Induction of Autophagy by Vasicinone Protects Neural Cells from Mitochondrial Dysfunction and Attenuates Paraquat-Mediated Parkinson’s Disease Associated α-Synuclein Levels
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Cell Culture and Treatments
2.3. MTT Assay
2.4. Detection of Mitochondrial ROS Generation
2.5. Detection of Mitochondrial Membrane Potential (Δψm)
2.6. Seperation of Mitochondrial Fractionation
2.7. Immunofluorescence Staining
2.8. Immunoblotting Assay
2.9. Statistical Analysis
3. Results
3.1. Vasicinone Ameliorated Paraquat Mediated Cytotoxicity in SH-SY5Y Cells
3.2. Vasicinone Prevented Paraquat Mediated Loss of Mitochondriyal Potential and ROS Generation in SH-SY5Y Cells
3.3. Vasicinone Protected SH-SY5Y Cells through Maintaining the Antioxidant Redox System
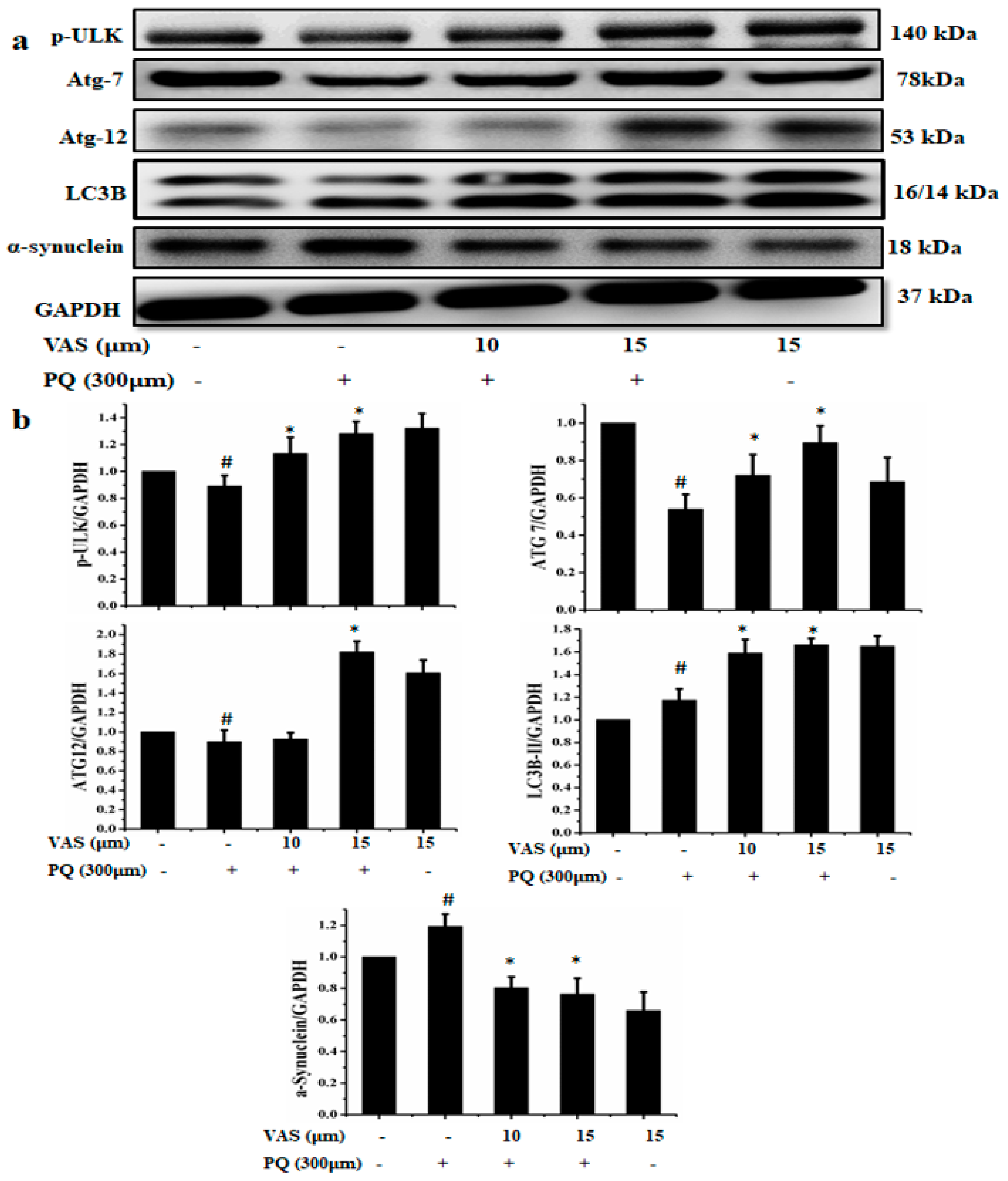
3.4. Vasicinone Enhanced the Clearance of α-Synuclein by Upregulating Autophagy
3.5. Vasicinone Protected SH-SY5Y Cells through PINK-1/Parkin-Mediated Mitophagy
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Lin, C.-Y.; Tsai, C.-W. Carnosic Acid Attenuates 6-Hydroxydopamine-Induced Neurotoxicity in SH-SY5Y Cells by Inducing Autophagy Through an Enhanced Interaction of Parkin and Beclin1. Mol. Neurobiol. 2016, 54, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Morizane, A.; Doi, D.; Magotani, H.; Onoe, H.; Hayashi, T.; Mizuma, H.; Takara, S.; Takahashi, R.; Inoue, H.; et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 2017, 548, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-S.; Geng, W.-S.; Jia, J.-J. Neurotoxin-Induced Animal Models of Parkinson Disease: Pathogenic Mechanism and Assessment. ASN Neuro 2018, 10. [Google Scholar] [CrossRef]
- Santiago, J.A.; Bottero, V.; Potashkin, J. Biological and Clinical Implications of Comorbidities in Parkinson’s Disease. Front. Aging Neurosci. 2017, 9. [Google Scholar] [CrossRef]
- Mythri, R.B.; Bharath, M.M. Curcumin: A potential neuroprotective agent in Parkinson’s disease. Curr. Pharm. Des. 2012, 18, 91–99. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Mariño, G.; Kroemer, G. Autophagy and Aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef]
- Wong, E.; Cuervo, A.M. Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 2010, 13, 805–811. [Google Scholar] [CrossRef]
- Pan, T.; Kondo, S.; Le, W.; Jankovic, J. The role of autophagy-lysosome pathway in neurode generation associated with Parkinson’s disease. Brain 2008, 131, 1969–19786. [Google Scholar] [CrossRef]
- Chu, Y.; Dodiya, H.B.; Aebischer, P.; Olanow, C.W.; Kordower, J.H. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: Relationship to alpha-synuclein inclusions. Neurobiol. Dis. 2009, 35, 385–398. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; MacLeod, K. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Van Laar, V.S.; Berman, S. The interplay of neuronal mitochondrial dynamics and bioenergetics: Implications for Parkinson’s disease. Neurobiol. Dis. 2012, 51, 43–55. [Google Scholar] [CrossRef]
- Von Stockum, S.; Nardin, A.; Schrepfer, E.; Ziviani, E. Mitochondrial dynamics and mitophagy in Parkinson’s disease: A fly point of view. Neurobiol. Dis. 2016, 90, 58–67. [Google Scholar] [CrossRef]
- Solesio, M.E.; Saez-Atienzar, S.; Jordan, J.; Galindo, M.F. Characterization of Mitophagy in the 6-Hydoxydopamine Parkinson’s Disease Model. Toxicol. Sci. 2012, 129, 411–420. [Google Scholar] [CrossRef]
- Miller, S.; Muqit, M.M.K. Therapeutic approaches to enhance PINK1/Parkin mediated mitophagy for the treatment of Parkinson’s disease. Neurosci Lett. 2019, 705, 7–13. [Google Scholar] [CrossRef]
- Cho, N.-H.; Nakamura, T.; Lipton, S.A. Mitochondrial dynamics in cell death and neurodegeneration. Cell. Mol. Life Sci. 2010, 67, 3435–3447. [Google Scholar] [CrossRef]
- Wu, W.; Xu, H.; Wang, Z.; Mao, Y.; Yuan, L.; Luo, W.; Cui, Z.; Cui, T.; Wang, X.L.; Shen, Y.H. PINK1-Parkin-Mediated Mitophagy Protects Mitochondrial Integrity and Prevents Metabolic Stress-Induced Endothelial Injury. PLoS ONE 2015, 10, e0132499. [Google Scholar] [CrossRef]
- Springer, W.; Kahle, P.J. Regulation of PINK1-Parkin-mediated mitophagy. Autophagy 2011, 7, 266–278. [Google Scholar] [CrossRef]
- Song, Y.; Du, Y.; Zou, W.; Luo, Y.; Zhang, X.; Fu, J. Involvement of impaired autophagy and mitophagy in Neuro-2a cell damage under hypoxic and/or high-glucose conditions. Sci. Rep. 2018, 8, 3301. [Google Scholar] [CrossRef]
- Jin, S.M.; Youle, R.J. PINK1- and Parkin-mediated mitophagy at a glance. J. Cell Sci. 2012, 125, 795–799. [Google Scholar] [CrossRef]
- Ashrafi, G.; Schwarz, T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2012, 20, 31–42. [Google Scholar] [CrossRef]
- Wu, H.; Wei, H.; Sehgal, S.A.; Liu, L.; Chen, Q. Mitophagy receptors sense stress signals and couple mitochondrial dynamic machinery for mitochondrial quality control. Free Radic. Boil. Med. 2016, 100, 199–209. [Google Scholar] [CrossRef]
- Briston, T.; Hicks, A.R. Mitochondrial dysfunction and neurodegenerative proteinopathies: Mechanisms and prospects for therapeutic intervention. Biochem. Soc. Trans. 2018, 46, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.H.; Mehta, D.R. A Bronchodilator Alkaloid (Vasicinone) from Adhatoda vasica Nees. Nature 1959, 184, 1317. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, R.A. Anti-inflammatory and antimicrobial properties of pyrroloquinazoline alkaloids from Adhatoda vasica Nees. Phytomedicine 2013, 20, 441–445. [Google Scholar] [CrossRef]
- Lahiri, P.K.; Pradhan, S.N. Pharmacological investigation of vasicinol, an alkaloid from Adhatoda vasica Nees. Ind. J. Exp. Biol. 1964, 2, 219. [Google Scholar]
- Kulkarni, A.A. Ray of hope for cancer patients. In Proceedings of the International Seminar on Holistic Management of Cancer, Jerusalem, Geneva, 2 July 2019; Ayurvedic Education: Albuquerque, NM, USA, 1998; Volume 67, pp. 5–11. [Google Scholar]
- Roja, G.; Vikrant, B.; Sandur, S.K.; Sharma, A.; Pushpa, K. Accumulation of vasicine and vasicinone in tissue cultures of Adhatoda vasica and evaluation of the free radical-scavenging activities of the various crude extracts. Food Chem. 2011, 126, 1033–1038. [Google Scholar] [CrossRef]
- Pan, T.; Rawal, P.; Wu, Y.; Xie, W.; Jankovic, J.; Le, W. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience 2009, 164, 541–551. [Google Scholar] [CrossRef]
- Deng, Y.-N.; Shi, J.; Liu, J.; Qu, Q. Celastrol protects human neuroblastoma SH-SY5Y cells from rotenone-induced injury through induction of autophagy. Neurochem. Int. 2013, 63, 1–9. [Google Scholar] [CrossRef]
- Liu, K.; Shi, N.; Sun, Y.; Zhang, T.; Sun, X. Therapeutic effects of rapamycin on MPTP-induced Parkinsonism in mice. Neurochem. Res. 2013, 38, 201–207. [Google Scholar] [CrossRef]
- Dagda, R.K.; Das Banerjee, T.; Janda, E. How Parkinsonian Toxins Dysregulate the Autophagy Machinery. Int. J. Mol. Sci. 2013, 14, 22163–22189. [Google Scholar] [CrossRef] [PubMed]
- Cerri, S.; Blandini, F. Role of Autophagy in Parkinson’s Disease. Curr. Med. Chem. 2019, 26, 3702–3718. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.P.; Hirsch, E.C.; Hunot, S. Understanding Dopaminergic Cell Death Pathways in Parkinson Disease. Neuron 2016, 90, 675–691. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Emr, S.D. Autophagy as a Regulated Pathway of Cellular Degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zhu, J.X.; Xie, W.; Le, W.; Fan, Z.; Jankovic, J.; Pan, T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals 2011, 19, 163–174. [Google Scholar] [CrossRef]
- Polo, R.-A.G.; Niso-Santano, M.; Ortiz-Ortiz, M.A.; Gómez-Martín, A.; Moran, J.M.; García-Rubio, L.; Francisco-Morcillo, J.; Zaragoza, C.; Soler, G.; Fuentes, J.M. Inhibition of Paraquat-Induced Autophagy Accelerates the Apoptotic Cell Death in Neuroblastoma SH-SY5Y Cells. Toxicol. Sci. 2007, 97, 448–458. [Google Scholar] [CrossRef]
- Ryan, B.J.; Hoek, S.; Fon, E.A.; Wade-Martins, R. Mitochondrial dysfunction and mitophagy in Parkinson’s: From familial to sporadic disease. Trends Biochem. Sci. 2015, 40, 200–210. [Google Scholar] [CrossRef]
- Bose, A.; Beal, M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016, 139, 216–231. [Google Scholar] [CrossRef]
- Fang, C.; Gu, L.; Smerin, D.; Mao, S.; Xiong, X.-X. The Interrelation between Reactive Oxygen Species and Autophagy in Neurological Disorders. Oxid. Med. Cell. Longev. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Castello, P.R.; Drechsel, D.A.; Patel, M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J. Boil. Chem. 2007, 282, 14186–14193. [Google Scholar] [CrossRef]
- Drechsel, D.A.; Patel, M. Chapter 21 Paraquat-Induced Production of Reactive Oxygen Species in Brain Mitochondria. Methods Enzymol. 2009, 456, 381–393. [Google Scholar] [CrossRef]
- Geisler, S.; Holmstrom, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef]
- Narendra, D.; Tanaka, A.; Suen, D.-F.; Youle, R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Boil. 2008, 183, 795–803. [Google Scholar] [CrossRef]
- Eiyama, A.; Okamoto, K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr. Opin. Cell Biol. 2015, 33, 95–101. [Google Scholar] [CrossRef]
- Weissbach, A.; Bäumer, T.; Pramstaller, P.P.; Brüggemann, N.; Tadic, V.; Chen, R.; Klein, C.; Münchau, A. Abnormal premotor–motor interaction in heterozygous Parkin - and Pink1 mutation carriers. Clin. Neurophysiol. 2017, 128, 275–280. [Google Scholar] [CrossRef]
- Sun, Y.; Vashisht, A.A.; Tchieu, J.; Wohlschlegel, J.A.; Dreier, L. Voltage-dependent Anion Channels (VDACs) Recruit Parkin to Defective Mitochondria to Promote Mitochondrial Autophagy. J. Boil. Chem. 2012, 287, 40652–40660. [Google Scholar] [CrossRef]
- Shendelman, S.; Jonason, A.; Martinat, C.; Leete, T.; Abeliovich, A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Boil. 2004, 2, e362. [Google Scholar] [CrossRef]
- Krebiehl, G.; Ruckerbauer, S.; Burbulla, L.F.; Kieper, N.; Maurer, B.; Waak, J.; Wolburg, H.; Gizatullina, Z.; Gellerich, F.N.; Woitalla, D.; et al. Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson’s disease-associated protein DJ-1. PLoS ONE 2010, 5, e9367. [Google Scholar] [CrossRef]
- Bahmed, K.; Messier, E.M.; Zhou, W.; Tuder, R.M.; Freed, C.R.; Chu, H.W.; Kelsen, S.G.; Bowler, R.P.; Mason, R.J.; Kosmider, B. DJ-1 Modulates Nuclear Erythroid 2–Related Factor-2–Mediated Protection in Human Primary Alveolar Type II Cells in Smokers. Am. J. Respir. Cell Mol. Boil. 2016, 55, 439–449. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Allen, R.G.; Tresini, M. Oxidative stress and gene regulation. Free Radic. Biol. Med. 2000, 28, 463–499. [Google Scholar] [CrossRef]
- Zhu, T.-G.; Wang, X.-X.; Luo, W.; Zhang, Q.-L.; Huang, T.-T.; Xu, X.; Liu, C.-F. Protective effects of urate against 6-OHDA-induced cell injury in PC12 cells through antioxidant action. Neurosci. Lett. 2012, 506, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Biosa, A.; Sanchez-Martinez, A.; Filograna, R.; Terriente-Felix, A.; Alam, S.M.; Beltramini, M.; Bubacco, L.; Bisaglia, M.; Whitworth, A.J. Superoxide dismutating molecules rescue the toxic effects of PINK1 and parkin loss. Hum. Mol. Genet. 2018, 27, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.-T.; Sivalingam, K.; Kuo, W.-W.; Ho, T.-J.; Chang, R.-L.; Chung, L.-C.; Day, C.H.; Viswanadha, V.P.; Liao, P.-H.; Huang, C.-Y. Effect of Vasicinone against Paraquat-Induced MAPK/p53-Mediated Apoptosis via the IGF-1R/PI3K/AKT Pathway in a Parkinson’s Disease-Associated SH-SY5Y Cell Model. Nutrients 2019, 11, 1655. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Sivalingam, K.; Shibu, M.A.; Liao, P.-H.; Ho, T.-J.; Kuo, W.-W.; Chen, R.-J.; Day, C.-H.; Viswanadha, V.P.; Ju, D.-T. Induction of Autophagy by Vasicinone Protects Neural Cells from Mitochondrial Dysfunction and Attenuates Paraquat-Mediated Parkinson’s Disease Associated α-Synuclein Levels. Nutrients 2020, 12, 1707. https://doi.org/10.3390/nu12061707
Huang C-Y, Sivalingam K, Shibu MA, Liao P-H, Ho T-J, Kuo W-W, Chen R-J, Day C-H, Viswanadha VP, Ju D-T. Induction of Autophagy by Vasicinone Protects Neural Cells from Mitochondrial Dysfunction and Attenuates Paraquat-Mediated Parkinson’s Disease Associated α-Synuclein Levels. Nutrients. 2020; 12(6):1707. https://doi.org/10.3390/nu12061707
Chicago/Turabian StyleHuang, Chih-Yang, Kalaiselvi Sivalingam, Marthandam Asokan Shibu, Po-Hsiang Liao, Tsung-Jung Ho, Wei-Wen Kuo, Ray-Jade Chen, Cecilia-Hsuan Day, Vijaya Padma Viswanadha, and Da-Tong Ju. 2020. "Induction of Autophagy by Vasicinone Protects Neural Cells from Mitochondrial Dysfunction and Attenuates Paraquat-Mediated Parkinson’s Disease Associated α-Synuclein Levels" Nutrients 12, no. 6: 1707. https://doi.org/10.3390/nu12061707
APA StyleHuang, C.-Y., Sivalingam, K., Shibu, M. A., Liao, P.-H., Ho, T.-J., Kuo, W.-W., Chen, R.-J., Day, C.-H., Viswanadha, V. P., & Ju, D.-T. (2020). Induction of Autophagy by Vasicinone Protects Neural Cells from Mitochondrial Dysfunction and Attenuates Paraquat-Mediated Parkinson’s Disease Associated α-Synuclein Levels. Nutrients, 12(6), 1707. https://doi.org/10.3390/nu12061707




