Mulberry Fruit Extract Promotes Serum HDL-Cholesterol Levels and Suppresses Hepatic microRNA-33 Expression in Rats Fed High Cholesterol/Cholic Acid Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of High Hydrostatic Pressure-Treated Mulberry Fruit Extract
2.2. Animals and Experimental Design
2.3. Determination of Serum Metabolic Parameters
2.4. Hepatic and Fecal Lipid Analysis
2.5. Fecal Bile Acid Analysis
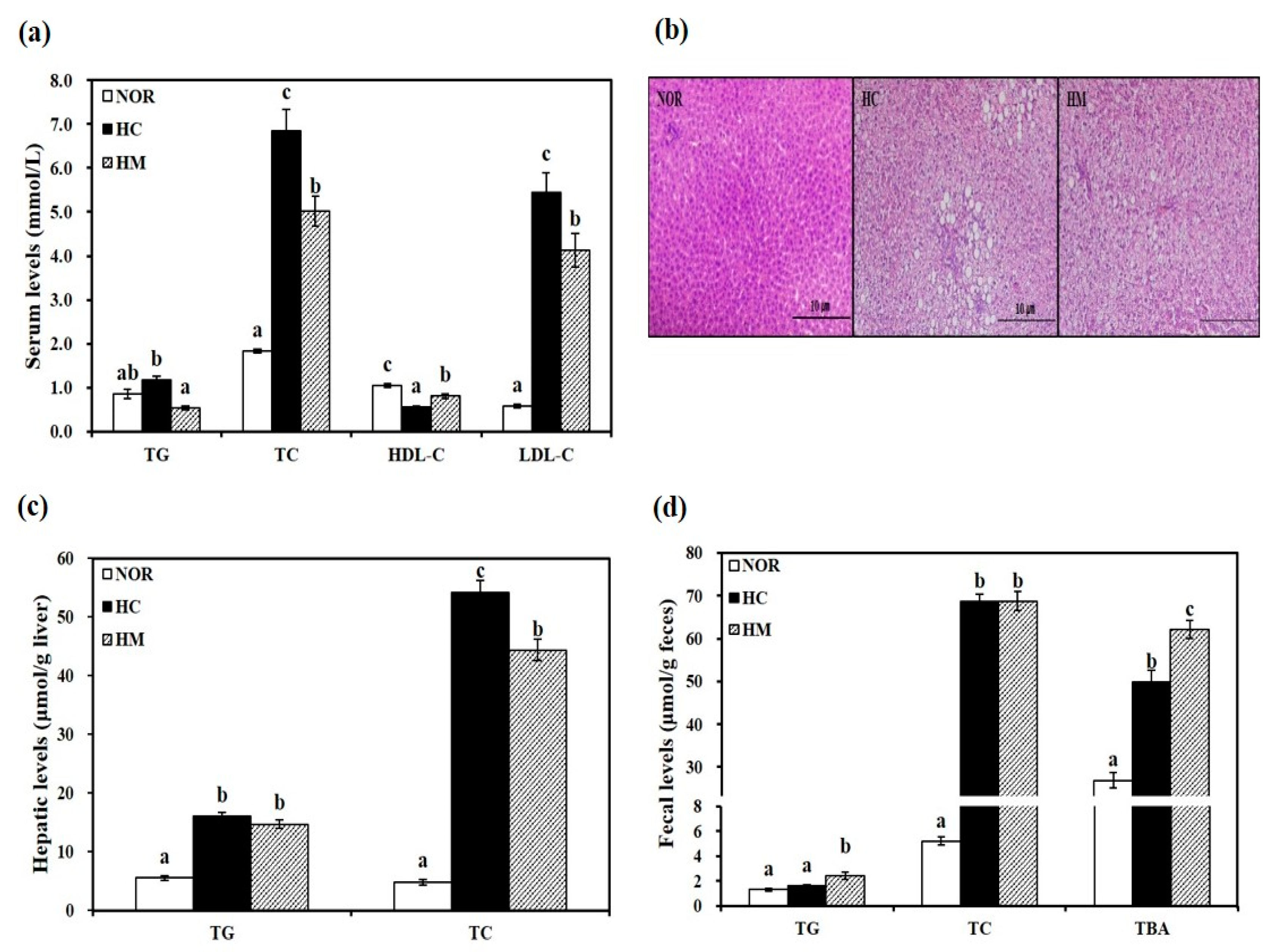
2.6. Histological Analysis
2.7. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Statistical Analysis
3. Results
3.1. Effects of Mulberry Fruit Extract on Body Weight, Food Intake, and Adipose Tissue Mass
3.2. Effects of Mulberry Fruit Extract on Liver Weight and Serum AST and ALT Activities
3.3. Effects of Mulberry Fruit Extract on Serum Lipid Parameters
3.4. Effects of Mulberry Fruit Extract on Hepatic Lipid Profiles
3.5. Effects of Mulberry Fruit Extract on Fecal Lipid Profiles and Bile Acid Excretion
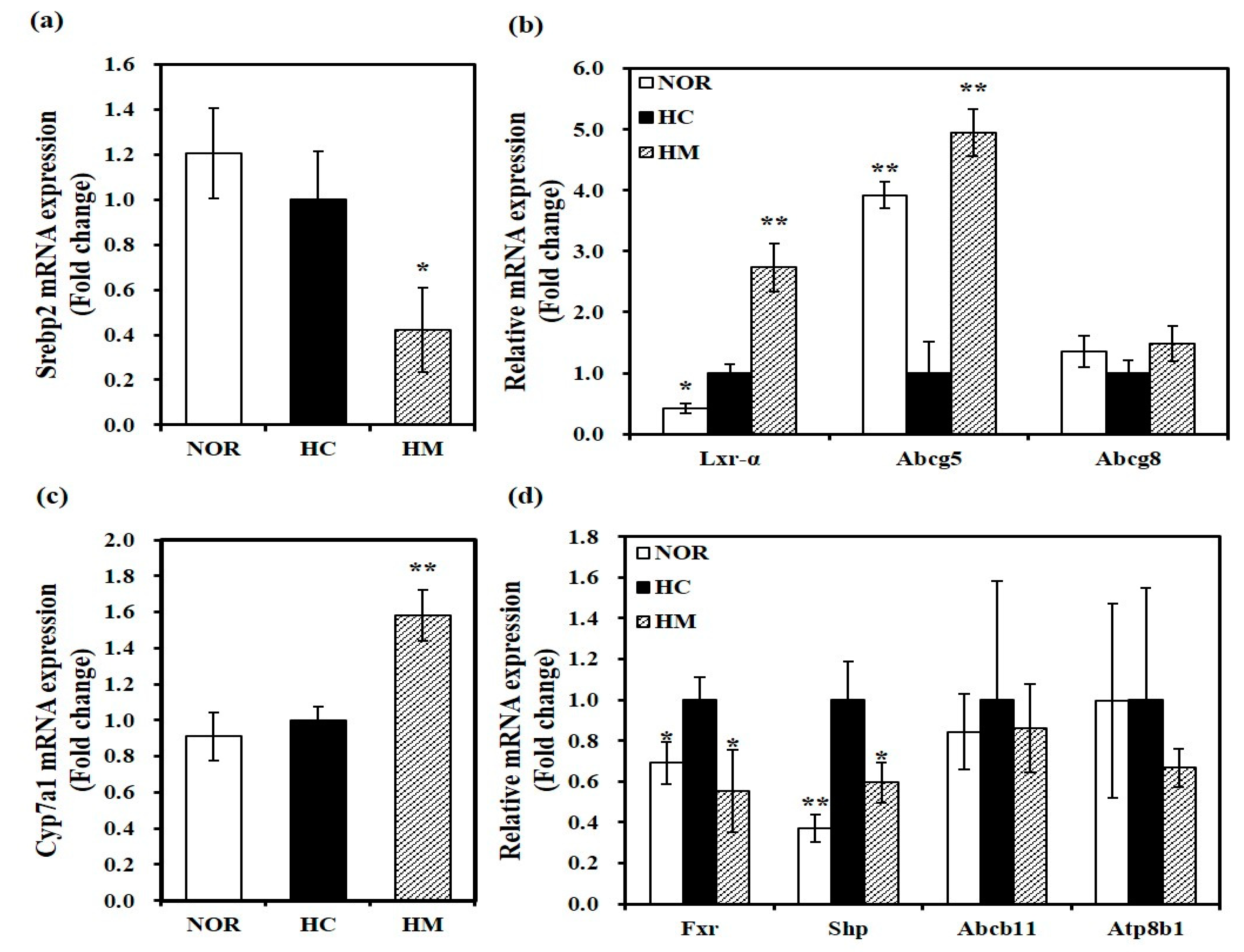
3.6. Effect of Mulberry Fruit Extract on Hepatic Gene Expression related to Cholesterol Metabolism and Bile Acid Synthesis
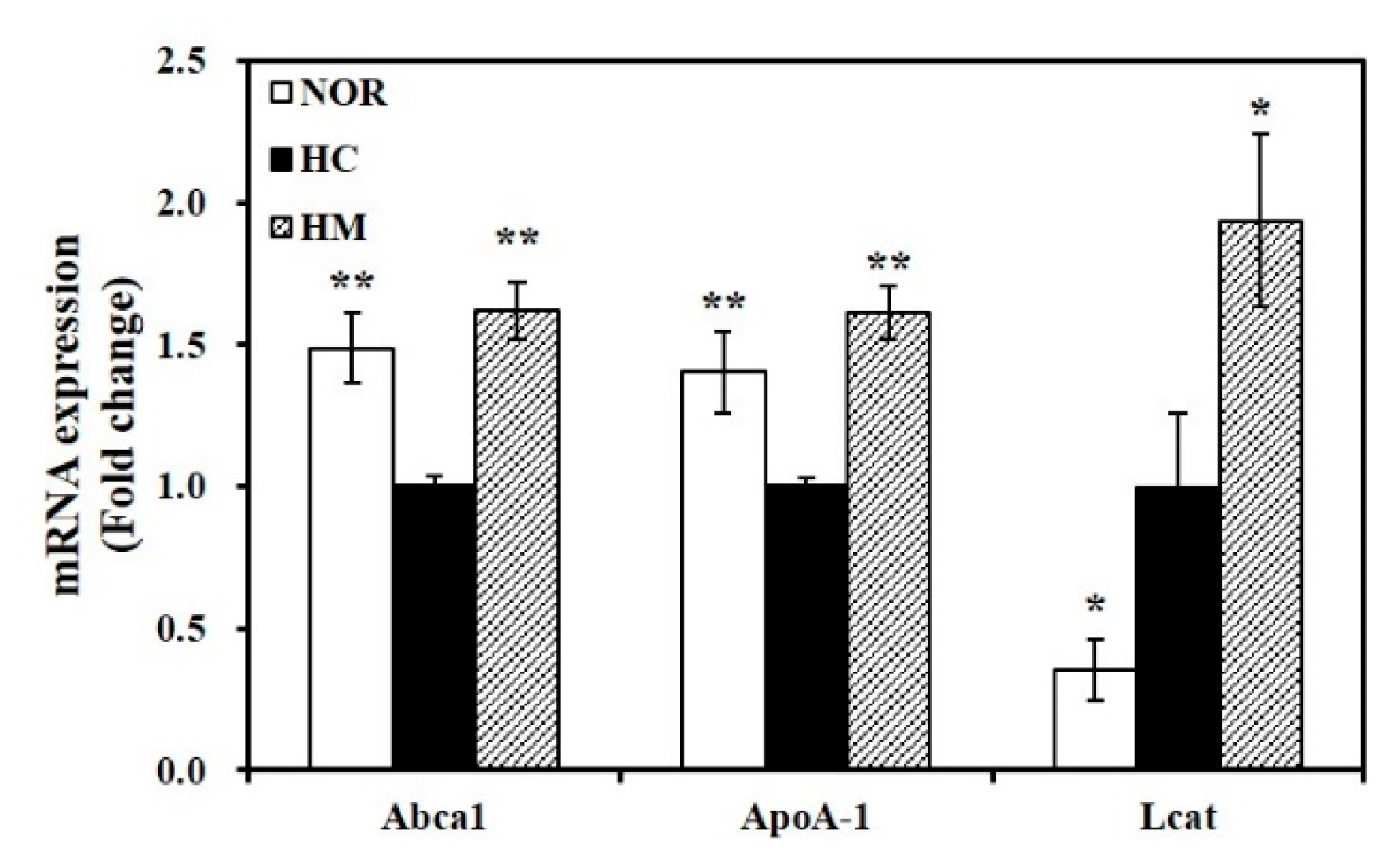
3.7. Effect of Mulberry Fruit Extract on Hepatic Gene Expression related to HDL Formation
3.8. Effect of Mulberry Fruit Extract on Hepatic miR-33 Expresion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barquera, S.; Pedroza-Tobias, A.; Medina, C.; Hernandez-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Hearts: Technical Package for Cardiovascular Disease Management in Primary Health Care; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, D.J.; Rifkind, B.M. High-density lipoprotein—The clinical implications of recent studies. N. Engl. J. Med. 1989, 321, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.; Gotto, A.M.; LaRosa, J.C.; Maroni, J.; Szarek, M.; Grundy, S.M.; Kastelein, J.J.; Bittner, V.; Fruchart, J.C. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 2007, 357, 1301–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rye, K.A.; Barter, P.J. Regulation of high-density lipoprotein metabolism. Circ. Res. 2014, 114, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Rosenson, R.S.; Brewer, H.B., Jr.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef]
- Groen, A.K.; Bloks, V.W.; Verkade, H.; Kuipers, F. Cross-talk between liver and intestine in control of cholesterol and energy homeostasis. Mol. Asp. Med. 2014, 37, 77–88. [Google Scholar] [CrossRef]
- Madison, B.B. Srebp2: A master regulator of sterol and fatty acid synthesis. J. Biol. Chem. 2016, 57, 333–335. [Google Scholar] [CrossRef] [Green Version]
- Repa, J.J.; Berge, K.E.; Pomajzl, C.; Richardson, J.A.; Hobbs, H.; Mangelsdorf, D.J. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 2002, 277, 18793–18800. [Google Scholar] [CrossRef] [Green Version]
- Zelcer, N.; Hong, C.; Boyadjian, R.; Tontonoz, P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 2009, 325, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Peet, D.J.; Turley, S.D.; Ma, W.; Janowski, B.A.; Lobaccaro, J.M.; Hammer, R.E.; Mangelsdorf, D.J. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell 1998, 93, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Sarenac, T.M.; Mikov, M. Bile Acid Synthesis: From Nature to the Chemical Modification and Synthesis and Their Applications as Drugs and Nutrients. Front. Pharmacol. 2018, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Rohrl, C.; Stangl, H. Cholesterol metabolism-physiological regulation and pathophysiological deregulation by the endoplasmic reticulum. Wien. Med. Wochenschr. 2018, 168, 280–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oram, J.F.; Heinecke, J.W. ATP-binding cassette transporter A1: A cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 2005, 85, 1343–1372. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gulshan, K.; Brubaker, G.; Hazen, S.L.; Smith, J.D. ABCA1 mediates unfolding of apolipoprotein AI N terminus on the cell surface before lipidation and release of nascent high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1197–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czarnecka, H.; Yokoyama, S. Regulation of cellular cholesterol efflux by lecithin:cholesterol acyltransferase reaction through nonspecific lipid exchange. J. Biol. Chem. 1996, 271, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit—A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Bao, T.; Gowd, V. Mulberry Fruit Extract Affords Protection against Ethyl Carbamate-Induced Cytotoxicity and Oxidative Stress. Oxid. Med. Cell. Longev. 2017, 2017, 1594963. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.H.; Lee, H.A.; Park, M.H.; Han, J.S. Mulberry (Morus alba L.) Fruit Extract Containing Anthocyanins Improves Glycemic Control and Insulin Sensitivity via Activation of AMP-Activated Protein Kinase in Diabetic C57BL/Ksj-db/db Mice. J. Med. Food 2016, 19, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, M.S.; Choi, A.J.; Kim, C.T.; Kim, Y. Anti-Inflammatory Effects of High Hydrostatic Pressure Extract of Mulberry (Morus alba) Fruit on LPS-Stimulated RAW264.7 Cells. Molecules 2019, 24, 1425. [Google Scholar] [CrossRef] [Green Version]
- Ju, W.-T.; Kwon, O.-C.; Lee, M.-K.; Kim, H.-B.; Sung, G.-B.; Kim, Y.-S. Quali-quantitative analysis of flavonoids for mulberry leaf and fruit of ‘Suhyang’. Korean J. Environ. Agric. 2017, 36, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K. Food processing by high hydrostatic pressure. Biosci. Biotechnol. Biochem. 2017, 81, 672–679. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Du, B.L.; Cui, Z.W.; Xu, L.P.; Li, C.Y. Effects of high hydrostatic pressure and thermal processing on bioactive compounds, antioxidant activity, and volatile profile of mulberry juice. Food Sci. Technol. Int. 2017, 23, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Karathanasis, S.K.; Freeman, L.A.; Gordon, S.M.; Remaley, A.T. The Changing Face of HDL and the Best Way to Measure It. Clin. Chem. 2017, 63, 196–210. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Bao, T.; Chen, W. Comparison of the protective effect of black and white mulberry against ethyl carbamate-induced cytotoxicity and oxidative damage. Food Chem. 2018, 243, 65–73. [Google Scholar] [CrossRef]
- Liu, C.J.; Lin, J.Y. Anti-inflammatory effects of phenolic extracts from strawberry and mulberry fruits on cytokine secretion profiles using mouse primary splenocytes and peritoneal macrophages. Int. Immunopharmacol. 2013, 16, 165–170. [Google Scholar] [CrossRef]
- Park, Y.-S.; Kang, S.-S.; Choi, H.-J.; Yang, S.-J.; Shon, H.-H.; Seo, H.-H.; Jeong, J.-M. Effect of mulberry (Morus alba L.) extract on blood flow improvement. J. Korean Soc. Food Sci. Nutr. 2014, 43, 498–506. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Dai, M.; Nie, W.-J.; Yang, X.-R.; Zeng, X.-C. Effects of the ethanol extract of black mulberry (Morus nigra L.) fruit on experimental atherosclerosis in rats. J. Ethnopharmacol. 2017, 200, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.K.; Chou, F.P.; Chen, Y.C.; Chyau, C.C.; Ho, H.H.; Wang, C.J. Effects of mulberry (Morus alba L.) extracts on lipid homeostasis in vitro and in vivo. J. Agric. Food Chem. 2009, 57, 7605–7611. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Dai, G.; Zheng, X. Mulberry anthocyanin extract ameliorates insulin resistance by regulating PI3K/AKT pathway in HepG2 cells and db/db mice. J. Nutr. Biochem. 2016, 36, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, F.; Nematbakhsh, M.; Monajemi, R.; Pezeshki, Z.; Talebi, A.; Zolfaghari, B.; Mansoori, A.; Saberi, S.; Dehghani, A.; Ashrafi, F. Effect of pomegranate flower extract on cisplatin-induced nephrotoxicity in rats. J. Nephropathol. 2014, 3, 133. [Google Scholar] [PubMed]
- Widowati, W.; Ratnawati, H.; Mozefis, T.; Pujimulyani, D.; Yelliantty, Y. Hypolipidemic and antioxidant effects of black tea extract and quercetin in atherosclerotic rats. Int. J. Med. Pharm. Sci. Eng. 2013, 7, 373–380. [Google Scholar]
- Loffredo, L.; Perri, L.; Nocella, C.; Violi, F. Antioxidant and antiplatelet activity by polyphenol-rich nutrients: Focus on extra virgin olive oil and cocoa. Br. J. Clin. Pharmacol. 2017, 83, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonardo, A.; Sookoian, S.; Chonchol, M.; Loria, P.; Targher, G. Cardiovascular and systemic risk in nonalcoholic fatty liver disease—Atherosclerosis as a major player in the natural course of NAFLD. Curr. Pharm. Des. 2013, 19, 5177–5192. [Google Scholar] [CrossRef]
- Lu, T.T.; Makishima, M.; Repa, J.J.; Schoonjans, K.; Kerr, T.A.; Auwerx, J.; Mangelsdorf, D.J. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell. 2000, 6, 507–515. [Google Scholar] [CrossRef]
- Guan, G.; Dai, P.; Shechter, I. Differential transcriptional regulation of the human squalene synthase gene by sterol regulatory element-binding proteins (SREBP) 1a and 2 and involvement of 5′ DNA sequence elements in the regulation. J. Biol. Chem. 1998, 273, 12526–12535. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Hammer, R.E.; Li-Hawkins, J.; Von Bergmann, K.; Lutjohann, D.; Cohen, J.C.; Hobbs, H.H. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. USA 2002, 99, 16237–16242. [Google Scholar] [CrossRef] [Green Version]
- Pullinger, C.R.; Eng, C.; Salen, G.; Shefer, S.; Batta, A.K.; Erickson, S.K.; Verhagen, A.; Rivera, C.R.; Mulvihill, S.J.; Malloy, M.J.; et al. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J. Clin. Investig. 2002, 110, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Matozel, M.; Boehme, S.; Kong, B.; Nilsson, L.M.; Guo, G.; Ellis, E.; Chiang, J.Y. Overexpression of cholesterol 7alpha-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology 2011, 53, 996–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figge, A.; Lammert, F.; Paigen, B.; Henkel, A.; Matern, S.; Korstanje, R.; Shneider, B.L.; Chen, F.; Stoltenberg, E.; Spatz, K.; et al. Hepatic overexpression of murine Abcb11 increases hepatobiliary lipid secretion and reduces hepatic steatosis. J. Biol. Chem. 2004, 279, 2790–2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulusma, C.C.; Groen, A.; Kunne, C.; Ho-Mok, K.S.; Spijkerboer, A.L.; Rudi de Waart, D.; Hoek, F.J.; Vreeling, H.; Hoeben, K.A.; van Marle, J.; et al. Atp8b1 deficiency in mice reduces resistance of the canalicular membrane to hydrophobic bile salts and impairs bile salt transport. Hepatology 2006, 44, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, P.; Hierro, L.; Jara, P.; Alvarez, L. Knockdown of ATP8B1 expression leads to specific downregulation of the bile acid sensor FXR in HepG2 cells: Effect of the FXR agonist GW4064. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1119–G1129. [Google Scholar] [CrossRef] [Green Version]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [Green Version]
- Saleheen, D.; Scott, R.; Javad, S.; Zhao, W.; Rodrigues, A.; Picataggi, A.; Lukmanova, D.; Mucksavage, M.L.; Luben, R.; Billheimer, J.; et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. Lancet Diabetes Endocrinol. 2015, 3, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.W.; Zheng, L.; Wang, Q. Regulation of cholesterol homeostasis by liver X receptors. Clinica Chimica Acta 2010, 411, 617–625. [Google Scholar] [CrossRef]
- Singaraja, R.R.; Fievet, C.; Castro, G.; James, E.R.; Hennuyer, N.; Clee, S.M.; Bissada, N.; Choy, J.C.; Fruchart, J.C.; McManus, B.M.; et al. Increased ABCA1 activity protects against atherosclerosis. J. Clin. Investig. 2002, 110, 35–42. [Google Scholar] [CrossRef]
- Brunham, L.R.; Singaraja, R.R.; Duong, M.; Timmins, J.M.; Fievet, C.; Bissada, N.; Kang, M.H.; Samra, A.; Fruchart, J.C.; McManus, B.; et al. Tissue-specific roles of ABCA1 influence susceptibility to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Paszty, C.; Maeda, N.; Verstuyft, J.; Rubin, E.M. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J. Clin. Investig. 1994, 94, 899–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tietjen, I.; Hovingh, G.K.; Singaraja, R.; Radomski, C.; McEwen, J.; Chan, E.; Mattice, M.; Legendre, A.; Kastelein, J.J.; Hayden, M.R. Increased risk of coronary artery disease in Caucasians with extremely low HDL cholesterol due to mutations in ABCA1, APOA1, and LCAT. Biochim. Biophys. Acta 2012, 1821, 416–424. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Marquart, T.J.; Allen, R.M.; Ory, D.S.; Baldan, A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 12228–12232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafi-Shoushtari, S.H.; Kristo, F.; Li, Y.; Shioda, T.; Cohen, D.E.; Gerszten, R.E.; Naar, A.M. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 2010, 328, 1566–1569. [Google Scholar] [CrossRef] [Green Version]
- Rayner, K.J.; Suarez, Y.; Davalos, A.; Parathath, S.; Fitzgerald, M.L.; Tamehiro, N.; Fisher, E.A.; Moore, K.J.; Fernandez-Hernando, C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science 2010, 328, 1570–1573. [Google Scholar] [CrossRef] [Green Version]

| Variables | NOR | HC | HM |
|---|---|---|---|
| Initial body weight (g) | 243.09 ± 3.18 | 242.18 ± 1.88 | 240.57 ± 1.26 |
| Final body weight (g) | 402.00 ± 6.70 | 404.97 ± 4.87 | 406.95 ± 4.59 |
| Body weight gain (g/4 week) | 158.90 ± 6.18 | 162.79 ± 4.83 | 166.39 ± 5.17 |
| Food intake (g/day) | 24.03 ± 0.49 | 24.06 ± 0.56 | 25.14 ± 0.19 |
| Energy intake (kcal/day) | 90.67 ± 1.83 | 89.37 ± 2.07 | 92.97 ± 0.71 |
| Food efficiency 1) | 0.24 ± 0.01 | 0.24 ± 0.00 | 0.24 ± 0.01 |
| Epididymal fat weight (g/100g body weight) | 1.52 ± 0.10 | 1.39 ± 0.06 | 1.37 ± 0.03 |
| Liver weight (g/100g body weight) | 3.28 ± 0.06 a | 5.17 ± 0.09 b | 5.46 ± 0.18 b |
| Serum ALT (IU/L) | 6.02 ± 0.25 | 8.75 ± 0.95 | 7.13 ± 0.91 |
| Serum AST (IU/L) | 35.88 ± 1.50 a | 41.54 ± 2.55 ab | 45.47 ± 3.00 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lee, M.-S.; Chang, E.; Lee, Y.; Lee, J.; Kim, J.; Kim, C.-T.; Kim, I.-H.; Kim, Y. Mulberry Fruit Extract Promotes Serum HDL-Cholesterol Levels and Suppresses Hepatic microRNA-33 Expression in Rats Fed High Cholesterol/Cholic Acid Diet. Nutrients 2020, 12, 1499. https://doi.org/10.3390/nu12051499
Lee S, Lee M-S, Chang E, Lee Y, Lee J, Kim J, Kim C-T, Kim I-H, Kim Y. Mulberry Fruit Extract Promotes Serum HDL-Cholesterol Levels and Suppresses Hepatic microRNA-33 Expression in Rats Fed High Cholesterol/Cholic Acid Diet. Nutrients. 2020; 12(5):1499. https://doi.org/10.3390/nu12051499
Chicago/Turabian StyleLee, Soojin, Mak-Soon Lee, Eugene Chang, Yoonjin Lee, Jaerin Lee, Jiyeon Kim, Chong-Tai Kim, In-Hwan Kim, and Yangha Kim. 2020. "Mulberry Fruit Extract Promotes Serum HDL-Cholesterol Levels and Suppresses Hepatic microRNA-33 Expression in Rats Fed High Cholesterol/Cholic Acid Diet" Nutrients 12, no. 5: 1499. https://doi.org/10.3390/nu12051499
APA StyleLee, S., Lee, M.-S., Chang, E., Lee, Y., Lee, J., Kim, J., Kim, C.-T., Kim, I.-H., & Kim, Y. (2020). Mulberry Fruit Extract Promotes Serum HDL-Cholesterol Levels and Suppresses Hepatic microRNA-33 Expression in Rats Fed High Cholesterol/Cholic Acid Diet. Nutrients, 12(5), 1499. https://doi.org/10.3390/nu12051499





