Intra-Articular Route for the System of Molecules 14G1862 from Centella asiatica: Pain Relieving and Protective Effects in a Rat Model of Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the System of Molecules 14G1862 from Centella asiatica
2.2. Characterization Method of the System of Molecules 14G1862 from Centella asiatica
2.3. Cell Viability Assay
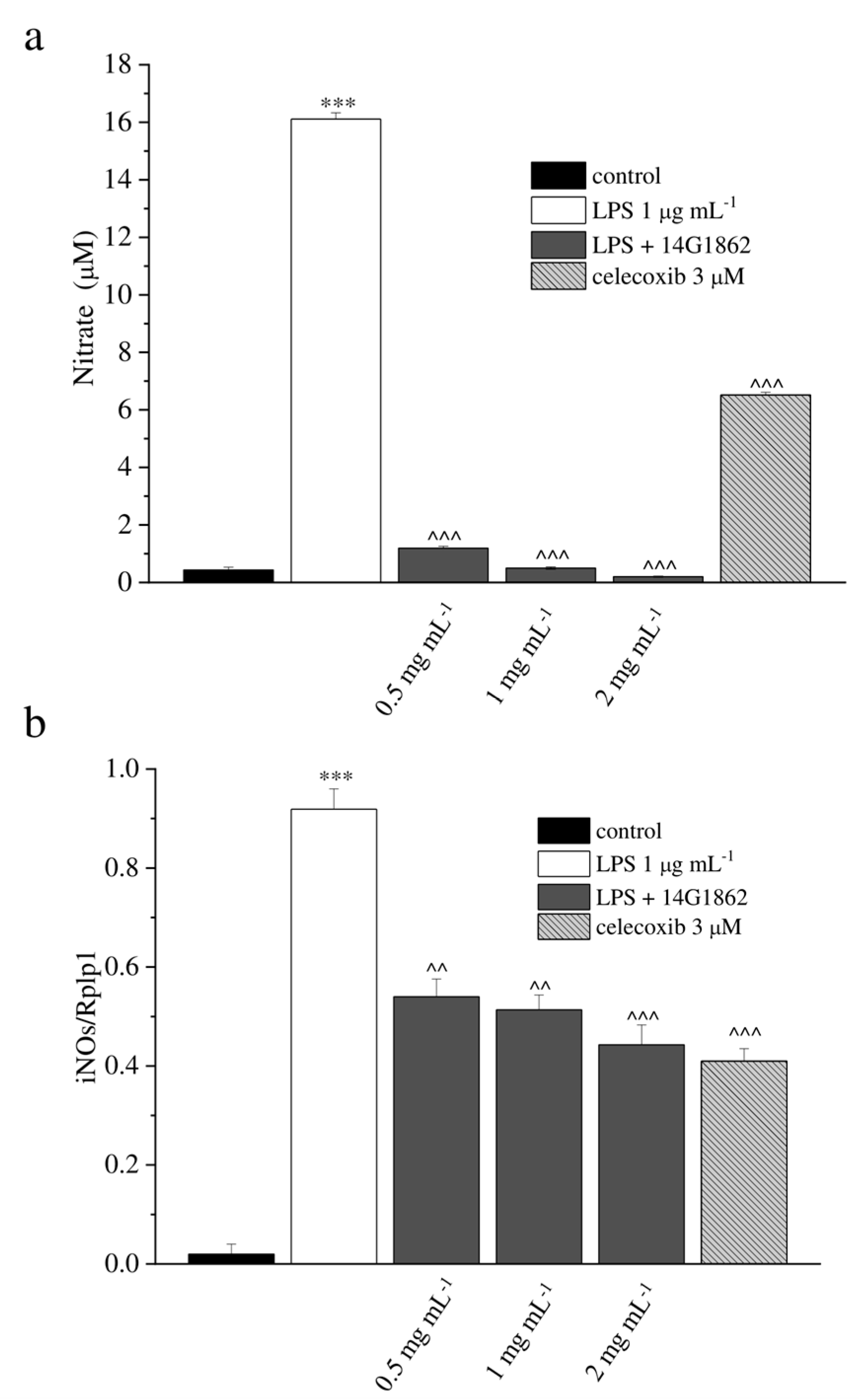
2.4. In Vitro Determination of Nitricoxide (NO) Production
2.5. RT-PCR
2.6. Animals
2.7. MIA-Induced Osteoarthritis
2.8. Treatment with the System of Molecules 14G1862 from Centella asiatica
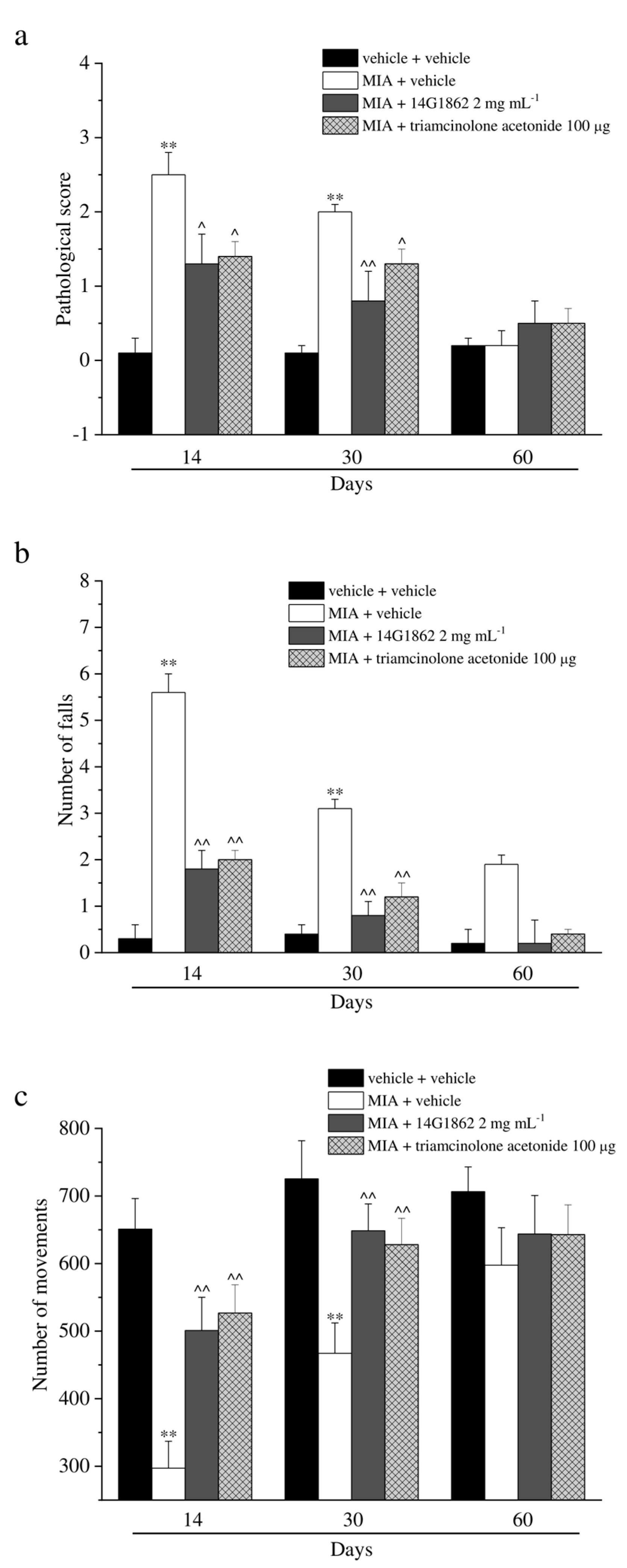
2.9. Paw Pressure Test
2.10. Von Frey Test
2.11. Incapacitance Test
2.12. Beam Balance Test
2.13. Rota Rod Test
2.14. Spontaneous Activity Meter (Animex Test)
2.15. Histological Evaluations
2.16. Statistical Analysis
3. Results
3.1. Characterization of the System of Molecules 14G1862 from Centella asiatica
3.2. In Vitro Evaluation
3.3. Behavioural Evaluation
3.4. Histological Analysis
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Chronic Rheumatic Conditions. 2018. Available online: http://www.who.int/chp/topics/rheumatic/en/GoogleScholar (accessed on 2 March 2020).
- Carr, A.J. Beyond disability: Measuring the social and personal consequences of osteoarthritis. Osteoarthr. Cartil. 1999, 7, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, V.Y.; Chan, L.; Carruthers, K.J. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: Stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch. Phys. Med. Rehabil. 2014, 95, 986.e1–995.e1. [Google Scholar] [PubMed] [Green Version]
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar] [PubMed]
- Sinusas, K. Osteoarthritis: Diagnosis and treatment. Am. Fam. Physician 2012, 85, 49–56. [Google Scholar] [PubMed]
- Zhang, W.; Moskowitz, R.W.; Nuki, G.; Abramson, S.; Altman, R.D.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008, 16, 137–162. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Doherty, M.; Arden, N.; Bannwarth, B.; Bijlsma, J.; Hauselmann, H.J.; Herrero-Beaumont, G.; Jordan, K.; Kaklamanis, P.; Leeb, B.; et al. EULAR evidence based recommendations for the management of hip osteoarthritis: Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann. Rheum. Dis. 2005, 64, 69–81. [Google Scholar] [CrossRef]
- Conaghan, P.G.; Dickson, J.; Grant, R.L. Care and management of osteoarthritis in adults: Summary of NICE guidance. BMJ 2008, 336, 502–503. [Google Scholar] [CrossRef] [Green Version]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierna-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [Green Version]
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15 (Suppl. 3), S2. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.H.; Kraus, V.B.; Setton, L.A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 2014, 10, 11–22. [Google Scholar] [CrossRef] [Green Version]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: A randomized clinical trial. J. Am. Med. Assoc. 2017, 317, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Mobasheri, A. Natural products for promoting joint health and managing osteoarthritis. Curr. Rheumatol. Rep. 2018, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Incandela, L.; Cesarone, M.R.; DeSanctis, M.T.; Belcaro, G.; Dugall, M.; Acerbi, G. Treatment of diabetic microangiopathy and edema with HR (Paroven, Venoruton; O-(β-hydroxyethyl)-rutosides): A prospective, placebo-controlled, randomized study. J. Cardiovasc. Pharmacol. Ther. 2002, 7 (Suppl. S1), S11–S15. [Google Scholar] [CrossRef]
- Subathra, M.; Shila, S.; Devi, M.A.; Panneerselvam, C. Emerging role of Centellaasiatica in improving age-related neurological antioxidant status. Exp. Gerontol. 2005, 40, 707–715. [Google Scholar] [CrossRef]
- Chong, N.J.; Aziz, Z. A systematic review of the efficacy of Centella asiatica for improvement of the signs and symptoms of chronic venous insufficiency. Evid. Based Complement. Altern. Med. 2013, 2013, 627182. [Google Scholar] [CrossRef] [Green Version]
- Pulito, C.; Mori, F.; Sacconi, A.; Casadei, L.; Ferraiuolo, M.; Valerio, M.C.; Santoro, R.; Goeman, F.; Maidecchi, A.; Mattoli, L.; et al. Cynara scolymus affects malignant pleural mesothelioma by promoting apoptosis and restraining invasion. Oncotarget 2015, 6, 18134–18150. [Google Scholar] [CrossRef] [Green Version]
- Council of Europe. European Pharmacopoeia, 9th ed.; Council of Europe: St. Petersburg, France, 2016. [Google Scholar]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [Green Version]
- Di Cesare Mannelli, L.; Micheli, L.; Zanardelli, M.; Ghelardini, C. Low dose native type II collagen prevents pain in a rat osteoarthritis model. BMC Musculoskelet. Disord. 2013, 14, 228. [Google Scholar] [CrossRef] [Green Version]
- Maresca, M.; Micheli, L.; Cinci, L.; Bilia, A.R.; Ghelardini, C.; Di Cesare Mannelli, L. Pain relieving and protective effects of Astragalus hydroalcoholic extract in rat arthritis models. J. Pharm. Pharmacol. 2017, 69, 1858–1870. [Google Scholar] [CrossRef] [Green Version]
- Micheli, L.; Ghelardini, C.; Lucarini, E.; Parisio, C.; Trallori, E.; Cinci, L.; Di Cesare Mannelli, L. Intra-articular mucilages: Behavioural and histological evaluations for a new model of articular pain. J. Pharm. Pharmacol. 2019, 71, 971–981. [Google Scholar] [CrossRef] [Green Version]
- Leighton, G.E.; Rodriguez, R.E.; Hill, R.G.; Hughes, J. κ-Opioid agonist produce antinociception after i.v. and i.c.v. but not intrathecal administration in the rat. Br. J. Pharmacol. 1988, 93, 553–560. [Google Scholar]
- Bird, M.F.; Cerlesi, M.C.; Brown, M.; Malfacini, D.; Vezzi, V.; Molinari, P.; Micheli, L.; Di Cesare Mannelli, L.; Ghelardini, C.; Guerrini, R.; et al. Characterisation of the Novel Mixed Mu-NOP Peptide Ligand Dermorphin-N/OFQ (DeNo). PLoS ONE 2016, 11, e0156897. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, M.; Egashira, N.; Kawashiri, T.; Yano, T.; Ikesue, H.; Oishi, R. Oxaliplatin-induced neuropathy in the rat: Involvement of oxalate in cold hyperalgesia but not mechanical allodynia. Pain 2009, 147, 165–174. [Google Scholar] [CrossRef]
- Baptista-de-Souza, D.; Di Cesare Mannelli, L.; Zanardelli, M.; Micheli, L.; Nunes-de-Souza, R.L.; Canto-de-Souza, A.; Ghelardini, C. Serotonergic modulation in neuropathy induced by oxaliplatin: Effect on the 5HT2C receptor. Eur. J. Pharmacol. 2014, 735, 141–149. [Google Scholar] [CrossRef]
- Bove, S.E.; Calcaterra, S.L.; Brooker, R.M.; Huber, C.M.; Guzman, R.E.; Juneau, P.L.; Schrier, D.J.; Kilgore, K.S. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr. Cartil. 2003, 11, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Maresca, M.; Micheli, L.; Di Cesare Mannelli, L.; Tenci, B.; Innocenti, M.; Khatib, M.; Mulinacci, N.; Ghelardini, C. Acute effect of Capparis spinosa root extracts on rat articular pain. J. Ethnopharmacol. 2016, 193, 456–465. [Google Scholar] [CrossRef]
- Ding, Y.; Li, J.; Lai, Q.; Rafols, J.A.; Luan, X.; Clark, J.; Diaz, F.G. Motor balance and coordination training enhances functional outcome in rat with transient middlecerebral artery occlusion. Neuroscience 2004, 123, 667–674. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Maresca, M.; Micheli, L.; Farina, C.; Scherz, M.W.; Ghelardini, C. A rat model of FOLFOX-induced neuropathy: Effects of oral dimiracetam in comparison with duloxetine and pregabalin. Cancer Chemother. Pharmacol. 2017, 80, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Snekhalatha, U.; Anburajan, M.; Venkatraman, B.; Menaka, M. Evaluation of complete Freund’s adjuvant-induced arthritis in a Wistar rat model. Comparison of thermography and histopathology. Z. Rheumatol. 2013, 72, 375–382. [Google Scholar] [CrossRef]
- Micheli, L.; Bozdag, M.; Akgul, O.; Carta, F.; Guccione, C.; Bergonzi, M.C.; Bilia, A.R.; Cinci, L.; Lucarini, E.; Parisio, C.; et al. Pain relieving effect of-NSAIDs-CAIs hybrid molecules: Systemic and intra-articular treatments against rheumatoid arthritis. Int. J. Mol. Sci. 2019, 20, 1923. [Google Scholar] [CrossRef]
- Bierma-Zeinstra, S.M.; Verhagen, A.P. Osteoarthritis subpopulations and implications for clinical trial design. Arthritis Res. Ther. 2011, 13, 213. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Xu, J.; Hunter, D.J.; Ding, C. Investigational drugs for the treatment of osteoarthritis. Expert Opin. Investig. Drugs 2015, 24, 1539–1556. [Google Scholar] [CrossRef]
- Richards, M.M.; Maxwell, J.S.; Weng, L.; Mathew, G.; Golzarian, J. Intra-articular treatment of knee osteoarthritis: From anti-inflammatories to products of regenerative medicine. Phys. Sports Med. 2017, 44, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American College Of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Jevsevar, D.; Donnelly, P.; Brown, G.; Cummins, D. Viscosupplementation for osteoarthritis of the knee: A systematic review of the evidence. J. Bone Joint Surg. Am. 2015, 97, 2047–2060. [Google Scholar] [CrossRef]
- Chandler, G.N.; Wright, V. Deleterious effects of intra-articular hydrocortisone. Lancet 1958, 2, 661–663. [Google Scholar] [CrossRef]
- McGarry, J.G.; Daruwalla, Z.J. The efficacy, accuracy and complications of corticosteroid injections of the knee joint. Knee Surg. Sport Traumatol. Arthrosc. 2011, 19, 1649–1654. [Google Scholar] [CrossRef]
- Pomonis, J.D.; Boulet, J.M.; Gottshall, S.L.; Phillips, S.; Sellers, R.; Bunton, T.; Walker, K. Development and pharmacological characterization of a rat model of osteoarthritis pain. Pain 2005, 114, 339–346. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Bani, D.; Bencini, A.; Brandi, M.L.; Calosi, L.; Cantore, M.; Carossino, A.M.; Ghelardini, C.; Valtancoli, B.; Failli, P. Therapeutic effects of the superoxide dismutase mimetic compound MnIIMe2DO2A on experimental articular pain in rats. Mediat. Inflamm. 2013, 2013, 905360. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Imaizumi, R.; Sumichika, H.; Tanaka, H.; Goda, M.; Fukunari, A.; Komatsu, H. Sodium iodoacetate-induced experimental osteoarthritis and associated pain model in rats. J. Vet. Med. Sci. 2003, 65, 1195–1199. [Google Scholar] [CrossRef] [Green Version]
- Guingamp, C.; Gegout-Pottie, P.; Philippe, L.; Terlain, B.; Netter, P.; Gillet, P. Mono iodoacetate-induced experimental osteoarthritis: A dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 1997, 40, 1670–1679. [Google Scholar] [CrossRef]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: An animal model of osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef]
- Fuchs, S.; Skwara, A.; Bloch, M.; Dankbar, B. Differential induction and regulation of matrix metalloproteinases in osteoarthritic tissue and fluid synovial fibroblasts. Osteoarthr. Cartil. 2004, 12, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Janusz, M.J.; Little, C.B.; King, L.E.; Hookfin, E.B.; Brown, K.K.; Heitmeyer, S.A.; Caterson, B.; Poole, A.R.; Taiwo, Y.O. Detection of aggrecanase- and MMP-generated catabolic neoepitopes in the rat iodoacetate model of cartilage degeneration. Osteoarthr. Cartil. 2004, 12, 720–728. [Google Scholar] [CrossRef] [Green Version]
- Scanzello, C.R.; Umoh, E.; Pessler, F.; Diaz-Torne, C.; Miles, T.; DiCarlo, E.; Potter, H.G.; Mandl, L.; Marx, R.; Rodeo, S.; et al. Local cytokine profiles in knee osteoarthritis: Elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthr. Cartil. 2009, 17, 1040–1048. [Google Scholar] [CrossRef] [Green Version]
- Pozgan, U.; Caglic, D.; Rozman, B.; Nagase, H.; Turk, V.; Turk, B. Expression and activity profiling of selected cysteine cathepsins and matrix metalloproteinases in synovial fluids from patients with rheumatoid arthritis and osteoarthritis. Biol. Chem. 2010, 391, 571–579. [Google Scholar] [CrossRef]
- Nakki, A.; Rodriguez-Fontenla, C.; Gonzalez, A.; Harilainen, A.; Leino-Arjas, P.; Heliovaara, M.; Eriksson, J.G.; Tallroth, K.; Videman, T.; Kaprio, J.; et al. Association study of MMP8 gene in osteoarthritis. Connect. Tissue Res. 2016, 57, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Mobasheri, A.; Matta, C.; Zakany, R.; Musumeci, G. Chondrosenescence: Definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas 2015, 80, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Tiku, M.L.; Liesch, J.B.; Robertson, F.M. Production of hydrogen peroxide by rabbit articular chondrocytes. Enhancement by cytokines. J. Immunol. 1990, 1990, 690–696. [Google Scholar]
- Rathakrishnan, C.; Tiku, K.; Raghavan, A.; Tiku, M.L. Release of oxygen radicals by articular chondrocytes: A study of luminol-dependent chemiluminescence and hydrogen peroxide secretion. J. Bone Miner. Res. 1992, 7, 1139–1148. [Google Scholar] [CrossRef]
- Sakurai, H.; Kohsaka, H.; Liu, M.F.; Higashiyama, H.; Hirata, Y.; Kanno, K.; Saito, I.; Miyasaka, N. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J. Clin. Investig. 1995, 96, 2357–2363. [Google Scholar] [CrossRef] [Green Version]
- Ostalowska, A.; Birkner, E.; Wiecha, M.; Kasperczyk, S.; Kasperczyk, A.; Kapolka, D.; Zon-Giebel, A. Lipid peroxidation and antioxidant enzymes in synovial fluid of patients with primary and secondary osteoarthritis of the knee joint. Osteoarthr. Cartil. 2006, 14, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.M.; Gilbert, S.J.; Caterson, B.; Sandell, L.J.; Archer, C.W. Oxidative stress induces expression of osteoarthritis markers procollagen IIA and 3B3(-) in adult bovine articular cartilage. Osteoarthr. Cartil. 2008, 16, 698–707. [Google Scholar] [CrossRef] [Green Version]
- Hiran, T.S.; Moulton, P.J.; Hancock, J.T. Detection of superoxide and NADPH oxidase in porcine articular chondrocytes. Free Radic. Biol. Med. 1997, 23, 736–743. [Google Scholar] [CrossRef]
- Regan, E.; Flannelly, J.; Bowler, R.; Tran, K.; Nicks, M.; Carbone, B.D.; Glueck, D.; Heijnen, H.; Mason, R.; Crapo, J. Extracellular superoxide dismutase and oxidant damage in osteoarthritis. Arthritis Rheumatol. 2005, 52, 3479–3491. [Google Scholar] [CrossRef] [Green Version]
- Aigner, T.; Fundel, K.; Saas, J.; Gebhard, P.M.; Haag, J.; Weiss, T.; Zien, A.; Obermayr, F.; Zimmer, R.; Bartnik, E. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheumatol. 2006, 54, 3533–3544. [Google Scholar] [CrossRef]
- Scott, J.L.; Gabrielides, C.; Davidson, R.K.; Swingler, T.E.; Clark, I.M.; Wallis, G.A.; Boot-Handford, R.P.; Kirkwood, T.B.; Taylor, R.W.; Young, D.A. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann. Rheum. Dis. 2010, 69, 1502–1510. [Google Scholar] [CrossRef] [Green Version]
- Altay, M.A.; Erturk, C.; Bilge, A.; Yapti, M.; Levent, A.; Aksoy, N. Evaluation of prolidase activity and oxidative status in patients with knee osteoarthritis: Relationships with radiographic severity and clinical parameters. Rheumatol. Int. 2015, 35, 1725–1731. [Google Scholar] [CrossRef]
- Johnson, K.; Jung, A.; Murphy, A.; Andreyev, A.; Dykens, J.; Terkeltaub, R. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheumatol. 2000, 43, 1560–1570. [Google Scholar] [CrossRef]
- Maneiro, E.; Lopez-Armada, M.J.; de Andres, M.C.; Carames, B.; Martin, M.A.; Bonilla, A.; Del Hoyo, P.; Galdo, F.; Arenas, J.; Blanco, F.J. Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann. Rheum. Dis. 2005, 64, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Rachek, L.I.; Grishko, V.I.; Ledoux, S.P.; Wilson, G.L. Role of nitric oxide-induced mtDNA damage in mitochondrial dysfunction and apoptosis. Free Radic. Biol. Med. 2006, 40, 754–762. [Google Scholar] [CrossRef] [PubMed]
- van Acker, S.A.; Tromp, M.N.; Haenen, G.R.; van der Vijgh, W.J.; Bast, A. Flavonoids as scavengers of nitric oxide radical. Biochem. Biophys. Res. Commun. 1995, 214, 755–759. [Google Scholar] [CrossRef]
- Yao, F.; Xue, Q.; Li, K.; Cao, X.; Sun, L.; Liu, Y. Phenolic compounds and ginsenosides in ginseng shoots and their antioxidant and anti-inflammatory capacities in LPS-induced RAW264.7 mouse macrophages. Int. J. Mol. Sci. 2019, 20, 2951. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Z.; Ye, G.; Huang, B. Kaempferol Alleviates the Interleukin-1β-Induced Inflammation in Rat Osteoarthritis Chondrocytes via Suppression of NF-κB. Med. Sci. Monit. 2017, 23, 3925–3931. [Google Scholar] [CrossRef] [Green Version]
- Feng, K.; Chen, Z.; Pengcheng, L.; Zhang, S.; Wang, X. Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J. Cell Physiol. 2019, 234, 18192–18205. [Google Scholar] [CrossRef]
- Borghi, S.M.; Mizokami, S.S.; Pinho-Ribeiro, F.A.; Fattori, V.; Crespigio, J.; Clemente-Napimoga, J.T.; Napimoga, M.H.; Pitol, D.L.; Issa, J.P.M.; Fukada, S.Y.; et al. The flavonoid quercetin inhibits titanium dioxide (TiO2)-induced chronic arthritis in mice. J. Nutr. Biochem. 2018, 53, 81–95. [Google Scholar] [CrossRef]
- Gou, K.J.; Zeng, R.; Dong, Y.; Hu, Q.Q.; Hu, H.W.; Maffucci, K.G.; Dou, Q.L.; Yang, Q.B.; Qin, X.H.; Qu, Y. Anti-inflammatory and analgesic effects of Polygonum orientale L. extracts. Front. Pharmacol. 2017, 8, 562. [Google Scholar] [CrossRef]
- Khatkar, A.; Sharma, K.K. Phenylpropanoids and its derivatives: Biological activities and its role in food, pharmaceutical and cosmetic industries. Crit. Rev. Food Sci. Nutr. 2019, 1–21. [Google Scholar] [CrossRef]
- Kao, J.H.; Lin, S.H.; Lai, C.H.; Lin, Y.C.; Kong, Z.L.; Wong, C.S. Shea nut oil triterpene concentrate attenuates knee osteoarthritis development in rats: Evidence from knee joint histology. PLoS ONE 2016, 11, e0162022. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Ma, C.; Xu, L.; Ran, J.; Jiang, L.; He, Y.; Adel Abdo Moqbel, S.; Wang, Z.; Wu, L. Polygalacic acid inhibits MMPs expression and osteoarthritis via Wnt/b-catenin and MAPK signal pathways suppression. Int. Immunopharmacol. 2018, 63, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Parisio, C.; Lucarini, E.; Micheli, L.; Toti, A.; Di Cesare Mannelli, L.; Antonini, G.; Panizzi, E.; Maidecchi, A.; Giovagnoni, E.; Lucci, J.; et al. Researching new therapeutic approaches for abdominal visceral pain treatment: Preclinical effects of an assembled system of molecules of vegetal origin. Nutrients 2019, 12, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Methods | Compounds | % (g of Compound/100 g of Sample) |
|---|---|---|
| Phenols, Total | 2.3409 | |
| Of which Flavonoids, total | 0.430 | |
| Of which Flavonols, total | 0.2910 | |
| UHPLC q-ToF | Kaempferol-7-O-glucoside | 0.019 |
| UHPLC q-ToF | Quercetin-3-O-glucuronide | 0.18 |
| UHPLC q-ToF | Quercetin-3-O-glucopyranoside | 0.033 |
| UHPLC q-ToF | Rutin | 0.02 |
| UHPLC q-ToF | Isorhamnetin | 0.0390 |
| Of which Flavanons, total | 0.0100 | |
| UHPLC q-ToF | Flavanomarein | 0.01 |
| Of which Dihydrochalcones, total | 0.0140 | |
| UHPLC q-ToF | Phloridzin | 0.014 |
| Of which Flavones, total | 0.1150 | |
| UHPLC q-ToF | Luteolin-4’-O-glucoside | 0.091 |
| UHPLC q-ToF | Luteolin-7-O-β-D-glucoside | 0.012 |
| UHPLC q-ToF | Schaftoside | 0.012 |
| Of which Acid Phenol, total | 0.0230 | |
| UHPLC q-ToF | Protocatechuic acid | 0.023 |
| Of which Phenylpropanoid derivatives, total | 0.48344 | |
| Of which Hydroxycinnamic acids, total | 0.0228 | |
| GC-MS (HS) | Cinnamic acid ethyl ester | 0.00006212 |
| GC-MS (HS) | Cinnamic acid methyl ester | 0.00004905 |
| UHPLC q-ToF | Verbascoside | 0.0130 |
| UHPLC q-ToF | Rosmarinic acid | 0.0097 |
| Of which Monolignols, total | 0.0068 | |
| UHPLC q-ToF (pos) | Syringin (Eleutheroside B) | 0.0068 |
| Of which Coumarins, total | 0.1110 | |
| UHPLC q-ToF | Aesculetin | 0.064 |
| UHPLC q-ToF | Aesculin | 0.034 |
| UHPLC q-ToF | Fraxin | 0.013 |
| Of which Lignans and Phenylpropenes, total | 0.000770045 | |
| GC-QqQ (HS) | Acetyleugenol | 0.00011256 |
| GC-QqQ (HS) | β-Asarone | 0.00011564 |
| GC-QqQ (HS) | α-Asarone | 0.00006897 |
| GC-QqQ (HS) | Apiole | 0.00006139 |
| GC-QqQ (HS) | Dillapiole | 0.00006708 |
| GC-QqQ (HS) | Eugenol | 0.00017197 |
| GC-QqQ (HS) | Isoeugenyl acetate | 0.00013133 |
| GC-QqQ (HS) | Myristicin | 0.00004111 |
| Of which Caffeic acids derivatives, total | 0.342 | |
| UHPLC q-ToF | 3,4-Dicaffeoylquinic acid | 0.022 |
| UHPLC q-ToF | 4,5-Dicaffeoylquinic acid | 0.13 |
| UHPLC q-ToF | Chlorogenic Acid | 0.190 |
| Of which Salicylates, total | 0.027 | |
| UHPLC q-ToF | Salicylic acid | 0.027 |
| Of which Simple Phenols, total | 0.0000441 | |
| GC-QqQ (HS) | m-Cresyl Acetate | 0.00001636 |
| GC-QqQ (HS) | Guaiacol Methyl ether | 0.00002774 |
| Of which Xanthones, total | 0.0075 | |
| UHPLC q-ToF | Mangiferin | 0.0075 |
| SFM | Tannins, total | 1.37 |
| Terpenes, Total | 5.012665 | |
| Of which Monoterpenes, total | 0.004806 | |
| Of which Monoterpene simple, total | 0.001123 | |
| GC-QqQ (HS) | β-Curcumene | 0.00068904 |
| GC-QqQ (HS) | p-Cymene (4-Cymene) | 0.00017318 |
| GC-QqQ (HS) | α-Curcumene | 0.00007149 |
| GC-QqQ (HS) | Limonene | 0.00004334 |
| GC-QqQ (HS) | β-Myrcene (myrcene) | 0.00007774 |
| GC-QqQ (HS) | β-Pinene | 0.00003455 |
| GC-QqQ (HS) | ɣ-Terpinene | 0.00003338 |
| Of which Monoterpene alcohol, total | 0.002889101 | |
| GC-QqQ (HS) | trans-Anethole | 0.00004189 |
| GC-QqQ (HS) | Borneol | 0.00076609 |
| GC-QqQ (HS) | 1,8-Cineol (Eucalyptol) | 0.00002888 |
| GC-QqQ (HS) | Geranyl Acetate | 0.00037654 |
| GC-QqQ (HS) | Linalool | 0.00143459 |
| GC-QqQ (HS) | Terpinen-4-ol | 0.00020283 |
| GC-QqQ (HS) | Menthol | 0.00003828 |
| Of which Monoterpene ketones and aldehydes total | 0.00056306 | |
| GC-QqQ (HS) | Camphor | 0.00027296 |
| GC-QqQ (HS) | Carvone | 0.00029010 |
| Of which Monoterpene Phenols derivatives, total | 0.00023069 | |
| GC-QqQ (HS) | Carvacrol | 0.00012183 |
| GC-QqQ (HS) | Thymol | 0.00010886 |
| Of which Sesquiterpenes, total | 0.00050243 | |
| GC-QqQ (HS) | Guaiazulene | 0.00000929 |
| GC-QqQ (HS) | Valencene | 0.00000851 |
| GC-QqQ (HS) | β-Eudesmol | 0.00002983 |
| GC-QqQ (HS) | Cedrol | 0.00002645 |
| GC-QqQ (HS) | Guaiol | 0.00003122 |
| GC-QqQ (HS) | Alloaromadendrene | 0.00021006 |
| GC-QqQ (HS) | α-Humulene | 0.00011879 |
| GC-QqQ (HS) | α-Bisabolol | 0.00003985 |
| GC-QqQ (HS) | Azulene | 0.00001726 |
| GC-QqQ (HS) | Chamazulene | 0.00001117 |
| Of which Triterpenes, total | 5.00724 | |
| UHPLC q-ToF | Asiatic Acid | 0.087 |
| UHPLC q-ToF | Madecassic Acid | 0.660 |
| Of whichSaponins, total | 4.2602 | |
| UHPLC q-ToF | Asiaticoside | 1.96 |
| UHPLC q-ToF | Madecassoside | 2.30 |
| UHPLC q-ToF | Hederagenin | 0.00024 |
| Of which Apocarotenoids, total | 0.00011726 | |
| GC-QqQ (HS) | β-Ionone | 0.00008004 |
| GC-QqQ (HS) | α-Ionone | 0.00003722 |
| Aromatic Alcohols, Total | 0.00017948 | |
| GC-QqQ (HS) | 1-Phenylethanol | 0.00003749 |
| GC-QqQ (HS) | 4-Isopropyl Benzyl Alcohol | 0.00014199 |
| Aromatic Aldehydes, Total | 0.0003053 | |
| GC-QqQ (HS) | Anisaldehyde | 0.00026692 |
| GC-QqQ (HS) | Cuminaldehyde | 0.00003838 |
| Aromatic Acids, Aromatic Esters and Lactones, Total | 0.00023445 | |
| GC-QqQ (HS) | Phenylacetic Acid Ethyl ester | 0.00003081 |
| GC-QqQ (HS) | Benzoic acid Ethyl ester | 0.00002015 |
| GC-QqQ (HS) | Benzyl Acetate | 0.00002002 |
| GC-QqQ (HS) | Benzyl Benzoate | 0.00001766 |
| GC-QqQ (HS) | Benzoic acid Eugenyl Ester | 0.00001478 |
| GC-QqQ (HS) | Benzoic Acid Methyl ester | 0.00000798 |
| GC-QqQ (HS) | Cinnamyl Acetate | 0.00012305 |
| Aromatic Ketones Total | 0.00009413 | |
| GC-QqQ (HS) | 4-Chromanone | 0.0000718 |
| GC-QqQ (HS) | Acetophenone | 0.0000223 |
| Esters, Total | 0.00003215 | |
| GC-QqQ (HS) | Ethyl Decanoate | 0.00003215 |
| Organic Acids, Total | 0.16 | |
| Of which Monocarboxylic, total | 0.13 | |
| UHPLC q-ToF | Quinic acid | 0.13 |
| Of which Dicarboxylic, total | 0.032 | |
| UHPLC q-ToF | Azelaic acid | 0.032 |
| Fats, Total | 0.002828 | |
| Of which Saturated acids and derivatives, total | 0.0093 | |
| GC-QqQ (Der) | Lignoceric Acid | 0.00220 |
| GC-QqQ (Der) | Myristic Acid | 0.00160 |
| GC-QqQ (Der) | Behenic Acid | 0.00300 |
| GC-QqQ (Der) | Arachidic Acid | 0.00240 |
| GC-QqQ (Der) | Octanoic Acid | 0.00009 |
| Of which Unsaturated acids and derivatives, total | 0.018990 | |
| GC-QqQ (Der) | Oleic Acid | 0.00089 |
| GC-QqQ (Der) | Linoleic Acid | 0.01810 |
| HPLC-RID | Polysaccharides > 20KDa, Total | 5.74 |
| Vitamins, Total | 0.35 | |
| Of which Hydro-soluble Vitamins | 0.35 | |
| UHPLC q-ToF (pos) | Choline (Vit J) | 0.35 |
| Minerals, Total | 16.518931 | |
| Of which Macro-elements, Total | 8.9494 | |
| ICP-MS | Calcium | 0.0614 |
| ICP-MS | Magnesium | 0.2183 |
| ICP-MS | Potassium | 7.0843 |
| ICP-MS | Sodium | 1.5854 |
| Of which Micro- Oligo-elements, Total | 0.02184 | |
| ICP-MS | Cobalt | 0.000172 |
| ICP-MS | Copper | 0.000757 |
| ICP-MS | Iron | 0.0028 |
| ICP-MS | Manganese | 0.0053 |
| ICP-MS | Selenium | 0.000001 |
| ICP-MS | Zinc | 0.001016 |
| ICP-MS | Arsenic | 0.000006 |
| ICP-MS | Boron | 0.000439 |
| ICP-MS | Chromium | 0.000027 |
| ICP-MS | Nichel | 0.000608 |
| ICP-MS | Silicon | 0.0107 |
| ICP-MS | Vanadium | 0.0000140 |
| Of which Other elements, Total | 0.0293 | |
| ICP-MS | Rubidium | 0.02217 |
| ICP-MS | Lithium | 0.000063 |
| ICP-MS | Barium | 0.000103 |
| ICP-MS | Aluminium | 0.0069 |
| ICP-MS | Cadmium | 0.0000190 |
| ICP-MS | Thallium | 0.0000260 |
| ICP-MS | Gallium | 0.0000090 |
| ICP-MS | Selenium | 0.0000010 |
| Of which Anions, Total | 7.1584 | |
| IC-CD | Chloride | 6.8162 |
| IC-CD | Nitrate | 0.3251 |
| IC-CD | Phosphate | 0.2086 |
| IC-CD | Sulphate | 0.1685 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micheli, L.; Di Cesare Mannelli, L.; Mattoli, L.; Tamimi, S.; Flamini, E.; Garetto, S.; Lucci, J.; Giovagnoni, E.; Cinci, L.; D’Ambrosio, M.; et al. Intra-Articular Route for the System of Molecules 14G1862 from Centella asiatica: Pain Relieving and Protective Effects in a Rat Model of Osteoarthritis. Nutrients 2020, 12, 1618. https://doi.org/10.3390/nu12061618
Micheli L, Di Cesare Mannelli L, Mattoli L, Tamimi S, Flamini E, Garetto S, Lucci J, Giovagnoni E, Cinci L, D’Ambrosio M, et al. Intra-Articular Route for the System of Molecules 14G1862 from Centella asiatica: Pain Relieving and Protective Effects in a Rat Model of Osteoarthritis. Nutrients. 2020; 12(6):1618. https://doi.org/10.3390/nu12061618
Chicago/Turabian StyleMicheli, Laura, Lorenzo Di Cesare Mannelli, Luisa Mattoli, Sara Tamimi, Enrico Flamini, Stefano Garetto, Jacopo Lucci, Emiliano Giovagnoni, Lorenzo Cinci, Mario D’Ambrosio, and et al. 2020. "Intra-Articular Route for the System of Molecules 14G1862 from Centella asiatica: Pain Relieving and Protective Effects in a Rat Model of Osteoarthritis" Nutrients 12, no. 6: 1618. https://doi.org/10.3390/nu12061618
APA StyleMicheli, L., Di Cesare Mannelli, L., Mattoli, L., Tamimi, S., Flamini, E., Garetto, S., Lucci, J., Giovagnoni, E., Cinci, L., D’Ambrosio, M., Luceri, C., & Ghelardini, C. (2020). Intra-Articular Route for the System of Molecules 14G1862 from Centella asiatica: Pain Relieving and Protective Effects in a Rat Model of Osteoarthritis. Nutrients, 12(6), 1618. https://doi.org/10.3390/nu12061618







