Human Milk Oligosaccharide Supplementation Affects Intestinal Barrier Function and Microbial Composition in the Gastrointestinal Tract of Young Sprague Dawley Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Dietary Treatment
2.2. Oral Glucose Tolerance Test
2.3. Insulin Tolerance Test
2.4. Intestinal Permeability Test
2.5. Final Body Composition, and Blood and Tissue Collection
2.6. Bacterial DNA Extraction and Microbiota Analysis
2.7. Tissue Gene Expression Using Real-Time PCR
2.8. Statistical Analysis
3. Results
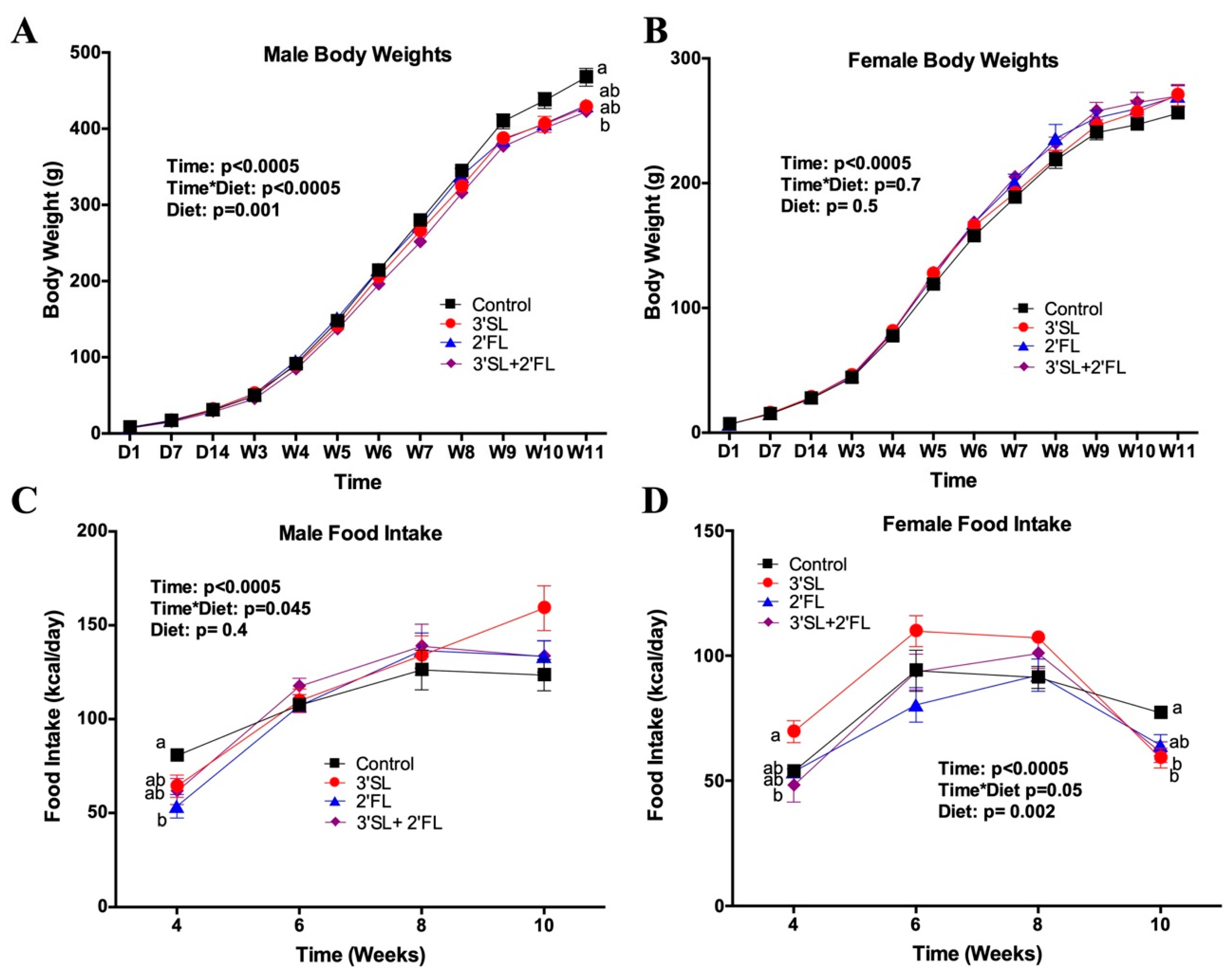
3.1. Bodyweight, Body Composition, Food Intake, and Serum Leptin
3.2. Intestinal Weight
3.3. Glucose and Insulin Tolerance Tests
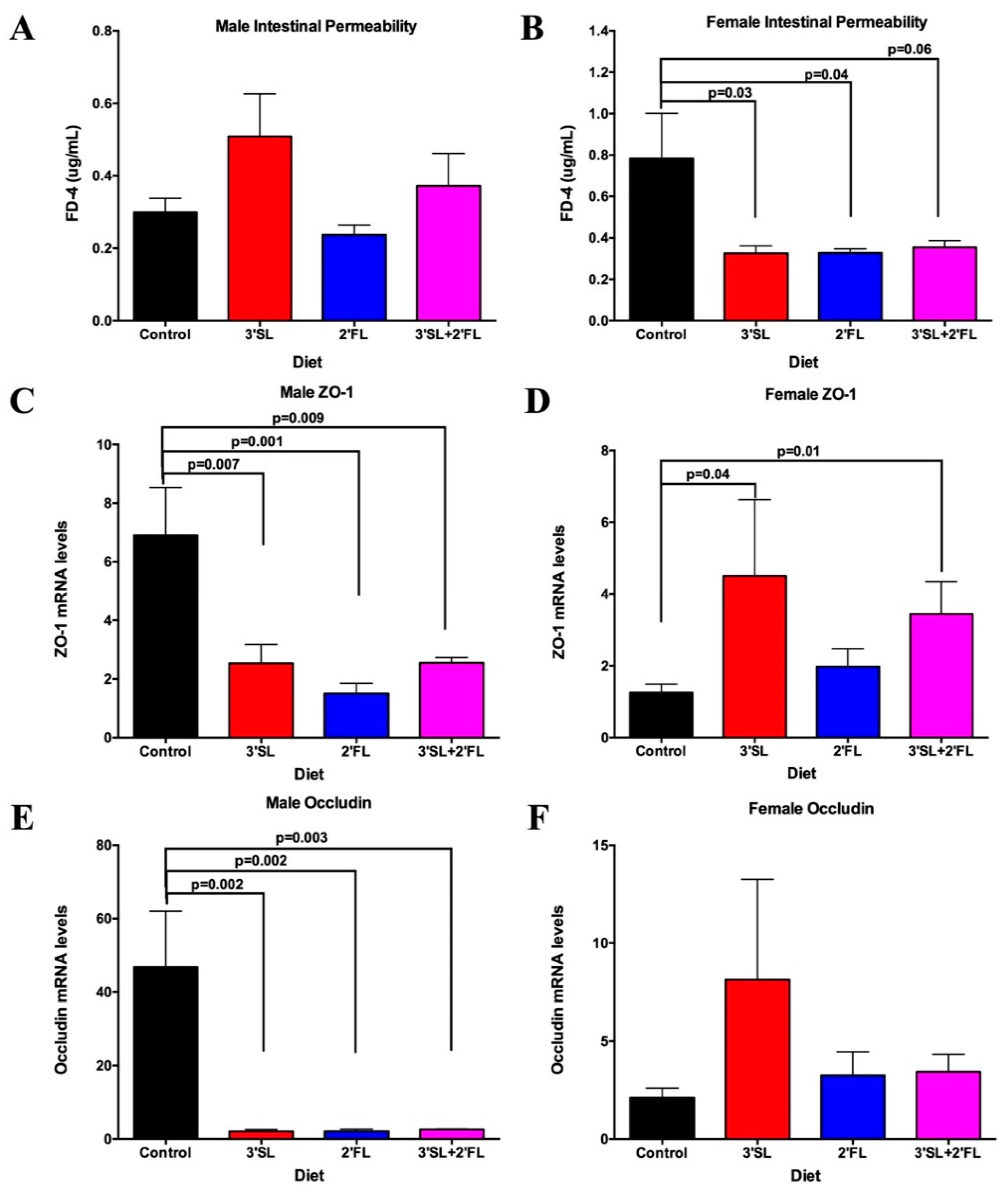
3.4. Intestinal Permeability and Inflammatory Biomarkers
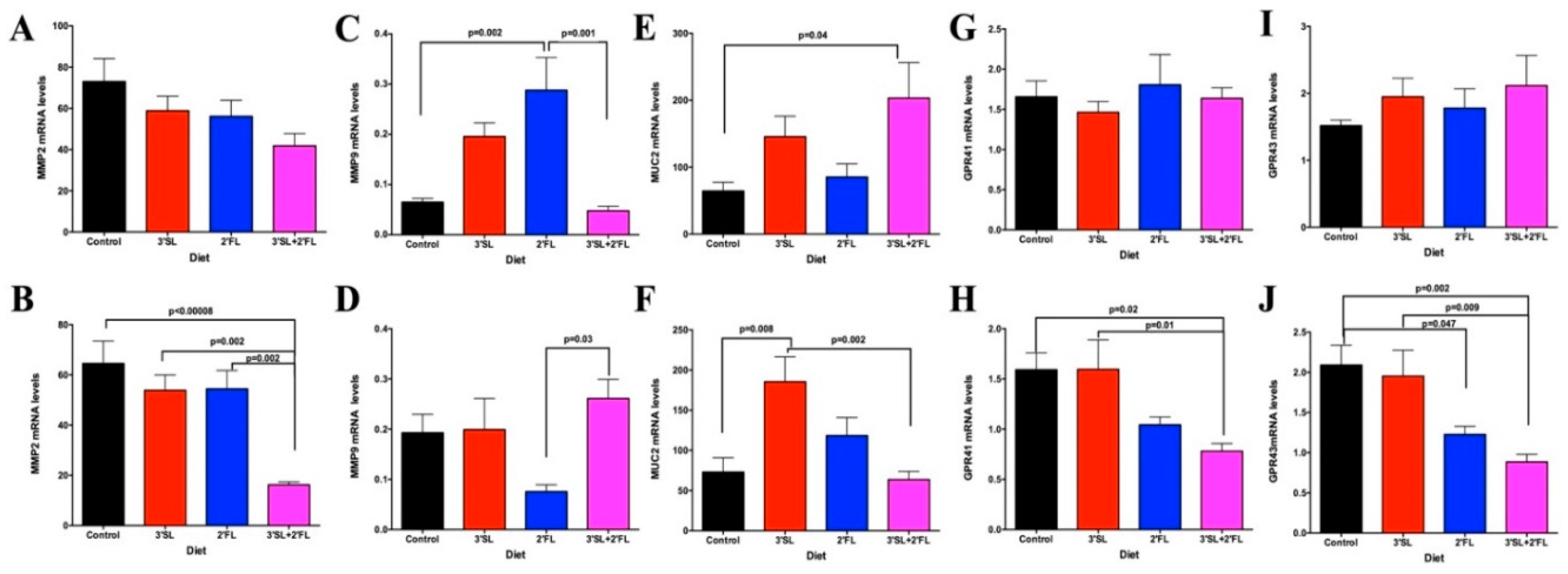
3.5. Colon and Jejunum PCR
3.6. Gut Microbial Profiling: qPCR and 16S rRNA Sequencing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chong, C.Y.L.; Bloomfield, F.H.; O’Sullivan, J.M. Factors Affecting Gastrointestinal Microbiome Development in Neonates. Nutrients 2018, 10, 274. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.N.; Basile, L.A.; Ebeling, M.; Wagner, C.L. Intestinal Permeability in Preterm Infants by Feeding Type: Mother’s Milk Versus Formula. Breastfeed. Med. 2009, 4, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Infant Formula Market Trends-Share Analysis Report 2019–2025. Available online: https://www.gminsights.com/industry-analysis/infant-formula-market (accessed on 16 December 2019).
- Cai, X.; Wardlaw, T.; Brown, D.W. Global trends in exclusive breastfeeding. Int. Breastfeed. J. 2012, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubold, A.M. Historical-qualitative analysis of breastfeeding trends in three OECD countries. Int. Breastfeed. J. 2019, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [Green Version]
- Zivkovic, A.M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc. Natl. Acad. Sci. USA 2011, 108, 4653–4658. [Google Scholar] [CrossRef] [Green Version]
- Marx, C.; Bridge, R.; Wolf, A.K.; Rich, W.; Kim, J.H.; Bode, L. Human milk oligosaccharide composition differs between donor milk and mother’s own milk in the NICU. J. Hum. Lact. Off. J. Int. Lact. Consult. Assoc. 2014, 30, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Sela, D.A.; Mills, D.A. Nursing our microbiota: Molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010, 18, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.C.C.; Totten, S.M.; Huang, J.O.; Nagshbandi, S.; Kirmiz, N.; Garrido, D.A.; Lewis, Z.T.; Wu, L.D.; Smilowitz, J.T.; German, J.B.; et al. Identification of Oligosaccharides in Feces of Breast-fed Infants and Their Correlation with the Gut Microbial Community. Mol. Cell. Proteom. MCP 2016, 15, 2987–3002. [Google Scholar] [CrossRef] [Green Version]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Morrow, A.L. Human Milk Glycans Protect Infants against Enteric Pathogens. Annu. Rev. Nutr. 2005, 25, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Nighot, P.; Al-Sadi, R.; Rawat, M.; Guo, S.; Watterson, D.M.; Ma, T. Matrix metalloproteinase 9-induced increase in intestinal epithelial tight junction permeability contributes to the severity of experimental DSS colitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 309, G988–G997. [Google Scholar] [CrossRef] [Green Version]
- Allaire, J.M.; Morampudi, V.; Crowley, S.M.; Stahl, M.; Yu, H.; Bhullar, K.; Knodler, L.A.; Bressler, B.; Jacobson, K.; Vallance, B.A. Frontline defenders: Goblet cell mediators dictate host-microbe interactions in the intestinal tract during health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G360–G377. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.Y.; Li, B.; Koike, Y.; Määttänen, P.; Miyake, H.; Cadete, M.; Johnson-Henry, K.C.; Botts, S.R.; Lee, C.; Abrahamsson, T.R.; et al. Human Milk Oligosaccharides Increase Mucin Expression in Experimental Necrotizing Enterocolitis. Mol. Nutr. Food Res. 2019, 63, 1800658. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chen, S.; Deng, B.; Tan, C.; Deng, J.; Zhu, G.; Yin, Y.; Ren, W. Implication of G Protein-Coupled Receptor 43 in Intestinal Inflammation: A Mini-Review. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52. [Google Scholar] [CrossRef]
- Lis-Kuberka, J.; Orczyk-Pawiłowicz, M. Sialylated Oligosaccharides and Glycoconjugates of Human Milk. The Impact on Infant and Newborn Protection, Development and Well-Being. Nutrients 2019, 11, 306. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, E.; Barranco, A.; Ramirez, M.; Gruart, A.; Delgado-Garcia, J.M.; Jimenez, M.L.; Buck, R.; Rueda, R. Dietary 2′-Fucosyllactose Enhances Operant Conditioning and Long-Term Potentiation via Gut-Brain Communication through the Vagus Nerve in Rodents. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Tarr, A.J.; Galley, J.D.; Fisher, S.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3′Sialyllactose and 6′Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut-brain axis. Brain. Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nettleton, J.E.; Klancic, T.; Schick, A.; Choo, A.C.; Shearer, J.; Borgland, S.L.; Chleilat, F.; Mayengbam, S.; Reimer, R.A. Low-Dose Stevia (Rebaudioside A) Consumption Perturbs Gut Microbiota and the Mesolimbic Dopamine Reward System. Nutrients 2019, 11, 1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bomhof, M.R.; Paul, H.A.; Geuking, M.B.; Eller, L.K.; Reimer, R.A. Improvement in adiposity with oligofructose is modified by antibiotics in obese rats. FASEB J. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, H.A.; Collins, K.H.; Bomhof, M.R.; Vogel, H.J.; Reimer, R.A. Potential Impact of Metabolic and Gut Microbial Response to Pregnancy and Lactation in Lean and Diet-Induced Obese Rats on Offspring Obesity Risk. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, T.K.; Hirota, C.L.; Menard, D.; MacNaughton, W.K.; Buret, A.G. Interleukin-18 disrupts tight junctions in gastric and intestinal epithelial monolayers. FASEB J. 2010, 24, 348–356. [Google Scholar] [CrossRef]
- German, J.B.; Freeman, S.L.; Lebrilla, C.B.; Mills, D.A. Human Milk Oligosaccharides: Evolution, Structures and Bioselectivity as Substrates for Intestinal Bacteria. Nestle Nutr. Workshop Ser. Paediatr. Programme 2008, 62, 205–222. [Google Scholar] [CrossRef] [Green Version]
- Agostoni, C.; Braegger, C.; Decsi, T.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Mihatsch, W.; Moreno, L.A.; Puntis, J.; Shamir, R.; et al. Breast-feeding: A Commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of Human Milk Oligosaccharides by Gut-Related Microbes. J. Agric. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef] [Green Version]
- Gueimonde, M.; Laitinen, K.; Salminen, S.; Isolauri, E. Breast milk: A source of bifidobacteria for infant gut development and maturation? Neonatology 2007, 92, 64–66. [Google Scholar] [CrossRef]
- Özcan, E.; Sela, D.A. Inefficient Metabolism of the Human Milk Oligosaccharides Lacto-N-tetraose and Lacto-N-neotetraose Shifts Bifidobacterium longum subsp. infantis Physiology. Front. Nutr. 2018, 5. [Google Scholar] [CrossRef]
- Bode, L. Human Milk Oligosaccharides in the Prevention of Necrotizing Enterocolitis: A Journey From in vitro and in vivo Models to Mother-Infant Cohort Studies. Front. Pediatr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Thongaram, T.; Hoeflinger, J.L.; Chow, J.; Miller, M.J. Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and lactobacilli. J. Dairy Sci. 2017, 100, 7825–7833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idota, T.; Kawakami, H.; Murakami, Y.; Sugawara, M. Inhibition of cholera toxin by human milk fractions and sialyllactose. Biosci. Biotechnol. Biochem. 1995, 59, 417–419. [Google Scholar] [CrossRef]
- Xiao, J.; Takahashi, S.; Nishimoto, M.; Odamaki, T.; Yaeshima, T.; Iwatsuki, K.; Kitaoka, M. Distribution of in vitro fermentation ability of lacto-N-biose I, a major building block of human milk oligosaccharides, in bifidobacterial strains. Appl. Environ. Microbiol. 2010, 76, 54–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, R.E.; Niñonuevo, M.; Mills, D.A.; Lebrilla, C.B.; German, J.B. In Vitro Fermentation of Breast Milk Oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Appl. Environ. Microbiol. 2006, 72, 4497–4499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [Green Version]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Schrenzel, J.; François, P.; Cani, P.D. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, L.; Ding, M.; Luo, Z.; Yuan, S.; Bansal, M.B.; Gilkeson, G.; Lang, R.; Jiang, W. Estrogen decreases tight junction protein ZO-1 expression in human primary gut tissues. Clin. Immunol. 2017, 183, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Weaver, L.T.; Laker, M.F.; Nelson, R.; Lucas, A. Milk feeding and changes in intestinal permeability and morphology in the newborn. J. Pediatr. Gastroenterol. Nutr. 1987, 6, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Bonucci, A.; Coppa, G.V.; Carlucci, A.; Giorgi, P.L. Intestinal permeability changes during the first month: Effect of natural versus artificial feeding. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.K.; Ronveaux, C.C.; Rust, B.M.; Newman, J.W.; Hawley, M.; Barile, D.; Mills, D.A.; Raybould, H.E. Prebiotic milk oligosaccharides prevent development of obese phenotype, impairment of gut permeability, and microbial dysbiosis in high fat-fed mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 312, G474–G487. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet–Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [Green Version]
- Eiwegger, T.; Stahl, B.; Schmitt, J.; Boehm, G.; Gerstmayr, M.; Pichler, J.; Dehlink, E.; Loibichler, C.; Urbanek, R.; Szépfalusi, Z. Human milk–derived oligosaccharides and plant-derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr. Res. 2004, 56, 536–540. [Google Scholar] [CrossRef] [Green Version]
- Bode, L.; Kunz, C.; Muhly-Reinholz, M.; Mayer, K.; Seeger, W.; Rudloff, S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb. Haemost. 2004, 92, 1402–1410. [Google Scholar] [CrossRef]
- Takahata, Y.; Takada, H.; Nomura, A.; Ohshima, K.; Nakayama, H.; Tsuda, T.; Nakano, H.; Hara, T. Interleukin-18 in Human Milk. Pediatr. Res. 2001, 50, 268–272. [Google Scholar] [CrossRef] [Green Version]
- Goehring, K.C.; Marriage, B.J.; Oliver, J.S.; Wilder, J.A.; Barrett, E.G.; Buck, R.H. Similar to Those Who Are Breastfed, Infants Fed a Formula Containing 2′-Fucosyllactose Have Lower Inflammatory Cytokines in a Randomized Controlled Trial. J. Nutr. 2016, 146, 2559–2566. [Google Scholar] [CrossRef] [Green Version]
- Kainonen, E.; Rautava, S.; Isolauri, E. Immunological programming by breast milk creates an anti-inflammatory cytokine milieu in breast-fed infants compared to formula-fed infants. Br. J. Nutr. 2013, 109, 1962–1970. [Google Scholar] [CrossRef] [Green Version]
- Pu, Z.; Che, Y.; Zhang, W.; Sun, H.; Meng, T.; Xie, H.; Cao, L.; Hao, H. Dual roles of IL-18 in colitis through regulation of the function and quantity of goblet cells. Int. J. Mol. Med. 2019, 43, 2291–2302. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Fontana, L.; Gil, A. Human Milk Oligosaccharides and Immune System Development. Nutrients 2018, 10, 1038. [Google Scholar] [CrossRef] [Green Version]
- Straub, R.H. The Complex Role of Estrogens in Inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016, 7, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudloff, S.; Kunz, C. Milk Oligosaccharides and Metabolism in Infants. Adv. Nutr. 2012, 3, 398S–405S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, S.; Schols, H.A.; van den Heuvel, E.G.H.M.; Voragen, A.G.J.; Gruppen, H. Occurrence of oligosaccharides in feces of breast-fed babies in their first six months of life and the corresponding breast milk. Carbohydr. Res. 2011, 346, 2540–2550. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Control | 3′SL | 2′FL | 3′SL + 2′FL | p-Value |
|---|---|---|---|---|---|
| Relative Abundance (%) | |||||
| Bacteroides/Prevotella spp. | 1.9 ± 0.3 | 1.4 ± 0.2 | 1.7 ± 0.3 | 1.3 ± 0.2 | 0.66 |
| Bifidobacterium spp. | 0.9 ± 0.2 ab | 0.8 ± 0.2 a | 3.1 ± 1.0 b | 2.3 ± 0.4 ab | 0.03 |
| Enterobacteriaceae | 0.2 ± 0.04 | 0.2 ± 0.1 | 0.1 ± 0.02 | 0.1 ± 0.02 | 0.31 |
| Lactobacillus spp. | 47.1 ± 6.6 | 57.4 ± 9.2 | 43.7 ± 9.6 | 38.2 ± 9.5 | 0.48 |
| Clostridium perfringens (cluster I) | 1.5 ± 0.2 a | 0.7 ± 0.2 b | 0.8 ± 0.2 b | 0.6 ± 0.1 b | 0.0004 |
| Clostridium leptum (cluster IV) | 9.1 ± 1.5 a | 5.1 ± 1.0 ab | 4.7 ± 0.9 b | 7.1 ± 1.2 ab | 0.04 |
| Clostridium difficile (cluster XI) | 0.4 ± 0.1 a | 0.1 ± 0.03 b | 0.1 ± 0.02 b | 0.1 ± 0.01 b | 0.002 |
| Clostridium coccoides (cluster XIV) | 16.3 ± 2.3 | 10.6 ± 1.4 | 10.4 ± 2.1 | 13.5 ± 1.9 | 0.12 |
| Roseburia spp. | 0.003 ± 0.002 | 0.004 ± 0.002 | 0.0006 ± 0.0001 | 0.003 ± 0.001 | 0.36 |
| Methanobrevibacter spp. | 0.005 ± 0.003 a | 0.003 ± 0.0003 b | 0.003 ± 0.0001 b | 0.004 ± 0.001 ab | 0.001 |
| Akkermansia muciniphila | 0.07 ± 0.03 a | 0.04 ± 0.01 ab | 0.02 ± 0.007 ab | 0.003 ± 0.001 b | 0.01 |
| Faecalibacterium prausnitzii | 0.09 ± 0.03 | 0.03 ± 0.008 | 0.04 ± 0.008 | 0.05 ± 0.005 | 0.20 |
| Collinsella aerofaciens | 0.005 ± 0.0004 a | 0.003 ± 0.0003 b | 0.003 ± 0.001 b | 0.004 ± 0.001 ab | 0.002 |
| Total bacteria (16S ribosomal RNA (rRNA) gene copies) | 30,736,150 ± 2,698,774 a | 53,509,340 ± 6,514,451 b | 49,386,585 ± 4698662 ab | 38,298,292 ± 5,529,141 ab | 0.02 |
| Treatment | Control | 3′SL | 2′FL | 3′SL + 2′FL | p-Value |
|---|---|---|---|---|---|
| Relative Abundance (%) | |||||
| Bacteroides/Prevotella spp. | 1.2 ± 0.1 | 1.9 ± 0.3 | 1.8 ± 0.3 | 1.4 ± 0.04 | 0.11 |
| Bifidobacterium spp. | 0.9 ± 0.4 | 1.2 ± 0.4 | 1.7 ± 0.5 | 1.3 ± 0.3 | 0.34 |
| Enterobacteriaceae | 0.1 ± 0.02 | 0.2 ± 0.04 | 0.1 ± 0.02 | 0.05 ± 0.009 | 0.06 |
| Lactobacillus spp. | 41.7 ± 5.3 | 42.0 ± 8.1 | 45.2 ± 5.6 | 45.7 ± 10.1 | 0.97 |
| Clostridium perfringens (cluster I) | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.06 | 0.60 |
| Clostridium leptum (cluster IV) | 6.5 ± 1.1 | 7.1 ± 1.3 | 10.7 ± 1.7 | 6.7 ± 1.2 | 0.10 |
| Clostridium difficile (cluster XI) | 0.1 ± 0.03 | 0.1 ± 0.03 | 0.1 ± 0.01 | 0.08 ± 0.02 | 0.35 |
| Clostridium coccoides (cluster XIV) | 10.9 ± 1.6 | 11.6 ± 1.5 | 12.2 ± 1.1 | 17.6 ± 2.8 | 0.06 |
| Roseburia spp. | 0.001 ± 0.0004 | 0.0006 ± 0.0001 | 0.0009 ± 0.0002 | 0.003 ± 0.001 | 0.20 |
| Methanobrevibacter spp. | 0.004 ± 0.001 | 0.005 ± 0.001 | 0.004 ± 0.001 | 0.006 ± 0.001 | 0.94 |
| Akkermansia muciniphila | 0.1 ± 0.07 a | 0.02 ± 0.006 b | 0.03 ± 0.01 b | 0.009 ± 0.004 b | 0.04 |
| Faecalibacterium prausnitzii | 0.07 ± 0.02 | 0.05 ± 0.01 | 0.04 ± 0.006 | 0.05 ± 0.009 | 0.61 |
| Collinsella aerofaciens | 0.003 ± 0.001 | 0.004 ± 0.001 | 0.004 ± 0.0004 | 0.004 ± 0.001 | 0.36 |
| Total bacteria (16S rRNA gene copies) | 47,474,463 ± 3,993,791 | 47,238,918 ± 6,656,759 | 47,704,183 ± 2,956,712 | 33,004,782 ± 4,106,583 | 0.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chleilat, F.; Klancic, T.; Ma, K.; Schick, A.; Nettleton, J.E.; Reimer, R.A. Human Milk Oligosaccharide Supplementation Affects Intestinal Barrier Function and Microbial Composition in the Gastrointestinal Tract of Young Sprague Dawley Rats. Nutrients 2020, 12, 1532. https://doi.org/10.3390/nu12051532
Chleilat F, Klancic T, Ma K, Schick A, Nettleton JE, Reimer RA. Human Milk Oligosaccharide Supplementation Affects Intestinal Barrier Function and Microbial Composition in the Gastrointestinal Tract of Young Sprague Dawley Rats. Nutrients. 2020; 12(5):1532. https://doi.org/10.3390/nu12051532
Chicago/Turabian StyleChleilat, Faye, Teja Klancic, Kyle Ma, Alana Schick, Jodi E. Nettleton, and Raylene A. Reimer. 2020. "Human Milk Oligosaccharide Supplementation Affects Intestinal Barrier Function and Microbial Composition in the Gastrointestinal Tract of Young Sprague Dawley Rats" Nutrients 12, no. 5: 1532. https://doi.org/10.3390/nu12051532
APA StyleChleilat, F., Klancic, T., Ma, K., Schick, A., Nettleton, J. E., & Reimer, R. A. (2020). Human Milk Oligosaccharide Supplementation Affects Intestinal Barrier Function and Microbial Composition in the Gastrointestinal Tract of Young Sprague Dawley Rats. Nutrients, 12(5), 1532. https://doi.org/10.3390/nu12051532







