Docosahexaenoic Acid-Rich Fish Oil Supplementation Reduces Kinase Associated with Insulin Resistance in Overweight and Obese Midlife Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design and Intervention
2.3. Outcome Measurements
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Compliance to the Study Products and Changes in the Participants’ Erythrocyte Membrane Fatty Acid Composition
3.3. Effect of DHA-Rich Fish Oil on GSK-3β
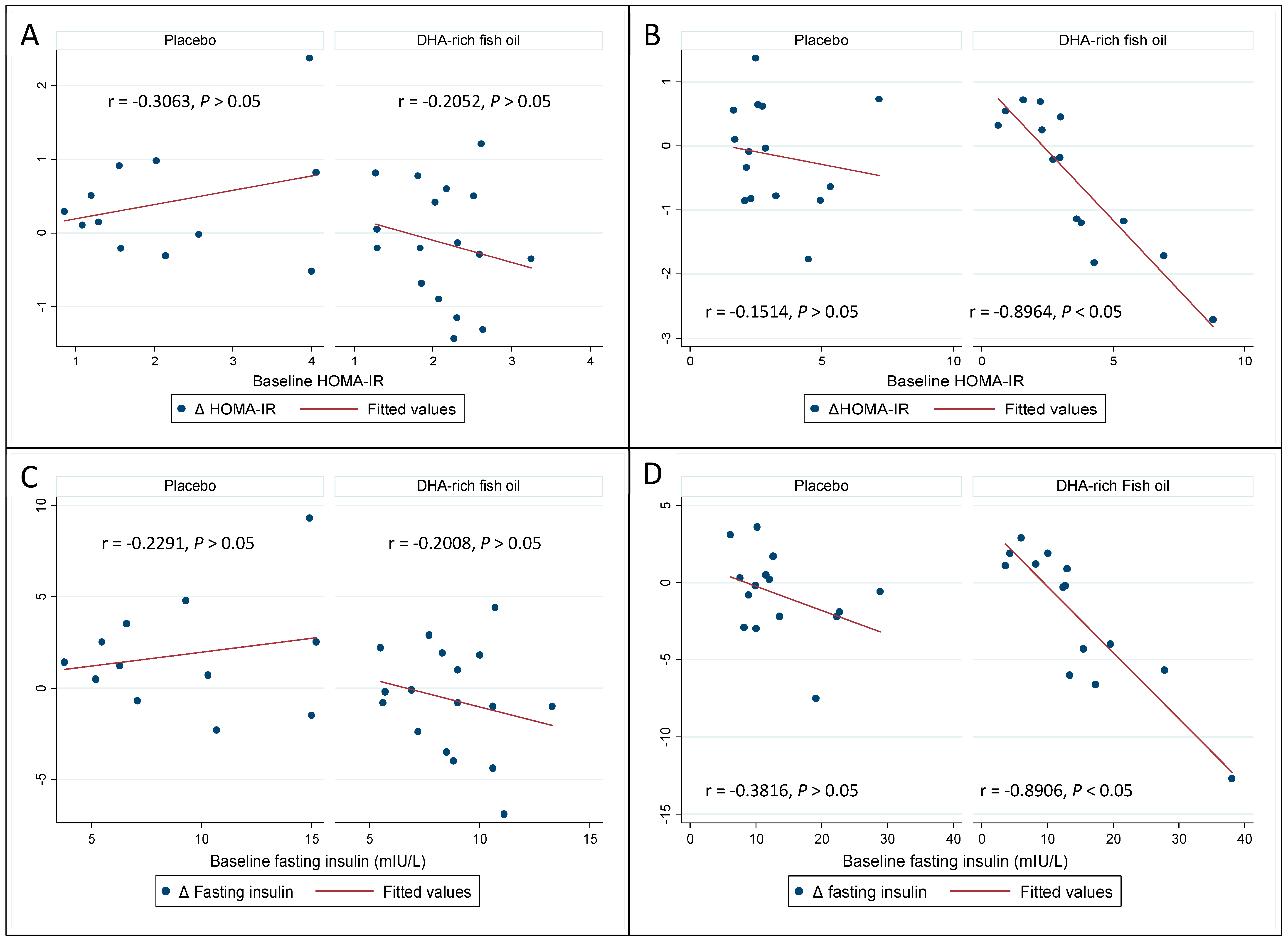
3.4. Effect of DHA-Rich Fish Oil on Fasting Insulin and Insulin Resistance
3.5. Effect of DHA-Rich Fish Oil on the Other Biomarkers
3.6. Effect of DHA-Rich Fish Oil Over GSK-3β, Insulin and HOMA-IR in Subgroups Based on Baseline Systemic Inflammation Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| BMI | Body mass index |
| CRP | C-reactive protein |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| FFM | Fat free mass |
| HOMA-IR | homeostatic model of assessment-insulin resistance |
| IPAQ | International physical activity questionnaire |
| IR | Insulin resistance |
| LCn-3PUFA | Long-chain omega-3 polyunsaturated fatty acid |
| T2D | Type 2 diabetes |
| WC | Waist circumference |
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Kelly, T.; Yang, W.; Chen, C.-S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kivipelto, M.; Ngandu, T.; Fratiglioni, L.; Viitanen, M.; Kåreholt, I.; Winblad, B.; Helkala, E.-L.; Tuomilehto, J.; Soininen, H.; Nissinen, A. Obesity and Vascular Risk Factors at Midlife and the Risk of Dementia and Alzheimer Disease. Arch. Neurol. 2005, 62, 1556–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westphal, S.A. Obesity, Abdominal Obesity, and Insulin Resistance. Clin. Cornerstone 2008, 9, 23–31. [Google Scholar] [CrossRef]
- Whitmer, R.; Gunderson, E.; Quesenberry, C.; Zhou, J.; Yaffe, K. Body Mass Index in Midlife and Risk of Alzheimer Disease and Vascular Dementia. Curr. Alzheimer Res. 2007, 4, 103–109. [Google Scholar] [CrossRef]
- Hugon, J.; Mouton-Liger, F.; Cognat, E.; Dumurgier, J.; Paquet, C. Blood-Based Kinase Assessments in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10. [Google Scholar] [CrossRef]
- Calon, F.; Cole, G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: Evidence from animal studies. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 287–293. [Google Scholar] [CrossRef]
- Fotuhi, M.; Mohassel, P.; Yaffe, K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: A complex association. Nat. Rev. Neurol. 2009, 5, 140–152. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7. [Google Scholar] [CrossRef] [Green Version]
- Demar, J.C.; Ma, K.; Chang, L.; Bell, J.M.; Rapoport, S.I. α-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J. Neurochem. 2005, 94, 1063–1076. [Google Scholar] [CrossRef]
- Gorjão, R.; Martins, A.K.A.; Rodrigues, H.G.; Abdulkader, F.; Arcisio-Miranda, M.; Procopio, J.; Curi, R. Comparative effects of DHA and EPA on cell function. Pharmacol. Ther. 2009, 122, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Claycombe, K.J.; Moustaid-Moussa, N. (n-3) Fatty Acids Alleviate Adipose Tissue Inflammation and Insulin Resistance: Mechanistic Insights12. Adv. Nutr. 2011, 2, 304–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Vicario, C.; Rius, B.; Alcaraz-Quiles, J.; García-Alonso, V.; Lopategi, A.; Titos, E.; Clària, J. Pro-resolving mediators produced from EPA and DHA: Overview of the pathways involved and their mechanisms in metabolic syndrome and related liver diseases. Eur. J. Pharmacol. 2016, 785, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Lalia, A.Z.; Lanza, I.R. Insulin-Sensitizing Effects of Omega-3 Fatty Acids: Lost in Translation? Nutrients 2016, 8, 329. [Google Scholar] [CrossRef]
- Takkunen, M.; the DPS Study Group; Schwab, U.S.; De Mello, V.D.; Eriksson, J.G.; Lindstrom, J.; Tuomilehto, J.; Uusitupa, M.I.J. Longitudinal associations of serum fatty acid composition with type 2 diabetes risk and markers of insulin secretion and sensitivity in the Finnish Diabetes Prevention Study. Eur. J. Nutr. 2015, 55, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Djoussé, L.; Biggs, M.L.; Lemaitre, R.N.; King, I.B.; Song, X.; Ix, J.H.; Mukamal, K.J.; Siscovick, D.S.; Mozaffarian, D. Plasma omega-3 fatty acids and incident diabetes in older adults. Am. J. Clin. Nutr. 2011, 94, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Abbott, K.A.; Veysey, M.; Lucock, M.; Niblett, S.; King, K.; Burrows, T.; Garg, M.L. Sex-dependent association between erythrocyte n-3 PUFA and type 2 diabetes in older overweight people. Br. J. Nutr. 2016, 115, 1379–1386. [Google Scholar] [CrossRef] [Green Version]
- Abbott, K.; Burrows, T.; Thota, R.; Acharya, S.; Garg, M.L. Do ω-3 PUFAs affect insulin resistance in a sex-specific manner? A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2016, 104, 1470–1484. [Google Scholar] [CrossRef]
- Akinkuolie, A.O.; Ngwa, J.S.; Meigs, J.B.; Djoussé, L. Omega-3 polyunsaturated fatty acid and insulin sensitivity: A meta-analysis of randomized controlled trials. Clin. Nutr. 2011, 30, 702–707. [Google Scholar] [CrossRef] [Green Version]
- Min, Y.; Nam, J.-H.; Ghebremeskel, K.; Kim, A.; Crawford, M. A distinctive fatty acid profile in circulating lipids of Korean gestational diabetics: A pilot study. Diabetes Res. Clin. Pr. 2006, 73, 178–183. [Google Scholar] [CrossRef]
- Rafraf, M.; Mohammadi, E.; Asghari-Jafarabadi, M.; Farzadi, L. Omega-3 Fatty Acids Improve Glucose Metabolism without Effects on Obesity Values and Serum Visfatin Levels in Women with Polycystic Ovary Syndrome. J. Am. Coll. Nutr. 2012, 31, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [PubMed]
- Young, S.E.; Mainous, A.G.; Carnemolla, M. Hyperinsulinemia and Cognitive Decline in a Middle-Aged Cohort. Diabetes Care 2006, 29, 2688–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.-Q.; Townsend, M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2009, 1792, 482–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willette, A.A.; Bendlin, B.B.; Starks, E.J.; Birdsill, A.C.; Johnson, S.C.; Christian, B.T.; Okonkwo, O.C.; La Rue, A.; Hermann, B.P.; Koscik, R.L.; et al. Association of Insulin Resistance With Cerebral Glucose Uptake in Late Middle-Aged Adults at Risk for Alzheimer Disease. JAMA Neurol. 2015, 72, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Lepretti, M.; Martucciello, S.; Burgos-Aceves, M.A.; Putti, R.; Lionetti, L. Omega-3 Fatty Acids and Insulin Resistance: Focus on the Regulation of Mitochondria and Endoplasmic Reticulum Stress. Nutrients 2018, 10, 350. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Geng, T.; Huang, T.; Zhao, Q. Fish oil supplementation and insulin sensitivity: A systematic review and meta-analysis. Lipids Heal. Dis. 2017, 16, 131. [Google Scholar] [CrossRef] [Green Version]
- Hooper, C.; Killick, R.; Lovestone, S. The GSK3 hypothesis of Alzheimer’s disease. J. Neurochem. 2008, 104, 1433–1439. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, E.J.; Dokken, B.B. Role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Curr. Drug Targets 2006, 7, 1435–1441. [Google Scholar] [CrossRef]
- Gingras, A.-A.; White, P.; Chouinard, P.Y.; Julien, P.; Davis, T.A.; Dombrowski, L.; Couture, Y.; Dubreuil, P.; Myre, A.; Bergeron, K.; et al. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J. Physiol. 2007, 579, 269–284. [Google Scholar] [CrossRef]
- Smith, G.; Atherton, P.; Reeds, M.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia–hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Wildman, R.P.; Hamm, L.L.; Muntner, P.; Reynolds, K.; Whelton, P.K.; He, J. Association between inflammation and insulin resistance in U.S. nondiabetic adults: Results from the Third National Health and Nutrition Examination Survey. Diabetes Care 2004, 27, 2960–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Li, C.; Lv, Y.; Zhang, Y.; Amakye, W.K.; Mao, L. DHA increases adiponectin expression more effectively than EPA at relative low concentrations by regulating PPARγ and its phosphorylation at Ser273 in 3T3-L1 adipocytes. Nutr. Metab. 2017, 14, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellmann, J.; Tang, Y.; Kosuri, M.; Bhatnagar, A.; Spite, M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011, 25, 2399–2407. [Google Scholar] [CrossRef] [Green Version]
- Browning, L.M.; Krebs, J.D.; Moore, C.S.; Mishra, G.D.; O’Connell, M.A.; Jebb, S.A. The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes, Obes. Metab. 2007, 9, 70–80. [Google Scholar] [CrossRef]
- Beurel, E. Regulation by Glycogen Synthase Kinase-3 of Inflammation and T Cells in CNS Diseases. Front. Mol. Neurosci. 2011, 4. [Google Scholar] [CrossRef] [Green Version]
- Crouch, P.J.; Cole, A.; Cousin, M.A.; Martínez, A.; Kanninen, K. Glycogen Synthase Kinase-3. Int. J. Alzheimer’s Dis. 2011, 2011, 1. [Google Scholar] [CrossRef] [Green Version]
- Jope, R.S.; Yuskaitis, C.J.; Beurel, E. Glycogen Synthase Kinase-3 (GSK3): Inflammation, Diseases, and Therapeutics. Neurochem. Res. 2006, 32, 577–595. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Huang, T.; Zheng, J.; Wu, K.; Li, D. Effect of Marine-Derived n-3 Polyunsaturated Fatty Acids on C-Reactive Protein, Interleukin 6 and Tumor Necrosis Factor α: A Meta-Analysis. PLoS ONE 2014, 9, e88103. [Google Scholar] [CrossRef] [Green Version]
- Rangel-Huerta, O.D.; Aguilera, C.M.; Mesa, M.-D.; Gil, A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: A systematic review of randomised clinical trials. Br. J. Nutr. 2012, 107, S159–S170. [Google Scholar] [CrossRef] [Green Version]
- Wallace, T.M.; Levy, J.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Characteristics | Total | Placebo | DHA-Rich |
|---|---|---|---|
| Fish Oil | |||
| (n = 58) | (n = 27) | (n = 31) | |
| Age (years) | 51.1 ± 1.7 | 48.8 ± 2.6 | 53.2 ± 2.5 |
| Males/females (n/n) | 20/38 | 9/19 | 11/19 |
| Anthropometry measures | |||
| Body weight (kg) | 89.4 ± 2.4 | 92.8 ± 4.1 | 86.3 ± 2.8 |
| Muscle mass (kg) | 31.5 ± 1.2 | 31.7 ± 1.2 | 31.4 ± 2.1 |
| Fat free mass (kg) | 55.2 ± 1.4 | 56.3 ± 2.0 | 54.3 ± 2.1 |
| Body mass index (kg·m−2) | 31.9 ± 0.8 | 33.5 ± 1.5 | 30.6 ± 0.8 |
| Waist circumference(cm) | 102.2 ± 2.4 | 106.0 ± 3.9 | 104.9 ± 2.9 |
| Plasma outcome measures | |||
| Fasting glucose (mmol/L) | 5.4 ± 0.1 | 5.3 ± 0.1 | 5.5 ± 0.1 |
| Fasting serum insulin (mIU/L) | 10.1 (6.1) | 10.2 (7.8) | 10 (5.8) |
| HOMA-IR | 2.3 (1.4) | 2.3 (2.3) | 2.3 (1.1) |
| CRP (mg/L) | 2.4 (4.6) | 3.2 (4.6) | 2.1 (4.9) |
| GSK-3β (ng/mL) | 2.5 (2.1) | 2.8 (1.9) | 2.1 (2.9) |
| Red blood cell measures | |||
| Eicosapentaenoic acid ** (%w/w) | 1.1 (0.4) | 1.0(0.4) | 1.2(0.4) |
| Docosahexaenoic acid ** (%w/w) | 6.1 ± 0.2 | 6.2 ± 0.3 | 6.0 ± 0.2 |
| LCn-3UFA (%w/w) | 7.1 (2.1) | 7.0(2.0) | 7.1(2.3) |
| Physical activity * (metabolic equivalents-min/week) | 2733 (4422) | 2504 (6066) | 2768 (3888) |
| Parameters | Post-Intervention | Change from Baseline | Between Group Differences |
|---|---|---|---|
| Mean Difference (95% CI) | |||
| Fasting glucose (mmol/L) | |||
| Normal range: 3.3–5.5 mmol/L | |||
| Placebo | 5.2 ± 0.1 | −0.04 ± 0.1 | |
| DHA-rich fish oil | 5.5 ± 0.1 | 0.04 ± 0.1 | 0.1 (−0.1, 0.3) |
| Fasting insulin (mIU/L) | |||
| Normal range: <10 mIU/L | |||
| Placebo | 12.0 ±1.1 | 0.4 ± 0.6 | |
| DHA-rich fish oil | 10.0 ± 0.9 | −1.3 ± 0.7 * | −1.7 (−3.5, 0.14) ** |
| HOMA-IR | |||
| Placebo | 2.9 ± 0.3 | 0.1 ± 0.2 | |
| DHA-rich fish oil | 2.4 ± 0.2 | −0.3 ± 0.2 | −0.4 (−0.9, 0.1) ** |
| C-reactive protein (mg/L) | |||
| Normal range: < 3.0 mg/L | |||
| Placebo | 4.6 ± 0.9 | −0.5 ± 0.4 | |
| DHA-rich fish oil | 3.4 ± 0.6 | −0.7 ± 0.6 | −0.2 (−1.8, 1.3) |
| Glycogen synthase kinase-3β (ng/mL) | |||
| Placebo | 2.8 ± 0.4 | −0.5 ± 0.4 | |
| DHA-rich fish oil | 2.4 ± 0.3 | −2.3 ± 0.3 * | −1.8 (−2.7, −0.9) ** |
| Eicosapentaenoic acid (%w/w) | |||
| Placebo | 1.0 ± 0.1 | −0.1 ± 0.1 | |
| DHA-rich fish oil | 1.8 ± 0.1 | 0.5 ± 0.1 * | 0.6 (0.4, 0.8) ** |
| Docosahexaenoic acid (%w/w) | |||
| Placebo | 6.0 (0.2) | −0.1 (0.9) | |
| DHA-rich fish oil | 9.7 (0.3) | 3.5 (1.7) * | 3.7 (3.1, 4.3) ** |
| LCn-3PUFA (%w/w) | |||
| Placebo | 7.1 (0.2) | −0.1 (1.04) | |
| DHA-rich fish oil | 11.1 (0.5) | 4.1 (2.1) * | 4.2 (3.6, 5.0) ** |
| C-Reactive Protein <2.4 mg/L | C-Reactive Protein ≥2.4 mg/L | |||
|---|---|---|---|---|
| Outcome Measures | Placebo | DHA-Rich Fish Oil | Placebo | DHA-Rich Fish Oil |
| Fasting glucose (mmol/L) | ||||
| Baseline | 5.2 ± 0.2 | 5.5 ± 0.1 | 5.3 ± 0.1 | 5.4 ± 0.2 |
| Δ Baseline | −0.1 ± 0.1 | −0.1 ± 0.1 | −0.03 ± 0.1 | 0.1 ± 0.1 |
| Fasting insulin (mIU/L) | ||||
| Baseline | 9.2 ± 1.2 | 8.7 ± 0.5 | 13.6 ± 1.7 | 14.4 ± 2.5 |
| Δ Baseline | 1.8 ± 0.9 | −0.6 ± 0.7 | −0.8 ± 0.7 | −2.1 ± 1.2 |
| HOMA-IR | ||||
| Baseline | 2.2 ± 0.3 | 2.1 ± 0.1 | 3.2 ± 0.4 | 3.5 ± 0.6 |
| Δ Baseline | 0.4 ± 0.2 | −1.3 ± 0.2 | −0.1 ± 0.2 | −0.5 ± 0.3 |
| Glycogen synthase kinase-β (ng/ML) | ||||
| Baseline | 2.5 ± 0.5 | 3.1 ± 0.5 | 3.4 ± 0.4 | 2.6 ± 0.4 |
| Δ Baseline | 0.18 ± 0.6 | −2.7 ± 0.3 | −1.1 ± 0.5 | −1.9 ± 0.4 |
| C-reactive protein (mg/L) | ||||
| Baseline | 1.3 (0.1) | 1.4(0.6) | 6(5.2) | 6.5(4.9) |
| Δ Baseline | 0 (0.8) | 0.1(0.6) | −0.9(2) | −0.8(2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thota, R.N.; Rosato, J.I.; Burrows, T.L.; Dias, C.B.; Abbott, K.A.; Martins, R.N.; Garg, M.L. Docosahexaenoic Acid-Rich Fish Oil Supplementation Reduces Kinase Associated with Insulin Resistance in Overweight and Obese Midlife Adults. Nutrients 2020, 12, 1612. https://doi.org/10.3390/nu12061612
Thota RN, Rosato JI, Burrows TL, Dias CB, Abbott KA, Martins RN, Garg ML. Docosahexaenoic Acid-Rich Fish Oil Supplementation Reduces Kinase Associated with Insulin Resistance in Overweight and Obese Midlife Adults. Nutrients. 2020; 12(6):1612. https://doi.org/10.3390/nu12061612
Chicago/Turabian StyleThota, Rohith N., Jessica I. Rosato, Tracy L. Burrows, Cintia B. Dias, Kylie A. Abbott, Ralph N. Martins, and Manohar L. Garg. 2020. "Docosahexaenoic Acid-Rich Fish Oil Supplementation Reduces Kinase Associated with Insulin Resistance in Overweight and Obese Midlife Adults" Nutrients 12, no. 6: 1612. https://doi.org/10.3390/nu12061612
APA StyleThota, R. N., Rosato, J. I., Burrows, T. L., Dias, C. B., Abbott, K. A., Martins, R. N., & Garg, M. L. (2020). Docosahexaenoic Acid-Rich Fish Oil Supplementation Reduces Kinase Associated with Insulin Resistance in Overweight and Obese Midlife Adults. Nutrients, 12(6), 1612. https://doi.org/10.3390/nu12061612








