Vertebral Bone Marrow Fat Is independently Associated to VAT but Not to SAT: KORA FF4—Whole-Body MR Imaging in a Population-Based Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Magnetic Resonance Imaging
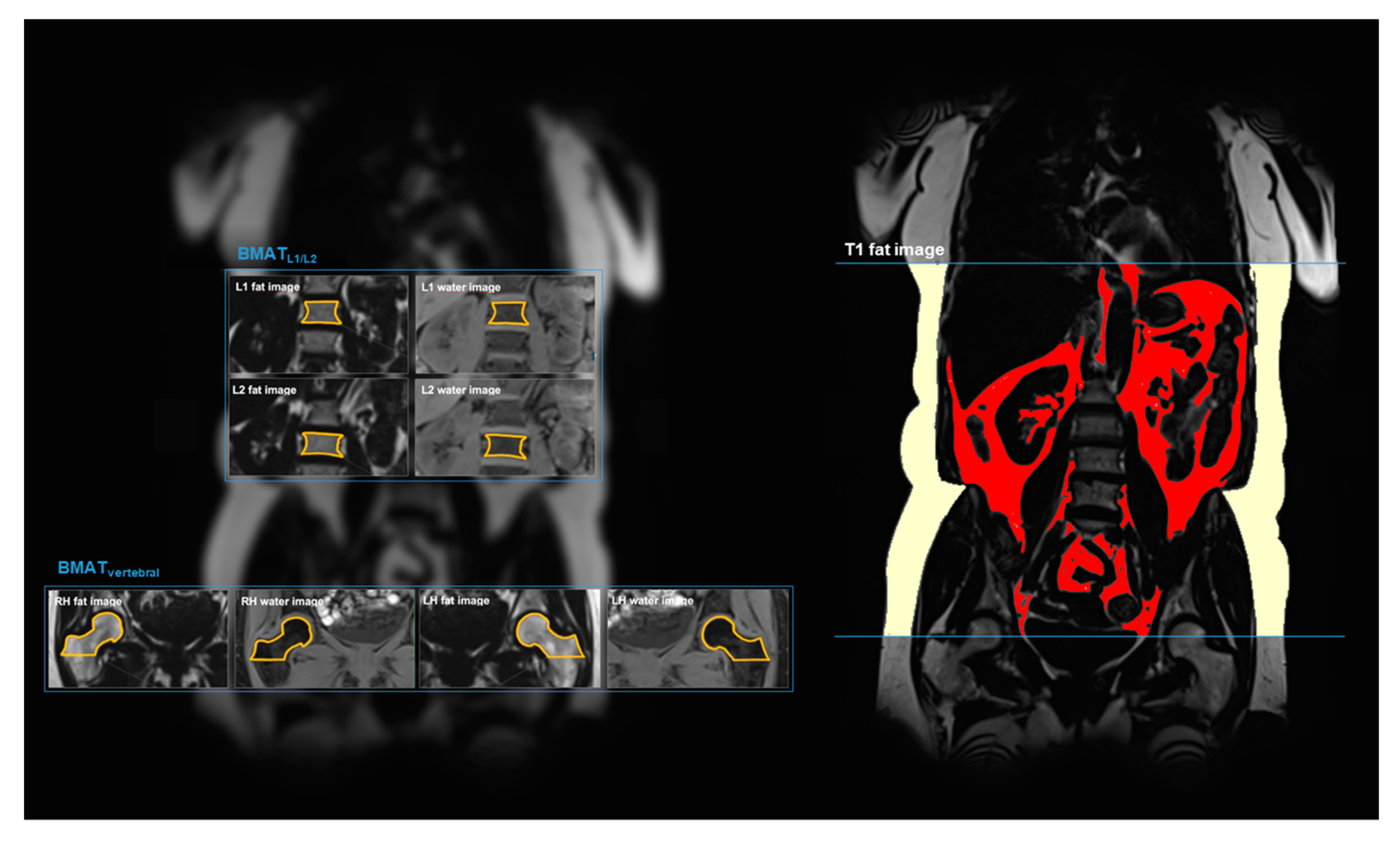
2.3. MR Image Analysis: Bone Marrow Adipose Tissue (BMAT)
2.4. MR Image Analysis: Visceral and Subcutaneous Adipose Tissue (VAT and SAT)
2.5. Demographics and Physical Activity
2.6. Statistical Analysis
3. Results
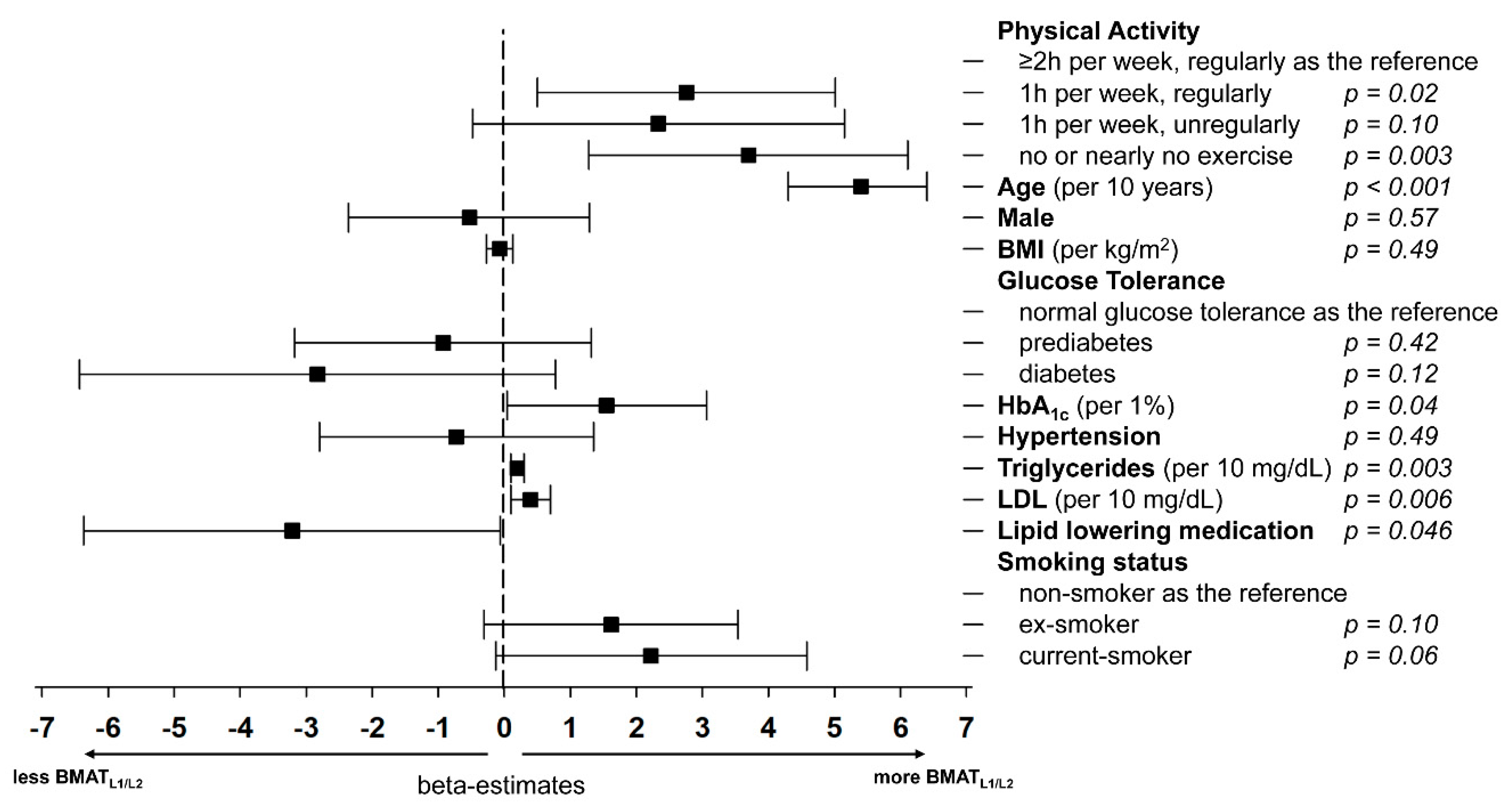
3.1. Vertebral BMAT and Its Association to Demographics and Risk Factors
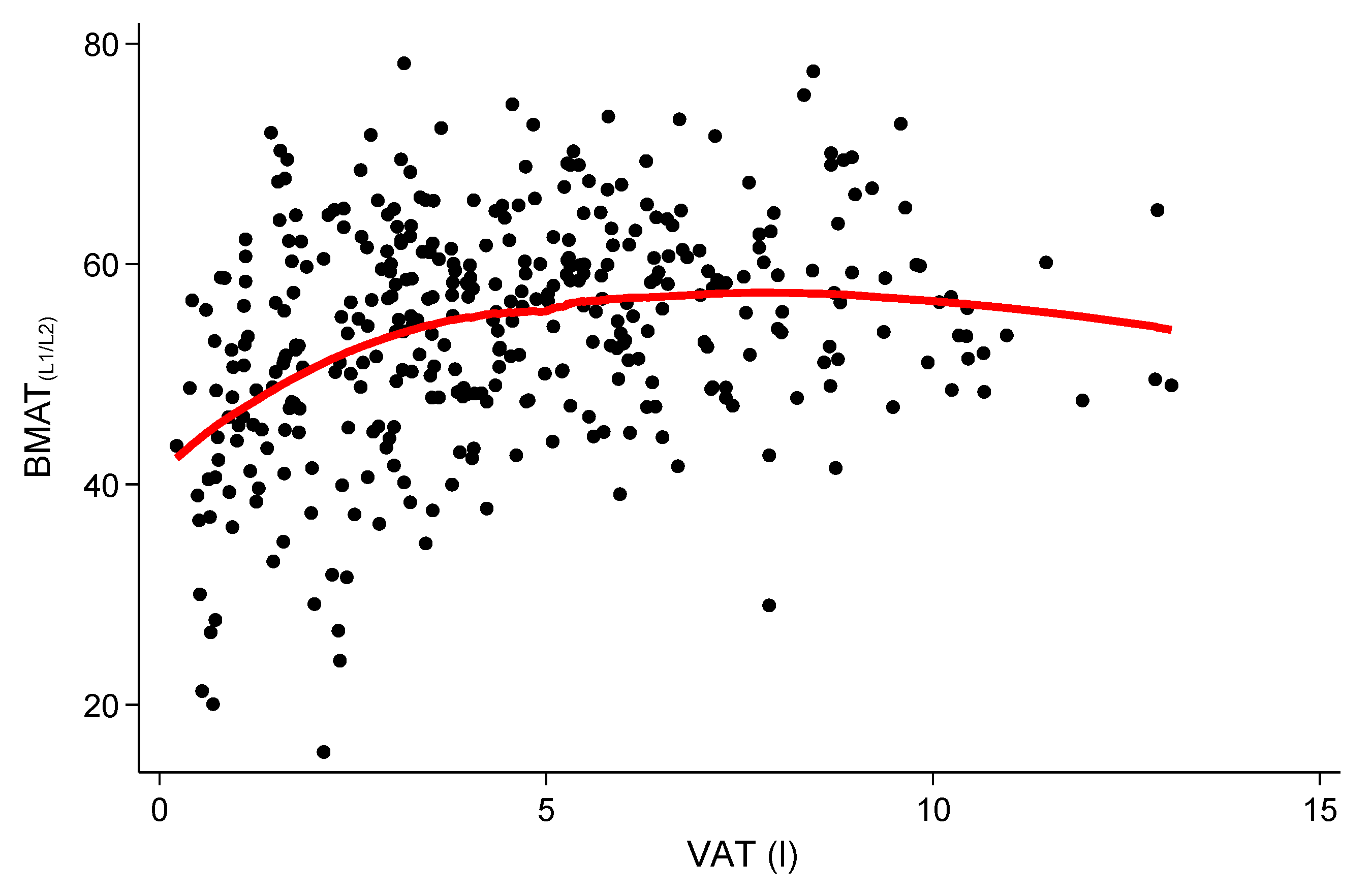
3.2. Relationship of Vertebral BMAT with VAT and SAT
3.3. Relationship of Femoral BMAT with VAT and SAT
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pitchai Balakumara, K.; Jagadeesh, G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 2016, 113, 600–609. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Horvat, P.M.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal Visceral and Subcutaneous Adipose Tissue Compartments Association With Metabolic Risk Factors in the Framingham Heart. Circulation 2007, 116, 39–48. [Google Scholar]
- Davidson, F.E.; Matsha, T.E.; Erasmus, R.T.; Kengne, A.P.; Goedecke, J.H. The discriminatory power of visceral adipose tissue area vs anthropometric measures as a diagnostic marker for metabolic syndrome in South African women. Diabetol. Metab. Syndr. 2019, 11, 93. [Google Scholar] [CrossRef]
- Abraham, T.M.; Pedley, A.; Massaro, J.M.; Hoffmann, U.; Fox, C.S. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation 2015, 132, 1639–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storz, C.; Heber, S.D.; Rospleszcz, S.; Machann, J.; Sellner, S.; Nikolaou, K.; Lorbeer, R.; Gatidis, S.; Elser, S.; Peters, A.; et al. The role of visceral and subcutaneous adipose tissue measurements and their ratio by magnetic resonance imaging in subjects with prediabetes, diabetes and healthy controls from a general population without cardiovascular disease. Br. J. Radiol. 2018, 91, 20170808. [Google Scholar] [CrossRef]
- Schneider, M.; Janas, G.; Lugauer, F.; Hoppe, E.; Nickel, D.; Dale, B.M.; Kiefer, B.; Maier, A.; Bashir, M.R. Accurate fatty acid composition estimation of adipose tissue in the abdomen based on bipolar multi-echo MRI. Magn. Reson. Med. 2019, 81, 2330–2346. [Google Scholar] [CrossRef]
- Schlett, C.L.; Hendel, T.; Weckbach, S.; Reiser, M.; Kauczor, H.U.; Nikolaou, K.; Gunther, M.; Forsting, M.; Hosten, N.; Volzke, H.; et al. Population-Based Imaging and Radiomics: Rationale and Perspective of the German National Cohort MRI Study. Rofo 2016, 188, 652–661. [Google Scholar] [CrossRef] [Green Version]
- Bamberg, F.; Kauczor, H.U.; Weckbach, S.; Schlett, C.L.; Forsting, M.; Ladd, S.C.; Greiser, K.H.; Weber, M.A.; Schulz-Menger, J.; Niendorf, T.; et al. Whole-Body MR Imaging in the German National Cohort: Rationale, Design, and Technical Background. Radiology 2015, 277, 206–220. [Google Scholar] [CrossRef] [Green Version]
- Bertheau, R.C.; Lorbeer, R.; Nattenmuller, J.; Wintermeyer, E.; Machann, J.; Linkohr, B.; Peters, A.; Bamberg, F.; Schlett, C.L. Bone marrow fat fraction assessment in regard to physical activity: KORA FF4-3-T MR imaging in a population-based cohort. Eur. Radiol. 2020, 1–12. [Google Scholar] [CrossRef]
- Hardouin, P.; Pansini, V.; Cortet, B. Bone Marrow Fat. Jt. Bone Spine 2014, 81, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, Y.; Kang, N. Marrow Adipose Tissue: Its Origin, Function, and Regulation in Bone Remodeling and Regeneration. Stem Cells Int. 2018, 2018, 7098456. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Leng, Y.; Gong, Y. Bone Marrow Fat and Hematopoiesis. Front. Endocrinol. 2018, 9, 694. [Google Scholar] [CrossRef]
- Holle, R.; Happich, M.; Löwel, H.; Wichmann, H.E.; MONICA/KORA Study Group. KORA—A research platform for population based health research. Gesundheitswesen 2005, 67, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamberg, F.; Hetterich, H.; Rospleszcz, S.; Lorbeer, R.; Auweter, S.D.; Schlett, C.L.; Schafnitzel, A.; Bayerl, C.; Schindler, A.; Saam, T.; et al. Subclinical Disease Burden as Assessed by Whole-Body MRI in Subjects with Prediabetes, Subjects with Diabetes, and Normal Control Subjects from the General Population: The KORA-MRI Study. Diabetes 2017, 66, 158–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trudel, G.; Payne, M.; Mädler, B.; Ramachandran, N.; Lecompte, M.; Wade, C.; Biolo, G.; Blanc, S.; Hughson, R.; Bear, L.; et al. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: The Women International Space Simulation for Exploration study. J. Appl. Physiol. 2009, 107, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Belavy, D.L.; Quittner, M.J.; Ridgers, N.D.; Shiekh, A.; Rantalainen, T.; Trudel, G. Specific Modulation of Vertebral Marrow Adipose Tissue by Physical Activity. J. Bone Miner. Res. 2018, 33, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Li, G.W.; Xu, Z.; Chen, Q.W.; Tian, Y.N.; Wang, X.Y.; Zhou, L.; Chang, S.X. Quantitative evaluation of vertebral marrow adipose tissue in postmenopausal female using MRI chemical shift-based water-fat separation. Clin. Radiol. 2014, 69, 254–262. [Google Scholar] [CrossRef]
- Fallah, F.; Machann, J.; Martirosian, P.; Bamberg, F.; Schick, F.; Yang, B. Comparison of T1-weighted 2D TSE, 3D SPGR, and two-point 3D Dixon MRI for automated segmentation of visceral adipose tissue at 3 Tesla. MAGMA 2017, 30, 139–151. [Google Scholar] [CrossRef]
- Di Iorgi, N.; Mittelman, S.D.; Gilsanz, V. Differtial Effect of Marrow Adiposity and Visceral and Subcuataneous Fat on Cardiovascular Risk in Young, Healthy Adults. Int. J. Obes. 2008, 32, 1854–1860. [Google Scholar] [CrossRef] [Green Version]
- Bredella, M.A.; Torriani, M.; Ghomi, R.H.; Thomas, B.J.; Brick, D.J.; Gerweck, A.V.; Rosen, C.J.; Klibanski, A.; Miller, K.K. Vertebral Bone Marrow Fat Is Positively Associated with Visceral Fat and Inversely Associated with IGF-1 in Obese Women. Obesity 2011, 19, 49–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baum, T.; Yap, S.P.; Karampinos, D.C.; Nardo, L.; Kuo, D.; Burghardt, A.J.; Masharani, U.B.; Schwartz, A.V.; Li, X.; Link, T.M. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J. Magn. Reson. Imaging 2012, 35, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardouin, P.; Rharass, T.; Lucas, S. Bone Marrow Adipose Tissue: To Be or Not To Be a Typical Adipose Tissue? Front. Endocrinol. 2016, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A.; Fazeli, P.K.; Daley, S.M.; Miller, K.K.; Rosen, C.J.; Klibanski, A.; Torriani, M. Marrow fat composition in anorexia nervosa. Bone 2014, 66, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Schafer, A.L.; Li, X.; Schwartz, A.V.; Tufts, L.S.; Wheeler, A.L.; Grunfeld, C.; Stewart, L.; Rogers, S.J.; Carter, J.T.; Posselt, A.M.; et al. Changes in Vertebral Bone Marrow Fat and Bone Mass after Gastric Bypass Surgery: A Pilot Study. Bone 2015, 74, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Machann, J.; Stefan, N.; Wagner, R.; Bongers, M.; Schleicher, E.; Fritsche, A.; Haring, H.U.; Nikolaou, K.; Schick, F. Intra- and interindividual variability of fatty acid unsaturation in six different human adipose tissue compartments assessed by (1) H-MRS in vivo at 3 T. NMR Biomed. 2017, 30, e3744. [Google Scholar] [CrossRef]
- Gilsanz, V.; Chalfant, J.; Mo, A.O.; Lee, D.C.; Dorey, F.J.; Mittelman, S.D. Reciprocal Relations of Subcutaneous and Visceral Fat to Bone Structure and Strength. J. Clin. Endocrinol. 2009, 94, 3387–3393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karampinos, D.C.; Melkus, G.; Baum, T.; Bauer, J.S.; Rummeny, E.J.; Krug, R. Bone marrow fat quantification in the presence of trabecular bone: Initial comparison between water-fat imaging and single-voxel MRS. Magn. Reson. Med. 2014, 71, 1158–1165. [Google Scholar] [CrossRef] [Green Version]
| All | Lower Tertile BMATL1/L2 | Mid Tertile BMATL1/L2 | Higher Tertile BMATL1/L2 | p-Value | |
|---|---|---|---|---|---|
| (15.7–50.7%) | (50.8–59.1%) | (59.2–78.2%) | |||
| N | 378 | 126 | 126 | 126 | |
| Age (years) | 56.0 (±9.1) | 50.6 (±7.9) | 56.9 (±8.7) | 60.6 (±7.6) | <0.001 |
| Female (%) | 159 (42.1%) | 54 (42.9%) | 48 (38.1%) | 57 (45.2%) | 0.51 |
| BMI (kg/m2) | 28.1 (±4.8) | 27.7 (±5.2) | 28.7 (±5.1) | 27.9 (±4) | 0.22 |
| Glucose tolerance | 0.02 | ||||
| Normal glucose tolerance | 230 (60.9%) | 90 (71.4%) | 67 (53.2%) | 73 (57.9%) | |
| Prediabetes | 95 (25.1%) | 27 (21.4%) | 38 (30.2%) | 30 (23.8%) | |
| Diabetes | 53 (14.0%) | 9 (7.1%) | 21 (16.7%) | 23 (18.3%) | |
| HbA1c (%) | 5.6 (±0.7) | 5.4 (±0.8) | 5.7 (±0.7) | 5.7 (±0.7) | <0.001 |
| Hypertension | 127 (33.6%) | 30 (23.8%) | 48 (38.1%) | 49 (38.9%) | 0.02 |
| Systolic RR (mmHg) | 121.1 (±16.6) | 117.5 (±16.5) | 122.2 (±18.0) | 123.5 (±14.7) | 0.01 |
| Diastolic RR (mmHg) | 75.6 (±9.9) | 74.3 (±9.8) | 76.6 (±10.8) | 76.0 (±9.0) | 0.16 |
| Antihypertensive medication | 93 (24.6%) | 22 (17.5%) | 32 (25.4%) | 39 (31%) | 0.04 |
| Triglyceride levels (mg/dL) | 132.4 (±85.8) | 112.1 (±62.3) | 138 (±85.9) | 147 (±101.4) | 0.003 |
| Total cholesterol (mg/dL) | 217.9 (±36.2) | 205.7 (±31.6) | 220.9 (±36.3) | 227.1 (±37.5) | <0.001 |
| HDL (mg/dL) | 61.6 (±17.4) | 62.0 (±18.4) | 60.5 (±17.8) | 62.1 (±16.1) | 0.72 |
| LDL (mg/dL) | 140 (±32.9) | 130.4 (±27.8) | 143.5 (±35.0) | 146 (±33.6) | 0.001 |
| Lipid lowering medication | 38 (10.1%) | 7 (5.6%) | 11 (8.7%) | 20 (15.9%) | 0.02 |
| Smoking status | 0.15 | ||||
| Non-smoker | 138 (36.5%) | 57 (45.2%) | 39 (31%) | 42 (33.3%) | |
| Ex-smoker | 163 (43.1%) | 48 (38.1%) | 60 (47.6%) | 55 (43.7%) | |
| Current-smoker | 77 (20.4%) | 21 (16.7%) | 27 (21.4%) | 29 (23%) |
| Vertebral Bone Marrow Adipose Tissue (BMATL1/L2) | ||
|---|---|---|
| β (95% CI) | p-Value | |
| Visceral Adipose Tissue (VAT) | ||
| Univariate | 1.07 (0.71–1.43) | <0.001 |
| Simple model | 0.66 (0.25–1.06) | 0.002 |
| Fully adjusted model | 0.53 (0.07–1.00) | 0.03 |
| Subcutaneous Adipose Tissue (SAT) | ||
| Univariate | 0.24 (−0.04–0.52) | 0.09 |
| Simple model | 0.10 (−0.16–0.35) | 0.44 |
| Fully adjusted model | 0.03 (−0.24–0.30) | 0.80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasic, D.; Lorbeer, R.; Bertheau, R.C.; Machann, J.; Rospleszcz, S.; Nattenmüller, J.; Rathmann, W.; Peters, A.; Bamberg, F.; Schlett, C.L. Vertebral Bone Marrow Fat Is independently Associated to VAT but Not to SAT: KORA FF4—Whole-Body MR Imaging in a Population-Based Cohort. Nutrients 2020, 12, 1527. https://doi.org/10.3390/nu12051527
Hasic D, Lorbeer R, Bertheau RC, Machann J, Rospleszcz S, Nattenmüller J, Rathmann W, Peters A, Bamberg F, Schlett CL. Vertebral Bone Marrow Fat Is independently Associated to VAT but Not to SAT: KORA FF4—Whole-Body MR Imaging in a Population-Based Cohort. Nutrients. 2020; 12(5):1527. https://doi.org/10.3390/nu12051527
Chicago/Turabian StyleHasic, Dunja, Roberto Lorbeer, Robert C. Bertheau, Jürgen Machann, Susanne Rospleszcz, Johanna Nattenmüller, Wolfgang Rathmann, Annette Peters, Fabian Bamberg, and Christopher L. Schlett. 2020. "Vertebral Bone Marrow Fat Is independently Associated to VAT but Not to SAT: KORA FF4—Whole-Body MR Imaging in a Population-Based Cohort" Nutrients 12, no. 5: 1527. https://doi.org/10.3390/nu12051527
APA StyleHasic, D., Lorbeer, R., Bertheau, R. C., Machann, J., Rospleszcz, S., Nattenmüller, J., Rathmann, W., Peters, A., Bamberg, F., & Schlett, C. L. (2020). Vertebral Bone Marrow Fat Is independently Associated to VAT but Not to SAT: KORA FF4—Whole-Body MR Imaging in a Population-Based Cohort. Nutrients, 12(5), 1527. https://doi.org/10.3390/nu12051527





