with and without PCOS, but further studies are required. | Study power less than 80%; may have missed some metabolic differences between groups; did not account for sun exposure or diet; did not measure circulating vitamin D | |
| Jedrzejuk, 2019 [110] | N = 63 women; 27 women with PCOS; 36 controls | No differences in VDBP between women with or without PCOS but VDBP was associated with BPA only in women with PCOS | Relationship between VDBP and BPA may reflect liver dysfunction in women with PCOS | Small sample size, lack of gold standard methods for measuring vitamin D and VDBP | |
| Endometriosis | Lee, 2011 [111] | N = 95 reproductive age women; 57 with endometriosis, 38 controls | Urinary VDBP was elevated in women with endometriosis | VDBP may be involved in the pathophysiology of endometriosis and be a valuable biomarker in detecting the disease alone or in combination with CA-125 | Majority of patients in the control group had various other benign diseases which may impact urinary VDBP levels, use of 2-DE methodology |
| Ferrero, 2005 [112] | N = 145 reproductive age women; 36 untreated mild endometriosis, 52 untreated severe endometriosis, 17 endometriosis treated with oral contraceptives, 40 controls | Reduced expression of one VDBP isoform in peritoneal fluid of women with endometriosis, but improved in women undergoing hormone treatment | VDBP as a biomarker for endometriosis and monitoring treatment of the disease | Small sample size, only patients with mild disease analysed, use of 2-DE methodology with low throughput | |
| Cho, 2019 [113]; and Baek, 2019 [114] | N = 32 women; 9 mild endometriosis; 7 advanced endometriosis; 16 healthy controls (both studies using the same sample, different groupings) | No differences in serum VDBP or in VDBP gene polymorphisms between controls and women with mild or advanced endometriosis | VDBP was not associated with severity of endometriosis; however, further studies are needed | Very small sample size; no assessment of some confounders including sun exposure | |
| Spontaneous miscarriage | Hou, 2020 [115] | N = 42 placentas; 20 from spontaneous miscarriages, 22 from normal pregnancies | VDBP was less expressed in the placenta and decidua in spontaneous miscarriages | VDBP as a potential biomarker for miscarriages and its implications in the pathophysiology of spontaneous miscarriage | Small sample size |
| Unexplained recurrent pregnancy loss (URPL) | Gharesi-Fard, 2014 [116] | N = 10 human placentas; 5 URPL, 5 gestation matched controls | VDBP had increased expression in URPL cases | Understanding into the pathophysiology of URPL and the potential use of VDBP as a biomarker | Very small sample size |
| Pre-eclampsia | Mekbeb, 1990 [117] | N = 239 pregnant women; 107 with pre-eclampsia, 132 controls | Increased expression of GC 2-1 phenotype in women with pre-eclampsia | GC 2-1 phenotype may be an early detection genetic biomarker for placental dysfunction | Modest sample size; outdated technology that has since been advanced with more accurate methods |
| Kolialexi, 2017 [118] | N = 10 pregnant women; 5 with pre-eclampsia, 5 controls | VDBP was upregulated in women with early-onset pre-eclampsia in the first trimester | VDBP as a biomarker for screening for early-onset pre-eclampsia | Lack of accuracy with 2D electrophoresis technique; very small sample size | |
| Ma, 2012 [89] | N = 22 human placentas; 11 from pre-eclamptic pregnancies, 11 from normal pregnancies | Increased oxidative stress as occurs in pre-eclampsia resulted in decreased expression of VDBP | VDBP as a biomarker of states of increased oxidative stress such as in pre-eclampsia | Small sample size; lack of correlation between immunostaining and Western blot results in snap-frozen tissues | |
| Behrouz, 2013 [119] | N = 40 women; 5 placentas, 20 sera from normotensive pregnancies, 20 sera from women with severe pre-eclampsia | VDBP of placental origin may be an autoimmune target in pre-eclampsia | Antibodies against VDBP may be involved in the pathophysiology of pre-eclampsia | Use of a 2D-PAGE technique lacks accuracy due to narrow dynamic range and low throughput; small sample size; small sample size | |
| Tannetta, 2014 [120] | N = 40 women; 10 non-pregnant, 10 normotensive pregnancies, 10 early onset pre-eclampsia, 10 late onset pre-eclampsia | Actin free VDBP was dysregulated in pre-eclampsia and lower in early onset pre-eclampsia than in normal pregnancies | VDBP as a biomarker of the underlying mechanisms of pre-eclampsia | Small, single-centre study; lack of statistical power | |
| Naidoo, 2019 [121] | N = 600 pregnant women; 246 normotensive, 167 early onset and 246 late onset pre-eclampsia | SNPs rs4588 and rs7041 were present more frequently in pre-eclamptic pregnancies | Genetic biomarkers for pre-eclampsia risk | HIV prevalent region; lack of ethnic diversity | |
| Emerson, 1983 [122] | N = 126 pregnant women; 26 with pre-eclampsia, 100 controls | Increased expression of GC:actin complexes in sera of complicated pregnancies compared with normal pregnancies | Reveals role of VDBP in pre-eclampsia and potential use of GC:actin complexes as biomarkers of complicated pregnancies | Small sample size; source of the actin in the GC:actin complexes in pregnant women could not be equivocally established; needs replication to confirm findings | |
| Albejante, 2020 [123] | N = 20 pregnant women; 15 with non-proteinuric pre-eclampsia; 5 with pre-eclampsia with proteinuria; 10 normotensive controls | Significant loss of VDBP in urine of women with pre-eclampsia with proteinuria | Proteinuria and resultant urinary loss of VDBP in preeclamptic pregnancies may promote vitamin D deficiency | Very small sample size and low statistical power | |
| Gestational diabetes mellitus (GDM) | Wang, 2015 [124] | N = 1985 pregnant women; 964 GDM cases, 1021 controls | GC rs16847024 and GC rs3733359 were associated with an increased GDM risk | GC alleles as potential early genetic biomarkers of GDM risk | Single ethnic group; 25(OH)D not measured in all participants; statistical power was insufficient to detect a small effect size |
| Karras, 2018 [125] | N = 70 pairs of neonates and their mothers | Maternal VDBP was strongly correlated with maternal adiponectin and neonatal VDBP and adiponectin | Potential independent interaction between VDBP and adiponectin in mothers and neonates. VDBP may be a marker of metabolic health | Small sample size, no association with birth weight | |
| Xia, 2019 [19] | N = 321; 107 GDM cases; 214 controls | Maternal VDBP was not associated with GDM risk at any gestational period (trimester) | No evidence to support the use of VDBP as a biomarker of GDM risk | Use of monoclonal assays to measure VDBP, potential confounding by sun exposure, diet, etc. | |
| Preterm birth | Kook, 2018 [126] | N = 251 pregnant women; 148 spontaneous preterm labour, 103 premature prelabour rupture of membranes | Increased CVF VDBP predicted imminent spontaneous preterm delivery within 48 h and intra-amniotic infection in women with preterm deliveries | VDBP may be a biomarker for intra-amniotic infection or impending preterm labour. CVF VDBP may regulate host response to intra-amniotic infection | Lacked comparative data to other predictive tests for preterm labour; molecular technique not used to detect microbes; samples not randomly analysed; confounders not assessed (e.g., recent intercourse, bacterial vaginosis) |
| D’Silva, 2020 [127] | N = 88 pregnancies; 44 pregnancies that delivered <37 weeks gestation, 44 pregnancies that delivered >37 weeks | Serum VDBP was significantly reduced in the preterm deliveries compared to the term deliveries | Serum VDBP as biomarker for preterm labour and delivery | 2DE technique, differences in cohort compared to initial study: delivered 3 weeks later, more ethnic diversity | |
| Pereira, 2007 [128,129] | N = 18 pregnant women; 6 preterm labour without preterm delivery, 6 spontaneous preterm birth without infection, 6 controls | VDBP was upregulated in the CVF of women with spontaneous preterm birth compared with controls | VDBP may be a novel biomarker for preterm birth and improved understanding of the pathophysiology involved in preterm labour and delivery | Small sample size; results may be due to genetic/ biological variation which was not accounted for | |
| Liong, 2013 [130] | N = 221 pregnant women; 48 preterm births, 173 normal term births | Increased expression of VDBP up to 3 days prior to premature labour compared to 15–28 days prior. Increased CVF VDBP in impending preterm and term labour. Unlike fetal fibronectin, CVF VDBP levels were not altered by recent sexual intercourse | VDBP levels may be a more accurate and specific biomarker for predicting labour compared with the gold-standard fetal fibronectin | Small sample size; included several multifetal gestations and did not consider the effects of this on VDBP concentrations | |
| Liong, 2013 [18] | N = 15 pregnant women; 5 with PROM, 10 controls | VDBP significantly increased in the women with PROM | VDBP as a potential biomarker for impending PROM | Small sample size; early and late PROM were combined despite likely different pathophysiologies; confounded by inclusion of both singleton and multifetal gestations | |
| Liong, 2015 [17] | N = 12 women with preterm deliveries; independent cohort of 129 women for ELISA validation | Albumin/VDBP ratio was more efficacious than fetal fibronectin in predicting spontaneous preterm labour in symptomatic women within 7 days | VDBP alone or in combination with albumin as a biomarker to predict preterm labour | Small sample size; confounders such as multifetal gestation, recent bleeding or intercourse were excluded but their impact on CVF expression of albumin and VDBP not determined; women with positive fetal fibronectin received tocolytic therapy which may have influenced results | |
| Hypo-vitaminosis D | Bouillon, 1977 [131] | N = 30 maternal–fetal pairs | Fetal cord blood had lower total 25(OH)D and VDBP but higher free vitamin D than maternal blood | Impaired transport of VDBP may result in neonatal vitamin D deficiency; low VDBP intrauterine state is not favourable for the storage of vitamin D in the fetus | Small sample size; outdated technology that has since been improved with more accurate methods |
| Fetal growth restriction (FGR) | Wookey, 2017 [132] | N = 35 human placentas; 18 from pregnancies complicated by FGR, 17 from normal pregnancies | Significantly lower placental VDBP in pregnancies complicated with FGR | VDBP as a potential biomarker for placental dysfunction and FGR | Small sample size; samples only obtained after delivery for analysis, well after peak expression of vitamin D and establishment of placental function; no patient-matched serum samples were used |
| Reduced birthweight | Chun, 2017 [84] | N = 356 paired pregnant women and their neonates | Reduced vitamin D was associated with low birthweight in carriers of GC rs12512631 allele | GC allelic variants as potential genetic predictive biomarkers for low birthweight | VDBP level not calculated; unclear mechanism by which certain GC variants modify the relationship between vitamin D and birthweight |
| Autism spectrum disorder | Schmidt, 2015 [133] | N = 1581 children and their parents; 341 children with autism, 1240 controls | rs4588 was associated with the development of Autism spectrum disorder | Potential use of GC allelic variants as risk or even diagnostic markers for autism | Some missing data on paternal genotypes; paternal vitamin D status and levels were not evaluated |
| Type 1 diabetes mellitus (T1DM) | Sorensen, 2016 [129] | N = 333 pregnant women; 113 whose offspring later developed T1DM, 220 controls | Low maternal VDBP in the third trimester was associated with an increased risk of T1DM in the offspring | VDBP as a potential biomarker for T1DM risk | Confounders such as ethnicity not considered; some samples underwent freeze-thaw cycles which may have altered sample integrity |
| Tapia, 2019 [134] | N= 767; 189 mother/child pairs where the child later developed T1DM, 576 random control mother/child pairs | Low maternal VDBP levels at birth were associated with an increased risk of T1DM in offspring | VDBP as a biomarker for metabolic risk in offspring such as T1DM | Observational study, possible presence of unknown confounding factors, low external validity due to primarily Caucasian population |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernando, M.; Ellery, S.J.; Marquina, C.; Lim, S.; Naderpoor, N.; Mousa, A. Vitamin D-Binding Protein in Pregnancy and Reproductive Health. Nutrients 2020, 12, 1489. https://doi.org/10.3390/nu12051489
Fernando M, Ellery SJ, Marquina C, Lim S, Naderpoor N, Mousa A. Vitamin D-Binding Protein in Pregnancy and Reproductive Health. Nutrients. 2020; 12(5):1489. https://doi.org/10.3390/nu12051489
Chicago/Turabian StyleFernando, Melinda, Stacey J. Ellery, Clara Marquina, Siew Lim, Negar Naderpoor, and Aya Mousa. 2020. "Vitamin D-Binding Protein in Pregnancy and Reproductive Health" Nutrients 12, no. 5: 1489. https://doi.org/10.3390/nu12051489






