Dietary Fibres Differentially Impact on the Production of Phenolic Acids from Rutin in an In Vitro Fermentation Model of the Human Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. In Vitro Fermentation Model
2.2.1. Donors, Sampling and Faecal Slurry Preparation
2.2.2. Fibres
2.2.3. Faecal Incubations
2.2.4. 13C Labelled Quercetin Study
2.3. Fibre-Phenolic Sequestration Experiment
2.4. Phenolic Acids Extraction
2.5. Phenolic Acid Analysis
2.6. Short Chain Fatty Acid Extraction and Analysis
2.7. Statistical Analysis
3. Results
3.1. GC-MS Method Optimisation and Validation
3.2. Phenolics and Their Metabolites Released from Fibres
3.3. Fibre-Phenolics Sequestration Interactions
3.4. Colonic Catabolism of Rutin in a Human Batch Fermentation Model
3.5. Effects of Fibres on Rutin Metabolites
3.6. Impact of Fibres and Rutin on pH and SCFA Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pérez-Jiménez, J.; Fezeu, L.; Touvier, M.; Arnault, N.; Manach, C.; Hercberg, S.; Galan, P.; Scalbert, A. Dietary intake of 337 polyphenols in French adults. Am. J. Clin. Nutr. 2011, 93, 1220–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brat, P.; Georgé, S.; Bellamy, A.; Du Chaffaut, L.; Scalbert, A.; Mennen, L.; Arnault, N.; Amiot, M.J. Daily polyphenol intake in France from fruit and vegetables. J. Nutr. 2006, 136, 2368–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L. Review: Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr. Cancer 2004, 50, 1–7. [Google Scholar] [CrossRef]
- Ovaskainen, M.-L.; Törrönen, R.; Koponen, J.M.; Sinkko, H.; Hellström, J.; Reinivuo, H.; Mattila, P. Dietary intake and major food sources of polyphenols in Finnish adults. J. Nutr. 2008, 138, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Andres-Lacueva, C.; Lamuela-Raventós, R.M.; Berenguer, T.; Jakszyn, P.; Barricarte, A.; Ardanaz, E.; Amiano, P.; Dorronsoro, M.; Larrañaga, N. Estimation of dietary sources and flavonoid intake in a Spanish adult population (EPIC-Spain). J. Am. Diet Assoc. 2010, 110, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, R.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010. [Google Scholar] [CrossRef] [PubMed]
- Wada, L.; Ou, B. Antioxidant activity and phenolic content of Oregon caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef]
- Hui, C.; Qi, X.; Qianyong, Z.; Xiaoli, P.; Jundong, Z.; Mantian, M. Flavonoids, flavonoid subclasses and breast cancer risk: A meta-analysis of epidemiologic studies. PLoS ONE 2013, 8, e54318. [Google Scholar] [CrossRef]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin reduces blood pressure in hypertensive subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; González-Sarrías, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.; Dumont, J. Impact of flavonols on cardiometabolic biomarkers: A meta-analysis of randomized controlled human trials to explore the role of inter-individual variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.-M.; O’leary, K.; Williamson, G.; Ojala, M.; Bailey, M.; Puupponen-Pimiä, R.; Nuutila, A.; Oksman-Caldentey, K.-M.; Poutanen, K. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J. Agric. Food Chem. 2002, 50, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Jaganath, I.B.; Mullen, W.; Lean, M.E.; Edwards, C.A.; Crozier, A. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free Radic. Biol. Med. 2009, 47, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Demigné, C.; Texier, O.; Régérat, F.; Rémésy, C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997, 409, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Baba, S.; Furuta, T.; Fujioka, M.; Goromaru, T. Studies on drug metabolism by use of isotopes XXVII: Urinary metabolites of rutin in rats and the role of intestinal microflora in the metabolism of rutin. J. Pharm. Sci. 1983, 72, 1155–1158. [Google Scholar] [CrossRef]
- Griffiths, L.; Barrow, A. Metabolism of flavonoid compounds in germ-free rats. Biochem. J. 1972, 130, 1161–1162. [Google Scholar] [CrossRef] [Green Version]
- Jaganath, I.B.; Jaganath, I.B.; Mullen, W.; Edwards, C.A.; Crozier, A. The relative contribution of the small and large intestine to the absorption and metabolism of rutin in man. Free Radic. Res. 2006, 40, 1035–1046. [Google Scholar] [CrossRef]
- Hollman, P.C.; Gaag, M.V.; Mengelers, M.J.; Van Trijp, J.M.; De Vries, J.H.; Katan, M.B. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic. Biol. Med. 1996, 21, 703–707. [Google Scholar] [CrossRef]
- Alkhaldy, A.; Edwards, C.A.; Combet, E. The urinary phenolic acid profile varies between younger and older adults after a polyphenol-rich meal despite limited differences in in vitro colonic catabolism. Eur. J. Nutr. 2019, 58, 1095–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhaldy, A.; Edwards, A.C.; Combet, E. Effect of ethnicity and a fermentable fibre on the in vitro colonic metabolism of polyphenols. Proc. Nutr. Soc. 2012, 71, E61. [Google Scholar] [CrossRef] [Green Version]
- Vlassopoulos, A.; Lean, M.E.J.; Combet, E. Role of oxidative stress in physiological albumin glycation: A neglected interaction. Free Radic. Biol. Med. 2013, 60, 318–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Nakashima, S.; Saiki, S.; Myoi, Y.; Abe, N.; Kuwazuru, S.; Zhu, B.; Ashida, H.; Murata, Y.; Nakamura, Y. 3,4-Dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides. Food Res. Int. 2016, 89, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Kroner, C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res. 2005, 116, 327–334. [Google Scholar] [CrossRef]
- Monagas, M.; Khan, N.; Andrés-Lacueva, C.; Urpí-Sardá, M.; Vázquez-Agell, M.; Lamuela-Raventós, R.M.; Estruch, R. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br. J. Nutr. 2009, 102, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Pashikanti, S.; de Alba, D.R.; Boissonneault, G.A.; Cervantes-Laurean, D. Rutin metabolites: Novel inhibitors of nonoxidative advanced glycation end products. Free Radic. Biol. Med. 2010, 48, 656–663. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B. Gut microbial catabolism of grape seed flavan-3-ols by human faecal microbiota. Targetted analysis of precursor compounds, intermediate metabolites and end-products. Food Chem. 2012, 131, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Jarosova, V.; Vesely, O.; Marsik, P.; Jaimes, J.D.; Smejkal, K.; Kloucek, P.; Havlik, J. Metabolism of stilbenoids by human faecal microbiota. Molecules 2019, 24, 1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef]
- Mansoorian, B.; Combet, E.; Alkhaldy, A.; Garcia, A.L.; Edwards, C.A. Impact of fermentable fibres on the colonic microbiota metabolism of dietary polyphenols rutin and quercetin. Int. J. Environ. Res. Pubic. Health 2019, 16, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koziolek, M.; Grimm, M.; Becker, D.; Iordanov, V.; Zou, H.; Shimizu, J.; Wanke, C.; Garbacz, G.; Weitschies, W. Investigation of pH and temperature profiles in the GI tract of fasted human subjects using the Intellicap® system. J. Pharm. Sci. 2015, 104, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Duncan, S.H.; Leitch, E.C.M.; Child, M.W.; Flint, H.J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crittenden, R.; Karppinen, S.; Ojanen, S.; Tenkanen, M.; Fagerström, R.; Mättö, J.; Saarela, M.; Mattila-Sandholm, T.; Poutanen, K. In vitro fermentation of cereal dietary fibre carbohydrates by probiotic and intestinal bacteria. J. Sci. Food Agric. 2002, 82, 781–789. [Google Scholar] [CrossRef]
- Brownlee, I.A. The physiological roles of dietary fibre. Food Hydrocoll. 2011, 25, 238–250. [Google Scholar] [CrossRef]
- Bazzocco, S.; Mattila, I.; Guyot, S.; Renard, C.M.; Aura, A.-M. Factors affecting the conversion of apple polyphenols to phenolic acids and fruit matrix to short-chain fatty acids by human faecal microbiota in vitro. Eur. J. Nutr. 2008, 47, 442–452. [Google Scholar] [CrossRef]
- Khossousi, A.; Binns, C.; Dhaliwal, S.; Pal, S. The acute effects of psyllium on postprandial lipaemia and thermogenesis in overweight and obese men. Br. J. Nutr. 2008, 99, 1068–1075. [Google Scholar] [CrossRef]
- Harris, H.C.; Edwards, C.A.; Morrison, D.J. Impact of glycosidic bond configuration on short chain fatty acid production from model fermentable carbohydrates by the human gut microbiota. Nutrients 2017, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Ladas, S.; Isaacs, P.; Murphy, G.; Sladen, G. Fasting and postprandial ileal function in adapted ileostomates and normal subjects. Gut 1986, 27, 906–912. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, L.; Harris, P. Protection against cancer by wheat bran: Role of dietary fibre and phytochemicals. Eur. J. Cancer Prev. 1999, 8, 17–26. [Google Scholar] [CrossRef]
- Combet, E.; Lean, M.E.; Boyle, J.G.; Crozier, A.; Davidson, D.F. Dietary flavonols contribute to false-positive elevation of homovanillic acid, a marker of catecholamine-secreting tumors. Clin. Chim. Acta 2011, 412, 165–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grün, C.H.; van Dorsten, F.A.; Jacobs, D.M.; Le Belleguic, M.; van Velzen, E.J.; Bingham, M.O.; Janssen, H.-G.; van Duynhoven, J.P. GC–MS methods for metabolic profiling of microbial fermentation products of dietary polyphenols in human and in vitro intervention studies. J. Chromatogr. B 2008, 871, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Jung, E.-A.; Sohng, I.-S.; Han, J.-A.; Kim, T.-H.; Han, M.J. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch. Pharm. Res. 1998, 21, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Vahouny, G.V.; Tombes, R.; Cassidy, M.M.; Kritchevsky, D.; Gallo, L.L. Dietary fibers: V. Binding of bile salts, phospholipids and cholesterol from mixed micelles by bile acid sequestrants and dietary fibers. Lipids 1980, 15, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Bouchet, B.; Renard, C. Non-covalent interaction between procyanidins and apple cell wall material. Part III: Study on model polysaccharides. Biophys. Acta Gen. Subj. 2005, 1725, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Tsitko, I.; Wiik-Miettinen, F.; Mattila, O.; Rosa-Sibakov, N.; Seppänen-Laakso, T.; Maukonen, J.; Nordlund, E.; Saarela, M. A small in vitro fermentation model for screening the gut microbiota effects of different fiber preparations. Int. J. Mol. Sci. 2019, 20, 1925. [Google Scholar] [CrossRef] [Green Version]
- Roberfroid, M.B.; Van Loo, J.A.; Gibson, G.R. The bifidogenic nature of chicory inulin and its hydrolysis products. J. Nutr. 1998, 128, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Salazar, N.; Dewulf, E.M.; Neyrinck, A.M.; Bindels, L.B.; Cani, P.D.; Mahillon, J.; de Vos, W.M.; Thissen, J.P.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; et al. Inulin-type fructans modulate intestinal Bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women. Clin. Nutr. 2015, 34, 501–507. [Google Scholar] [CrossRef]
- Lohner, S.; Jakobik, V.; Mihályi, K.; Soldi, S.; Vasileiadis, S.; Theis, S.; Sailer, M.; Sieland, C.; Berényi, K.; Boehm, G.; et al. Inulin-type fructan supplementation of 3-to 6-year-old children is associated with higher fecal Bifidobacterium concentrations and fewer febrile episodes requiring medical attention. J. Nutr. 2018, 148, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.A.; Soto-Vaca, A.; Nicolucci, A.C.; Mayengbam, S.; Park, H.; Madsen, K.L.; Menon, R.; Vaughan, E.E. Effect of chicory inulin-type fructan–containing snack bars on the human gut microbiota in low dietary fiber consumers in a randomized crossover trial. Am. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, N.; Vollmer, M.; Holtrop, G.; Farquharson, F.M.; Wefers, D.; Bunzel, M.; Duncan, S.H.; Drew, J.E.; Williams, L.M.; Milligan, G.; et al. Specific substrate-driven changes in human faecal microbiota composition contrast with functional redundancy in short-chain fatty acid production. ISME J. 2018, 12, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Blaut, M.; Schoefer, L.; Braune, A. Transformation of flavonoids by intestinal microorganisms. Int. J. Vitam. Nutr. Res. 2003, 73, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Reichardt, N.; Braune, A.; Kroh, L.W.; Blaut, M.; Rohn, S. The microbial degradation of onion flavonol glucosides and their roasting products by the human gut bacteria Eubacterium ramulus and Flavonifractor plautii. Food Res. Int. 2015, 67, 349–355. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure–function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Van Beek, S.; Priest, F.G. Decarboxylation of substituted cinnamic acids by lactic acid bacteria isolated during malt whisky fermentation. Appl. Environ. Microbiol. 2000, 66, 5322–5328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, H.; Landete, J.M.; Curiel, J.A.; de Las Rivas, B.; Mancheño, J.M.; Muñoz, R. Characterization of the p-coumaric acid decarboxylase from Lactobacillus plantarum CECT 748T. J. Agric. Food Chem. 2008, 56, 3068–3072. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Yang, C.S. Biological fates of tea polyphenols and their interactions with microbiota in the gastrointestinal tract: Implications on health effects. Crit. Rev. Food Sci. Nutr. 2019, 1–19. [Google Scholar] [CrossRef]
- Auclair, S.; Silberberg, M.; Gueux, E.; Morand, C.; Mazur, A.; Milenkovic, D.; Scalbert, A. Apple polyphenols and fibers attenuate atherosclerosis in apolipoprotein E-deficient mice. J. Agric. Food Chem. 2008, 56, 5558–5563. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Nakagawa, H.; Tsushida, T.; Hirayama, K.; Itoh, K. Effect of pectin enhancement on plasma quercetin and fecal flora in rutin-supplemented mice. J. Food Sci. 2007, 72, S648–S651. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, T.; Iwai, K.; Saito, Y.; Takida, Y.; Matsue, H. Chronic ingestion of apple pectin can enhance the absorption of quercetin. J. Agric. Food Chem. 2009, 57, 2583–2587. [Google Scholar] [CrossRef] [PubMed]
- Justesen, U.; Arrigoni, E. Electrospray ionisation mass spectrometric study of degradation products of quercetin, quercetin-3-glucoside and quercetin-3-rhamnoglucoside, produced by in vitro fermentation with human faecal flora. Rapid Commun. Mass Spectrom. 2001, 15, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Russell, W.R.; Duncan, S.H.; Scobbie, L.; Duncan, G.; Cantlay, L.; Calder, A.G.; Anderson, S.E.; Flint, H.J. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol. Nutr. Food Res. 2013, 57, 523–535. [Google Scholar] [CrossRef]
- Russell, W.R.; Hoyles, L.; Flint, H.J.; Dumas, M.-E. Colonic bacterial metabolites and human health. Curr. Opin. Microbiol. 2013, 16, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Valerio, F.; Lavermicocca, P.; Pascale, M.; Visconti, A. Production of phenyllactic acid by lactic acid bacteria: An approach to the selection of strains contributing to food quality and preservation. FEMS Microbiol. Lett. 2004, 233, 289–295. [Google Scholar] [CrossRef]
- Kaplan, H.; Hutkins, R.W. Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl. Environ. Microbiol. 2000, 66, 2682–2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chifiriuc, M.C.; Veronica, L.; Dracea, O.; Ditu, L.-M.; Smarandache, D.; Bucur, M.; Larion, C.; Ramona, C.; Sasarman, E. Drastic attenuation of Pseudomonas aeruginosa pathogenicity in a holoxenic mouse experimental model induced by subinhibitory concentrations of phenyllactic acid (PLA). Int. J. Mol. Sci. 2007, 8, 583–592. [Google Scholar] [CrossRef]
- Chifiriuc, M.-C.; Diţu, L.; Banu, O.; Bleotu, C.; Drăcea, O.; Bucur, M.; Larion, C.; Israil, A.M.; Lazăr, V. Subinhibitory concentrations of phenyl lactic acid interfere with the expression of virulence factors in Staphylococcus aureus and Pseudomonas aeruginosa clinical strains. Roum. Arch. Microbiol. Immunol. 2009, 68, 27–33. [Google Scholar] [PubMed]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, S48–S66. [Google Scholar] [CrossRef] [Green Version]
- Granado-Serrano, A.B.; Martín-Garí, M.; Sánchez, V.; Solans, M.R.; Berdún, R.; Ludwig, I.A.; Rubió, L.; Vilaprinyó, E.; Portero-Otín, M.; Serrano, J.C.E. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 2019, 9, 1772. [Google Scholar] [CrossRef]


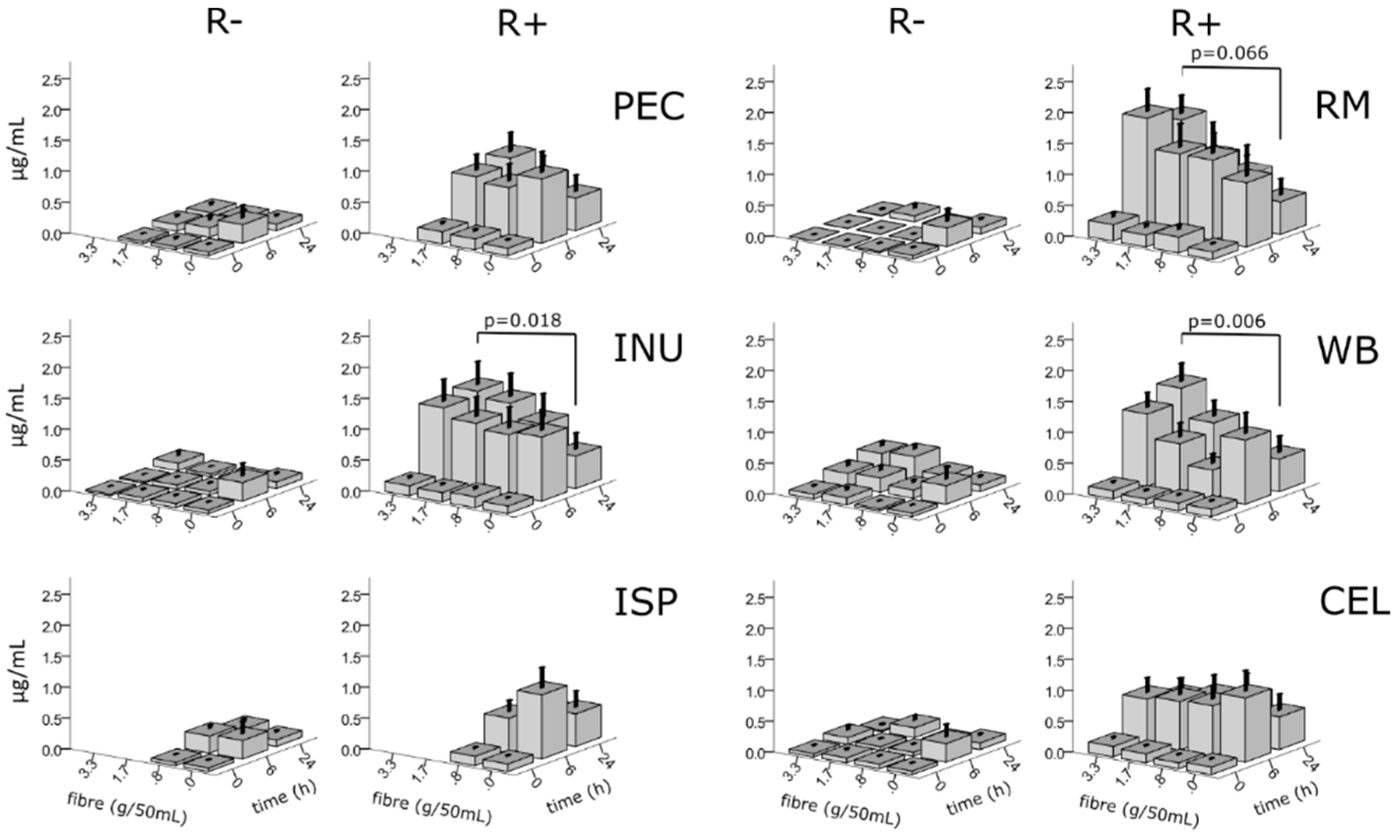
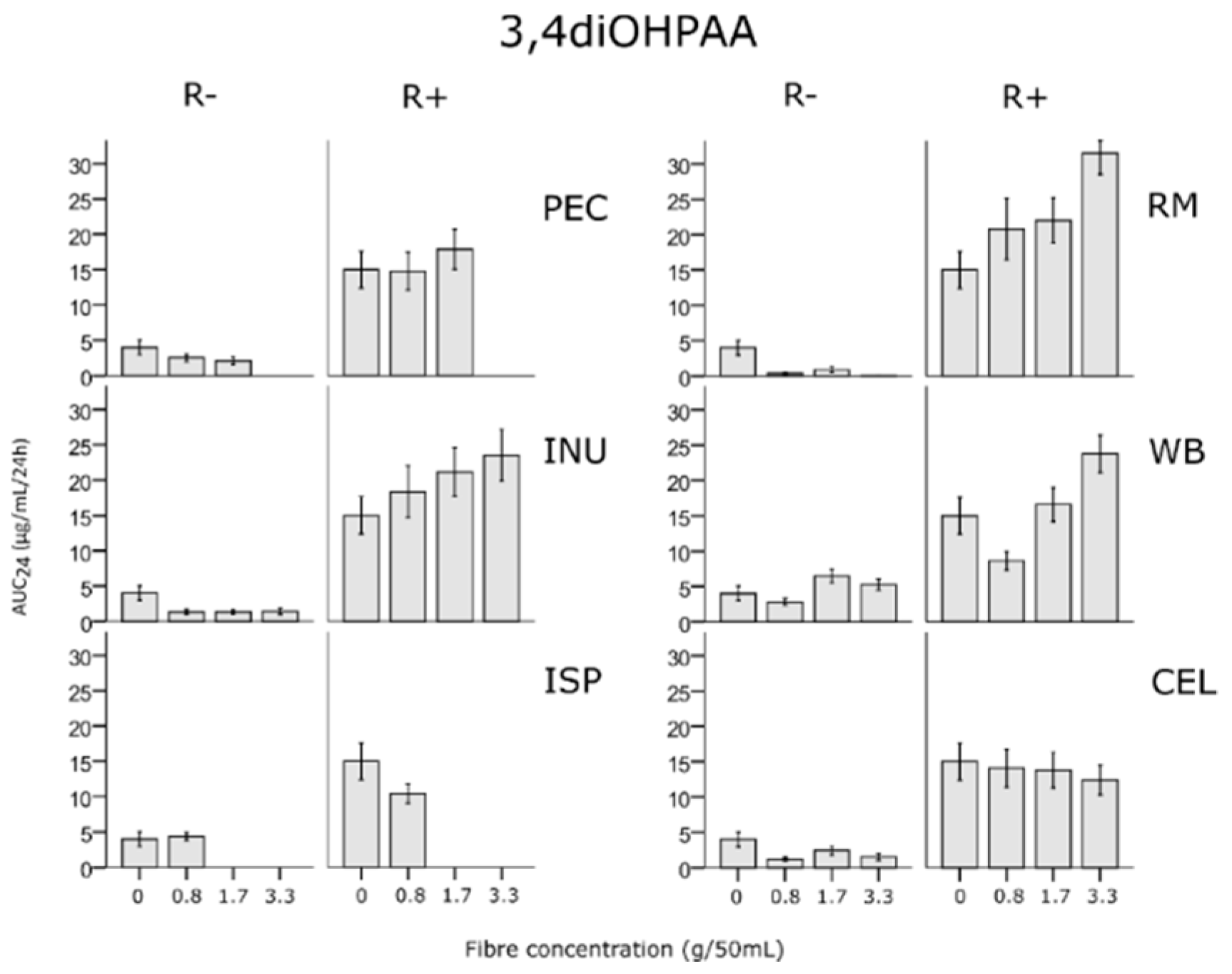

| Fibre | |||||||
|---|---|---|---|---|---|---|---|
| PEC | INU | ISP | RM # | WB | CEL | Blank ## | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| BA | 37.1 ± 4.4 | 49.7 ± 6.6 | 37.7±5.3* | 78.4 ± 15.7 | 48.0 ± 4.6 | 35.1 ± 6.1 | 33.3 ± 3.7 |
| PAA | 245.6 ± 20.9 ** | 205.1 ± 15.4 ** | 267.3 ± 19.1 ** | 233.2 ± 16.7 ** | 649.4 ± 53.6 | 510.7 ± 37.8 | 598.5 ± 40.9 |
| Pyrocatechol | 20.6 ± 4.5 | 58.7±8.6* | 16.8 ± 3.2 | 43.3±2.9** | 38.3±5.4** | 23.4 ± 5.2 | 15.3 ± 3.8 |
| Resorcinol | 8.0 ± 2.7 | 6.2 ± 2.5 | 7.2 ± 2.6 | 0.0 ± 0.0 | 9.2 ± 2.6 | 6.4 ± 2.2 | 5.1± 1.8 |
| 3PPA | 191.6 ± 21.6 | 139.6 ± 16.5 | 136.3 ± 11.8 | 129.3 ± 5.0 | 280.6 ± 32.8 | 139.6 ± 16.6 | 118.3 ± 12.4 |
| Mandelic acid | 5.3 ± 1.4 | 4.4 ± 1.2 | 10.3±1.8** | 1.4 ± 0.6 | 10.4±1.8* | 3.1 ± 0.8 | 2.5 ± 0.6 |
| Cinnamic acid | 4.6±1.7* | 2.8 ± 1.4 | 2.2 ± 1.3 | 0.0 ± 0.0 | 2.3 ± 1.2 | 1.8 ± 1.0 | 1.9 ± 1.0 |
| 3HBA | 3.2 ± 1.5 * | 6.6 ± 3.3 | 6.3 ± 3.0 | 10.7 ± 2.3 | 5.9 ± 3.0 | 3.7 ± 1.6 | 3.9 ± 2.0 |
| 3-Phenyllactic acid | 34.5 ± 6.6 | 120.6±15.5** | 11.9 ± 5.1 | 22.1 ± 9.9 | 7.2 ± 1.7 | 2.4 ± 0.6 | 4.6 ± 1.5 |
| 3OHPAA | 7.0 ± 1.2 | 7.1 ± 1.3 | 10.7 ± 1.4 | 8.9 ± 2.4 | 7.6 ± 1.2 | 6.7 ± 1.2 | 4.5 ± 0.8 |
| 4OHBA | 3.7 ± 0.6 | 4.9 ± 1.0 | 6.8 ± 0.8 | 10.4±1.8* | 12.8±1.1** | 6.1 ± 1.2 | 2.8 ± 0.6 |
| Phloroglucinol | 3.8 ± 1.3 | 10.9 ± 3.1 | 7.3 ± 2.0 | 6.4 ± 2.8 | 7.1 ± 2.1 | 6.4 ± 2.1 | 7.9 ± 1.8 |
| 4OHPAA | 14.1 ± 1.4 ** | 16.8 ± 2.3 ** | 12.4 ± 1.5 ** | 14.3 ± 3.1 ** | 62.4 ± 4.1 | 52.2 ± 3.7 | 50.0 ± 4.0 |
| 3,3OHPPA | 115.3±8.5** | 26.2 ± 3.9 ** | 37.3 ± 4.7 | 11.6 ± 3.4 ** | 128.5±17.7** | 36.3 ± 4.3 | 40.9 ± 6.8 |
| VAN + iso-VAN | 0.9 ± 0.3 | 1.2 ± 0.3 | 3.1 ± 0.6 | 2.4 ± 1.0 | 7.3±0.7* | 1.2 ± 0.3 | 0.8 ± 0.2 |
| 4-OHmandelic acid | 2.6 ± 1.2 | 2.9 ± 1.1 | 1.6 ± 0.7 | 0.0 ± 0.0 | 1.8 ± 0.7 | 3.2 ± 1.0 | 0.6 ± 0.1 |
| 3OH4MPAA + 4OH3MPAA | 7.2 ± 2.3 | 5.2 ± 1.5 | 7.1 ± 2.6 | 15.5 ± 6.9 | 11.1 ± 2.9 | 11.6 ± 4.3 | 6.1 ± 2.6 |
| 3,4OHPPA | 23.2 ± 3.4 | 16.9 ± 3.5 | 18.5 ± 3.6 | 14.1 ± 4.8 | 26.0 ± 3.7 | 32.3 ± 5.9 | 27.6 ± 5.5 |
| 3,4diOHBA | 1.0 ± 0.2 | 2.3 ± 0.4 | 1.5 ± 0.2 | 3.3±0.5** | 5.3±0.5** | 1.0 ± 0.3 | 3.1 ± 1.5 |
| 3,4diOHPAA | 2.5 ± 0.5 | 1.3 ± 0.4 | 4.3±0.6** | 0.4 ± 0.2 | 2.8 ± 0.5 | 1.2 ± 0.3 | 4.0 ± 1.0 |
| 3,3OH4MPPA | 132.5±12.0** | 0.5 ± 0.1 ** | 0.6 ± 0.2 | 0.0 ± 0.0 | 0.4 ± 0.2 | 0.4 ± 0.2 | 5.0 ± 2.1 |
| 3,4OH3MPPA | 2.1 ± 0.6 | 1.5 ± 0.6 | 1.8 ± 0.9 ** | 0.0 ± 0.0 ** | 26.3±3.2** | 2.1 ± 0.6 | 1.0 ± 0.4 |
| 3,3,4diOHPPA | 12.2 ± 3.7 | 4.0 ± 2.0 | 8.9 ± 2.6 | 2.2 ± 1.0 | 9.6±1.6** | 6.2 ± 2.3 | 5.8 ± 1.8 |
| p-Coumaric acid | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.0 ± 0.0 | 0.5 ± 0.1 | 0.1 ± 0.0 | 0.4 ± 0.1 |
| 3OH4M Cinnamic acid | 0.5 ± 0.3 | 0.1 ± 0.1 | 0.4 ± 0.1 | 0.0 ± 0.0 | 0.5 ± 0.2 | 0.0 ± 0.0 | 0.1 ± 0.1 |
| Ferulic acid | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.6 ± 0.2 | 0.0 ± 0.0 | 5.2±0.7** | 0.4 ± 0.1 | 0.6 ± 0.2 |
| Caffeic acid | 4.6 ± 2.0 | 4.2 ± 2.1 | 5.7 ± 1.9 | 0.8 ± 0.4 | 3.6 ± 1.0 | 2.9 ± 1.0 | 2.7 ± 1.3 |
| Quercetin | 6.0 ± 3.3 | 7.0 ± 3.8 | 11.7±2.9** | 0.0 ± 0.0 | 7.8 ± 3.2 | 3.0 ± 1.6 | 4.4 ± 1.8 |
| AUC24 | Rutin not Added | Rutin Added | p-Value | n (Total) |
|---|---|---|---|---|
| Mean ± S.D. | Mean ± S.D. | |||
| pH | 147.11 ± 19.42 | 148.68 ± 19.59 | 0.187 | 300 |
| C2 | 617.79 ± 351.04 | 618.37 ± 348.00 | 0.980 | 300 |
| C3 | 120.48 ± 66.61 | 143.05 ± 88.11 | 0.000 | 300 |
| IC4 | 92.24 ± 344.67 | 3.83 ± 3.83 | 0.000 | 300 |
| C4 | 202.45 ± 167.81 | 226.58 ± 177.94 | 0.032 | 300 |
| IC5 | 6.80 ± 6.57 | 6.87 ± 6.45 | 0.874 | 300 |
| C5 | 11.80 ± 8.15 | 14.35 ± 8.22 | 0.000 | 300 |
| IC6 | 0.01 ± 0.13 | 0.01 ± 0.11 | 0.574 | 300 |
| C6 | 2.50 ± 5.21 | 3.61 ± 6.64 | 0.005 | 300 |
| C7 | 0.12 ± 0.62 | 0.16 ± 0.67 | 0.361 | 300 |
| Sum C2-C5 | 1051.56 ± 578.89 | 1013.04 ± 516.17 | 0.285 | 300 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havlik, J.; Marinello, V.; Gardyne, A.; Hou, M.; Mullen, W.; Morrison, D.J.; Preston, T.; Combet, E.; Edwards, C.A. Dietary Fibres Differentially Impact on the Production of Phenolic Acids from Rutin in an In Vitro Fermentation Model of the Human Gut Microbiota. Nutrients 2020, 12, 1577. https://doi.org/10.3390/nu12061577
Havlik J, Marinello V, Gardyne A, Hou M, Mullen W, Morrison DJ, Preston T, Combet E, Edwards CA. Dietary Fibres Differentially Impact on the Production of Phenolic Acids from Rutin in an In Vitro Fermentation Model of the Human Gut Microbiota. Nutrients. 2020; 12(6):1577. https://doi.org/10.3390/nu12061577
Chicago/Turabian StyleHavlik, Jaroslav, Vittoria Marinello, Andrew Gardyne, Min Hou, William Mullen, Douglas J. Morrison, Thomas Preston, Emilie Combet, and Christine A. Edwards. 2020. "Dietary Fibres Differentially Impact on the Production of Phenolic Acids from Rutin in an In Vitro Fermentation Model of the Human Gut Microbiota" Nutrients 12, no. 6: 1577. https://doi.org/10.3390/nu12061577







