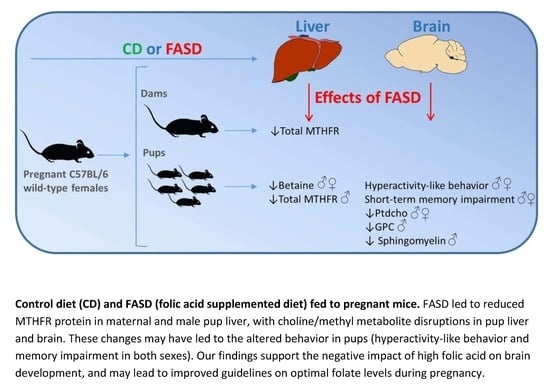
Moderate Folic Acid Supplementation in Pregnant Mice Results in Behavioral Alterations in Offspring with Sex-Specific Changes in Methyl Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Dosage Information
2.2. Behavioral Testing
2.2.1. OF Test
2.2.2. NOR Test
2.2.3. Reciprocal Social Interactions
2.2.4. Grip Strength Measurement
2.3. Western Blotting
2.4. Measurement of SAM, SAH and Choline Metabolites by Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry (LC-MS)
2.5. Measurement of PtdCho and SM by Matrix-Assisted Laser Desorption Ionization Imaging Mass Spectrometry (MALDI-IMS)
2.5.1. Sample Preparation
2.5.2. Imaging Mass Spectrometry (IMS)
2.6. Statistical Analysis
3. Results
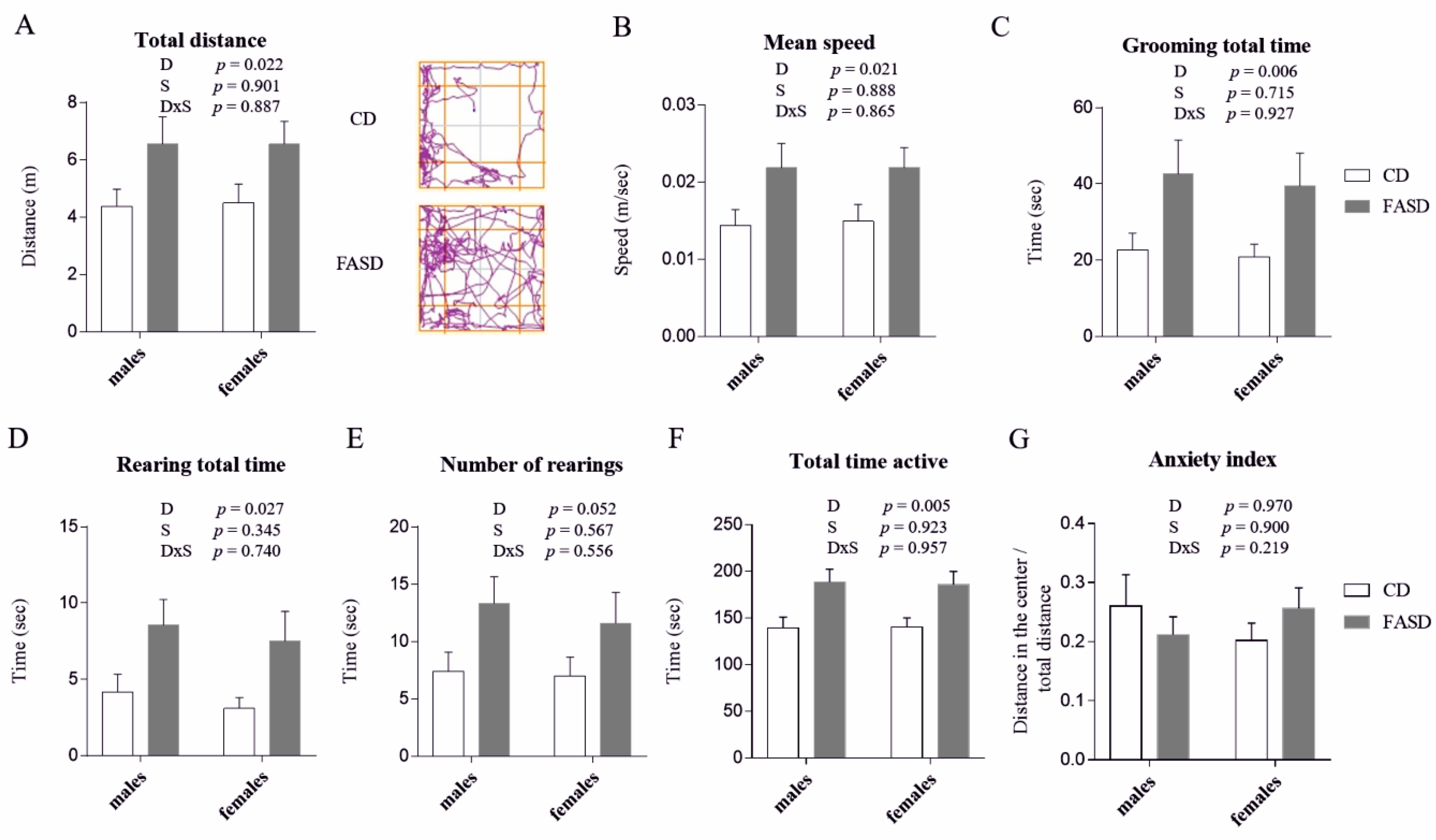
3.1. 5× FASD during Pregnancy and Lactation Leads to Behavioral Alterations in Offspring
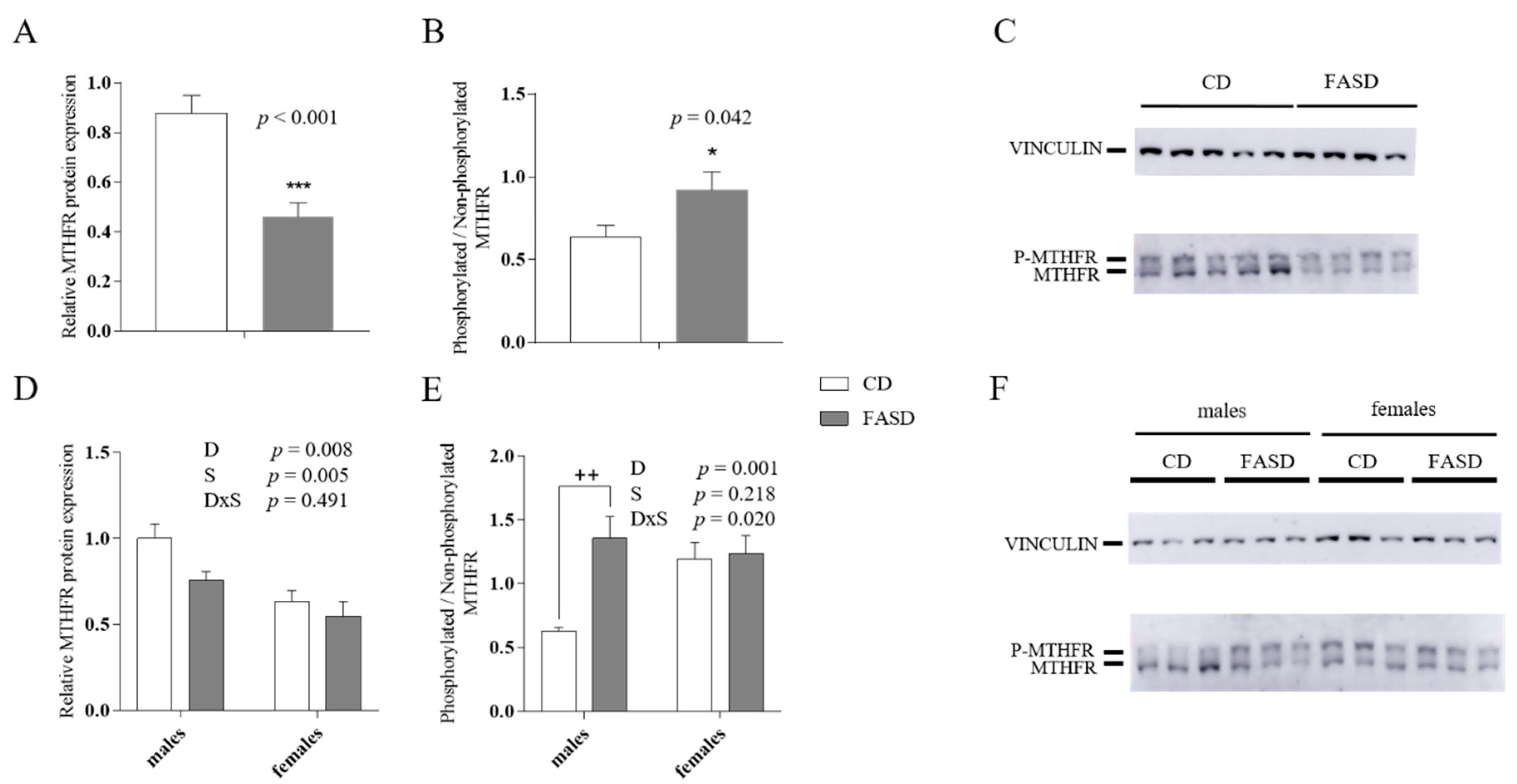
3.2. Reduced MTHFR Protein in Maternal and Male Offspring Liver
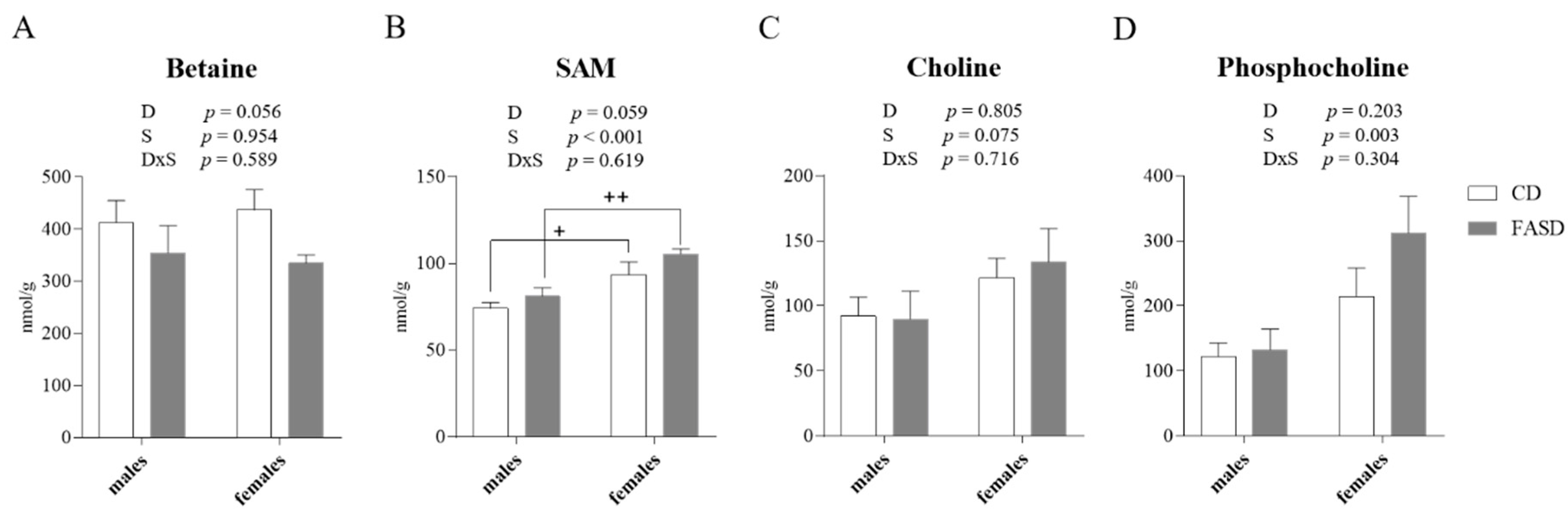
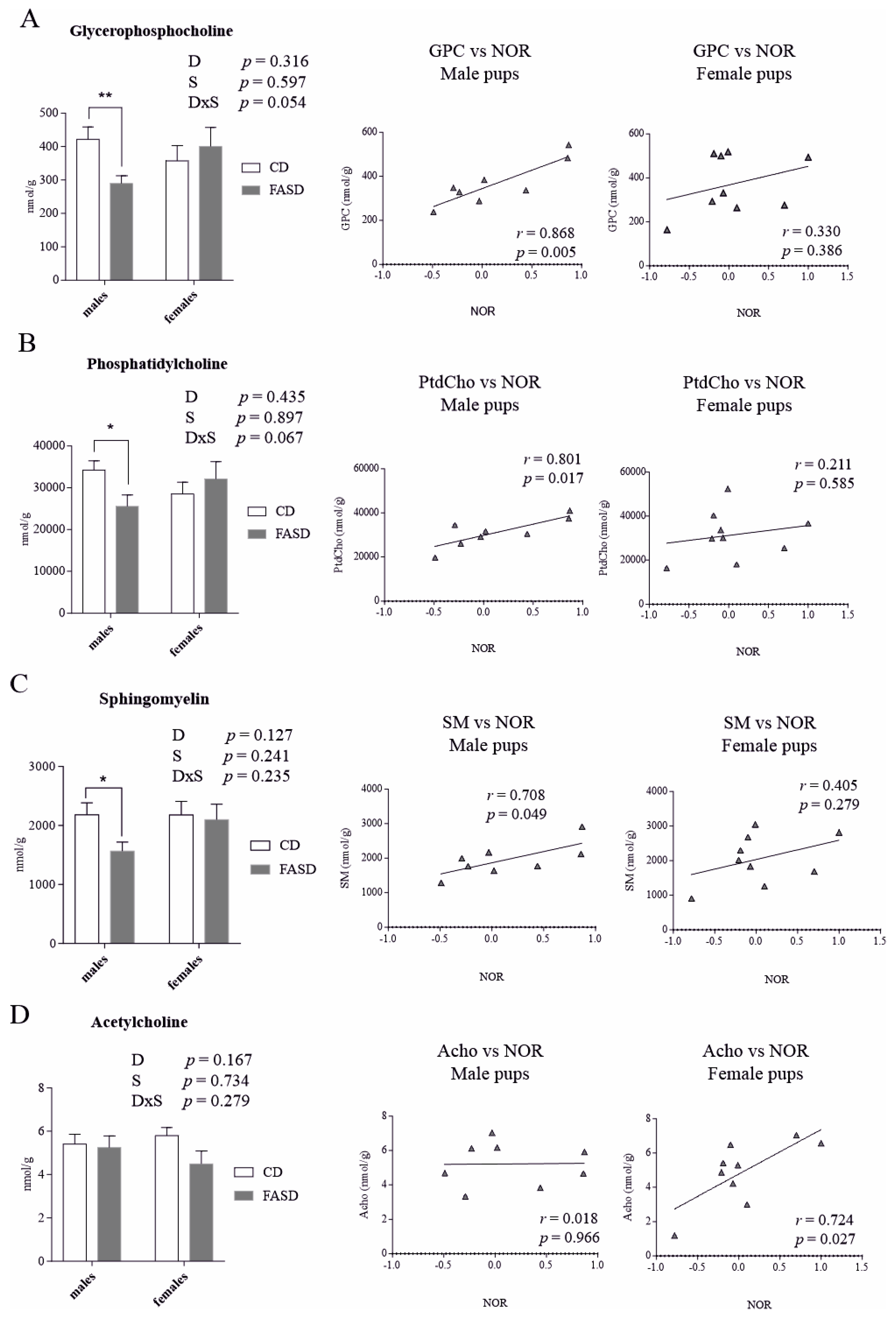
3.3. Altered Concentration of Choline-Derived Metabolites in Liver of FASD Offspring
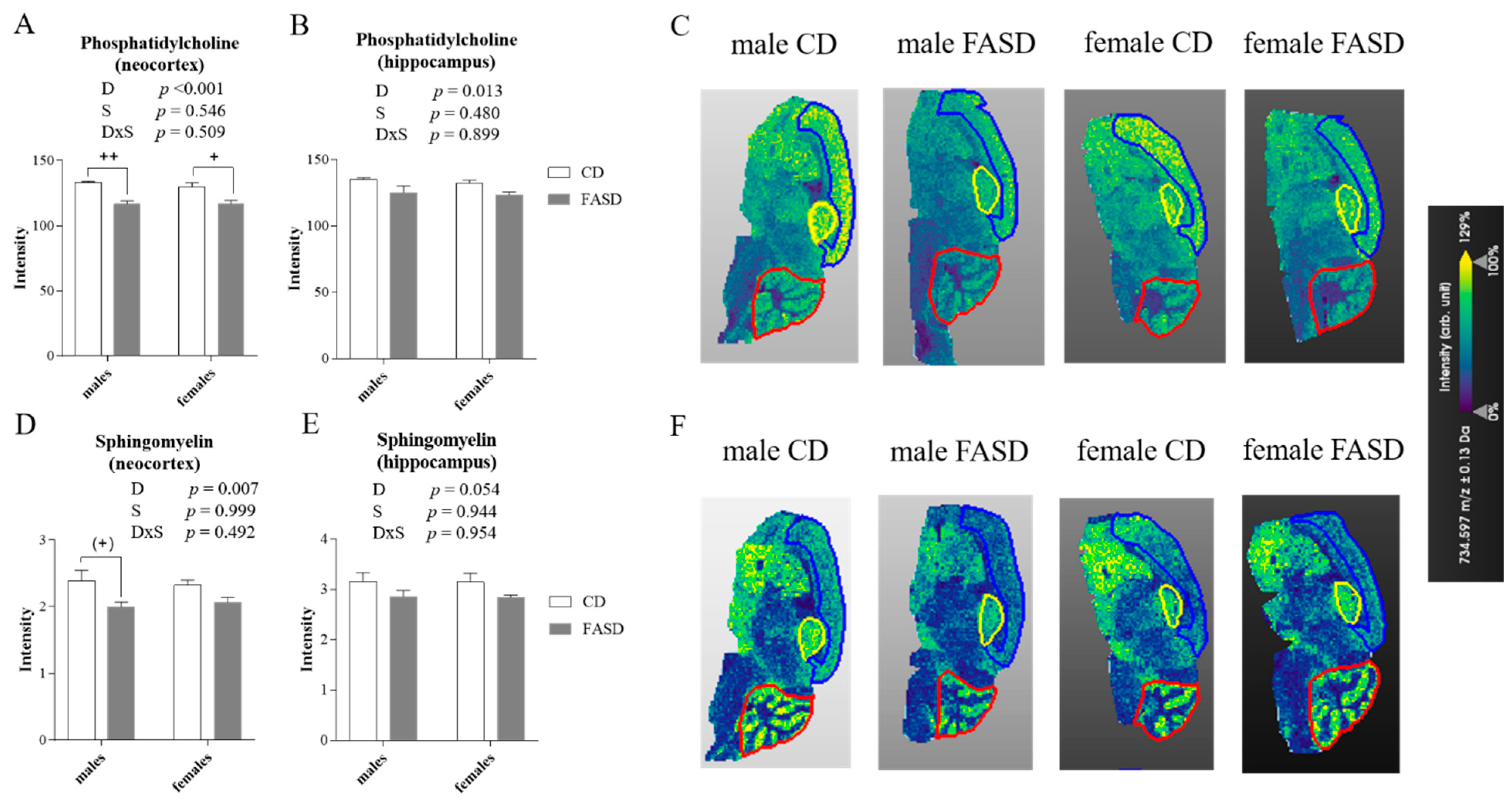
3.4. Altered Concentration of Choline-Derived Metabolites in Cerebral Cortex of FASD Offspring
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Georgieff, M.K. Nutrition and the developing brain: Nutrient priorities and measurement. Am. J. Clin. Nutr. 2007, 85, 614S–620S. [Google Scholar] [CrossRef]
- Wan, L.; Li, Y.; Zhang, Z.; Sun, Z.; He, Y.; Li, R. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl. Psychiatry 2018, 8, 242. [Google Scholar] [CrossRef]
- Ueland, P.M. MTHFR Polymorphisms and Disease; Taylor & Francis: Milton Park, UK, 2005; ISBN 978-1-58706-217-9. [Google Scholar]
- Plumptre, L.; Masih, S.P.; Ly, A.; Aufreiter, S.; Sohn, K.-J.; Croxford, R.; Lausman, A.Y.; Berger, H.; O’Connor, D.L.; Kim, Y.-I. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am. J. Clin. Nutr. 2015, 102, 848–857. [Google Scholar] [CrossRef]
- Murray, L.K.; Smith, M.J.; Jadavji, N.M. Maternal oversupplementation with folic acid and its impact on neurodevelopment of offspring. Nutr. Rev. 2018, 76, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Naninck, E.F.G.; Stijger, P.C.; Brouwer-Brolsma, E.M. The Importance of Maternal Folate Status for Brain Development and Function of Offspring. Adv. Nutr. 2019, 10, 502–519. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Chadman, K.K.; Kuizon, S.; Buenaventura, D.; Stapley, N.W.; Ruocco, F.; Begum, U.; Guariglia, S.R.; Brown, W.T.; Junaid, M.A. Increasing maternal or post-weaning folic acid alters gene expression and moderately changes behavior in the offspring. PLoS ONE 2014, 9, e101674. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Kuizon, S.; Brown, W.T.; Junaid, M.A. High Gestational Folic Acid Supplementation Alters Expression of Imprinted and Candidate Autism Susceptibility Genes in a sex-Specific Manner in Mouse Offspring. J. Mol. Neurosci. 2016, 58, 277–286. [Google Scholar] [CrossRef]
- Sable, P.; Randhir, K.; Kale, A.; Chavan-Gautam, P.; Joshi, S. Maternal micronutrients and brain global methylation patterns in the offspring. Nutr. Neurosci. 2015, 18, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Henzel, K.S.; Ryan, D.P.; Schröder, S.; Weiergräber, M.; Ehninger, D. High-dose maternal folic acid supplementation before conception impairs reversal learning in offspring mice. Sci. Rep. 2017, 7, 3098. [Google Scholar] [CrossRef]
- Barua, S.; Kuizon, S.; Brown, W.T.; Junaid, M.A. DNA Methylation Profiling at Single-Base Resolution Reveals Gestational Folic Acid Supplementation Influences the Epigenome of Mouse Offspring Cerebellum. Front. Neurosci. 2016, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Kale, A.; Dangat, K.; Sable, P.; Kulkarni, A.; Joshi, S. Maternal micronutrients (folic acid and vitamin B(12)) and omega 3 fatty acids: Implications for neurodevelopmental risk in the rat offspring. Brain Dev. 2012, 34, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Sable, P.S.; Kale, A.A.; Joshi, S.R. Prenatal omega 3 fatty acid supplementation to a micronutrient imbalanced diet protects brain neurotrophins in both the cortex and hippocampus in the adult rat offspring. Metabolism 2013, 62, 1607–1622. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.E.; Mikael, L.G.; Leung, K.-Y.; Lévesque, N.; Deng, L.; Wu, Q.; Malysheva, O.V.; Best, A.; Caudill, M.A.; Greene, N.D.E.; et al. High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am. J. Clin. Nutr. 2015, 101, 646–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahous, R.H.; Jadavji, N.M.; Deng, L.; Cosín-Tomás, M.; Lu, J.; Malysheva, O.; Leung, K.-Y.; Ho, M.-K.; Pallàs, M.; Kaliman, P.; et al. High dietary folate in pregnant mice leads to pseudo-MTHFR deficiency and altered methyl metabolism, with embryonic growth delay and short-term memory impairment in offspring. Hum. Mol. Genet. 2017, 26, 888–900. [Google Scholar] [CrossRef] [Green Version]
- Schwahn, B.C.; Chen, Z.; Laryea, M.D.; Wendel, U.; Lussier-Cacan, S.; Genest, J.; Mar, M.-H.; Zeisel, S.H.; Castro, C.; Garrow, T.; et al. Homocysteine-betaine interactions in a murine model of 5,10-methylenetetrahydrofolate reductase deficiency. FASEB J. 2003, 17, 512–514. [Google Scholar] [CrossRef] [PubMed]
- MA Caudill Folate and choline interrelationships: Metabolic and potential health implications. In Folate in Health and Disease; CRC Press: Bailey, CO, USA, 2009; Volume 1, pp. 449–465.
- Troen, A.M.; Chao, W.-H.; Crivello, N.A.; D’Anci, K.E.; Shukitt-Hale, B.; Smith, D.E.; Selhub, J.; Rosenberg, I.H. Cognitive impairment in folate-deficient rats corresponds to depleted brain phosphatidylcholine and is prevented by dietary methionine without lowering plasma homocysteine. J. Nutr. 2008, 138, 2502–2509. [Google Scholar] [CrossRef] [Green Version]
- Perlov, E.; Philipsen, A.; Matthies, S.; Drieling, T.; Maier, S.; Bubl, E.; Hesslinger, B.; Buechert, M.; Henning, J.; Ebert, D.; et al. Spectroscopic findings in attention-deficit/hyperactivity disorder: Review and meta-analysis. World J. Biol. Psychiatry 2009, 10, 355–365. [Google Scholar] [CrossRef]
- Hamlin, J.C.; Pauly, M.; Melnyk, S.; Pavliv, O.; Starrett, W.; Crook, T.A.; James, S.J. Dietary intake and plasma levels of choline and betaine in children with autism spectrum disorders. Autism Res. Treat. 2013, 2013, 578429. [Google Scholar] [CrossRef] [Green Version]
- Partearroyo, T.; Pérez-Miguelsanz, J.; Peña-Melián, Á.; Maestro-de-Las-Casas, C.; Úbeda, N.; Varela-Moreiras, G. Low and high dietary folic acid levels perturb postnatal cerebellar morphology in growing rats. Br. J. Nutr. 2016, 115, 1967–1977. [Google Scholar] [CrossRef] [Green Version]
- May, T.; Adesina, I.; McGillivray, J.; Rinehart, N.J. Sex differences in neurodevelopmental disorders. Curr. Opin. Neurol. 2019, 32, 622–626. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef]
- Spijker, S. Dissection of Rodent Brain Regions. In Neuroproteomics; Li, K.W., Ed.; Neuromethods; Humana Press: Totowa, NJ, USA, 2011; pp. 13–26. ISBN 978-1-61779-111-6. [Google Scholar]
- Jadavji, N.M.; Deng, L.; Leclerc, D.; Malysheva, O.; Bedell, B.J.; Caudill, M.A.; Rozen, R. Severe methylenetetrahydrofolate reductase deficiency in mice results in behavioral anomalies with morphological and biochemical changes in hippocampus. Mol. Genet. Metab. 2012, 106, 149–159. [Google Scholar] [CrossRef]
- Rodriguiz, R.M.; Colvin, J.S.; Wetsel, W.C. Neurophenotyping genetically modified mice for social behavior. Methods Mol. Biol. 2011, 768, 343–363. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, R.M.; Steward, O. A bilateral cervical contusion injury model in mice: Assessment of gripping strength as a measure of forelimb motor function. Exp. Neurol. 2010, 221, 38–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweatt, J.D. Rodent Behavioral Learning and Memory Models. In Mechanisms of Memory; Academic Press: Cambridge, MA, USA, 2009; ISBN 978-0-08-095919-1. [Google Scholar]
- Frosst, P.; Blom, H.J.; Milos, R.; Goyette, P.; Sheppard, C.A.; Matthews, R.G.; Boers, G.J.; den Heijer, M.; Kluijtmans, L.A.; van den Heuvel, L.P. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.I.; Ueland, P.M.; Kvalheim, G.; Lien, E.A. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin. Chem. 2003, 49, 286–294. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, X.; West, A.A.; Perry, C.A.; Malysheva, O.V.; Devapatla, S.; Pressman, E.; Vermeylen, F.; Stabler, S.P.; Allen, R.H.; et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am. J. Clin. Nutr. 2012, 95, 1060–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koc, H.; Mar, M.-H.; Ranasinghe, A.; Swenberg, J.A.; Zeisel, S.H. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal. Chem. 2002, 74, 4734–4740. [Google Scholar] [CrossRef]
- Kim, J.K.; Harada, K.; Bamba, T.; Fukusaki, E.; Kobayashi, A. Stable isotope dilution-based accurate comparative quantification of nitrogen-containing metabolites in Arabidopsis thaliana T87 cells using in vivo (15)N-isotope enrichment. Biosci. Biotechnol. Biochem. 2005, 69, 1331–1340. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Strahler, J.R.; Andrews, P.C.; Matthews, R.G. Regulation of human methylenetetrahydrofolate reductase by phosphorylation. Proc. Natl. Acad. Sci. USA 2005, 102, 10454–10459. [Google Scholar] [CrossRef] [Green Version]
- Christensen, K.E.; Wu, Q.; Wang, X.; Deng, L.; Caudill, M.A.; Rozen, R. Steatosis in mice is associated with gender, folate intake, and expression of genes of one-carbon metabolism. J. Nutr. 2010, 140, 1736–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottiglieri, T.; Reynolds, E. Folate and Neurological Disease: Basic Mechanisms. In Folate in Health and Disease; CRC Press: Boca Raton, FL, USA, 2009; pp. 355–381. [Google Scholar]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Acetylcholine in the cerebral cortex: Effects and clinical implications. Neurology 2010, 75, 659–665. [Google Scholar] [CrossRef]
- Veloso, A.; Fernández, R.; Astigarraga, E.; Barreda-Gómez, G.; Manuel, I.; Giralt, M.T.; Ferrer, I.; Ochoa, B.; Rodríguez-Puertas, R.; Fernández, J.A. Distribution of lipids in human brain. Anal. Bioanal. Chem. 2011, 401, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, M.S.; Oliveros, L.B.; Gomez, N.N. Nutritional Deficiencies and Phospholipid Metabolism. Int. J. Mol. Sci. 2011, 12, 2408–2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparvero, L.J.; Amoscato, A.A.; Dixon, C.E.; Long, J.B.; Kochanek, P.M.; Pitt, B.R.; Bayir, H.; Kagan, V.E. Mapping of Phospholipids by MALDI Imaging (MALDI-MSI): Realities and Expectations. Chem Phys. Lipids 2012, 165, 545–562. [Google Scholar] [CrossRef] [Green Version]
- Valera-Gran, D.; de la Hera, M.G.; Navarrete-Muñoz, E.M.; Fernandez-Somoano, A.; Tardón, A.; Julvez, J.; Forns, J.; Lertxundi, N.; Ibarluzea, J.M.; Murcia, M.; et al. Folic acid supplements during pregnancy and child psychomotor development after the first year of life. JAMA Pediatr. 2014, 168, e142611. [Google Scholar] [CrossRef] [Green Version]
- Valera-Gran, D.; Navarrete-Muñoz, E.M.; de la Hera, M.G.; Fernández-Somoano, A.; Tardón, A.; Ibarluzea, J.; Balluerka, N.; Murcia, M.; González-Safont, L.; Romaguera, D.; et al. Effect of maternal high dosages of folic acid supplements on neurocognitive development in children at 4–5 y of age: The prospective birth cohort Infancia y Medio Ambiente (INMA) study. Am. J. Clin. Nutr. 2017, 106, 878–887. [Google Scholar] [CrossRef] [Green Version]
- Jadavji, N.M.; Deng, L.; Malysheva, O.; Caudill, M.A.; Rozen, R. MTHFR deficiency or reduced intake of folate or choline in pregnant mice results in impaired short-term memory and increased apoptosis in the hippocampus of wild-type offspring. Neuroscience 2015, 300, 1–9. [Google Scholar] [CrossRef]
- Ars, C.L.; Nijs, I.M.; Marroun, H.E.; Muetzel, R.; Schmidt, M.; Steenweg-de Graaff, J.; van der Lugt, A.; Jaddoe, V.W.; Hofman, A.; Steegers, E.A.; et al. Prenatal folate, homocysteine and vitamin B12 levels and child brain volumes, cognitive development and psychological functioning: The Generation R Study. Br. J. Nutr. 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Martinussen, R.; Hayden, J.; Hogg-Johnson, S.; Tannock, R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 377–384. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, H.G.; Kusek, G.K.; Yang, M.; Phoenix, J.L.; Bolivar, V.J.; Crawley, J.N. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008, 7, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Patterson, P.H. Modeling Autistic Features in Animals. Pediatr Res. 2011, 69, 34R–40R. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Riley, A.W.; Volk, H.; Caruso, D.; Hironaka, L.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol. 2018, 32, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Ganz, A.B.; Shields, K.; Fomin, V.G.; Lopez, Y.S.; Mohan, S.; Lovesky, J.; Chuang, J.C.; Ganti, A.; Carrier, B.; Yan, J.; et al. Genetic impairments in folate enzymes increase dependence on dietary choline for phosphatidylcholine production at the expense of betaine synthesis. FASEB J. 2016, 30, 3321–3333. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S.H. Choline: An important nutrient in brain development, liver function and carcinogenesis. J. Am. Coll. Nutr. 1992, 11, 473–481. [Google Scholar] [CrossRef]
- Devlin, A.M.; Chau, C.M.Y.; Dyer, R.; Matheson, J.; McCarthy, D.; Yurko-Mauro, K.; Innis, S.M.; Grunau, R.E. Developmental Outcomes at 24 Months of Age in Toddlers Supplemented with Arachidonic Acid and Docosahexaenoic Acid: Results of a Double Blind Randomized, Controlled Trial. Nutrients 2017, 9, 975. [Google Scholar] [CrossRef] [Green Version]
- Aoki, Y.; Yoncheva, Y.N.; Chen, B.; Nath, T.; Sharp, D.; Lazar, M.; Velasco, P.; Milham, M.P.; Di Martino, A. Association of White Matter Structure with Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder. JAMA Psychiatry 2017, 74, 1120–1128. [Google Scholar] [CrossRef]
- Deoni, S.; Dean, D.; Joelson, S.; O’Regan, J.; Schneider, N. Early nutrition influences developmental myelination and cognition in infants and young children. Neuroimage 2018, 178, 649–659. [Google Scholar] [CrossRef]
- Orenbuch, A.; Fortis, K.; Taesuwan, S.; Yaffe, R.; Caudill, M.A.; Golan, H.M. Prenatal Nutritional Intervention Reduces Autistic-Like Behavior Rates among Mthfr-Deficient Mice. Front. Neurosci. 2019, 13, 383. [Google Scholar] [CrossRef] [Green Version]
- Parikshak, N.N.; Swarup, V.; Belgard, T.G.; Irimia, M.; Ramaswami, G.; Gandal, M.J.; Hartl, C.; Leppa, V.; de la Torre Ubieta, L.; Huang, J.; et al. Genome-wide changes in lncRNA, splicing, and regional gene expression patterns in autism. Nature 2016, 540, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.W.; Ayling, J.E. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc. Natl. Acad. Sci. USA 2009, 106, 15424–15429. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosín-Tomás, M.; Luan, Y.; Leclerc, D.; Malysheva, O.V.; Lauzon, N.; Bahous, R.H.; Christensen, K.E.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Behavioral Alterations in Offspring with Sex-Specific Changes in Methyl Metabolism. Nutrients 2020, 12, 1716. https://doi.org/10.3390/nu12061716
Cosín-Tomás M, Luan Y, Leclerc D, Malysheva OV, Lauzon N, Bahous RH, Christensen KE, Caudill MA, Rozen R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Behavioral Alterations in Offspring with Sex-Specific Changes in Methyl Metabolism. Nutrients. 2020; 12(6):1716. https://doi.org/10.3390/nu12061716
Chicago/Turabian StyleCosín-Tomás, Marta, Yan Luan, Daniel Leclerc, Olga V. Malysheva, Nidia Lauzon, Renata H. Bahous, Karen E. Christensen, Marie A. Caudill, and Rima Rozen. 2020. "Moderate Folic Acid Supplementation in Pregnant Mice Results in Behavioral Alterations in Offspring with Sex-Specific Changes in Methyl Metabolism" Nutrients 12, no. 6: 1716. https://doi.org/10.3390/nu12061716
APA StyleCosín-Tomás, M., Luan, Y., Leclerc, D., Malysheva, O. V., Lauzon, N., Bahous, R. H., Christensen, K. E., Caudill, M. A., & Rozen, R. (2020). Moderate Folic Acid Supplementation in Pregnant Mice Results in Behavioral Alterations in Offspring with Sex-Specific Changes in Methyl Metabolism. Nutrients, 12(6), 1716. https://doi.org/10.3390/nu12061716