Effects of Cocoa-Rich Chocolate on Blood Pressure, Cardiovascular Risk Factors, and Arterial Stiffness in Postmenopausal Women: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Study Participants and Recruitment
2.3. Sample Size
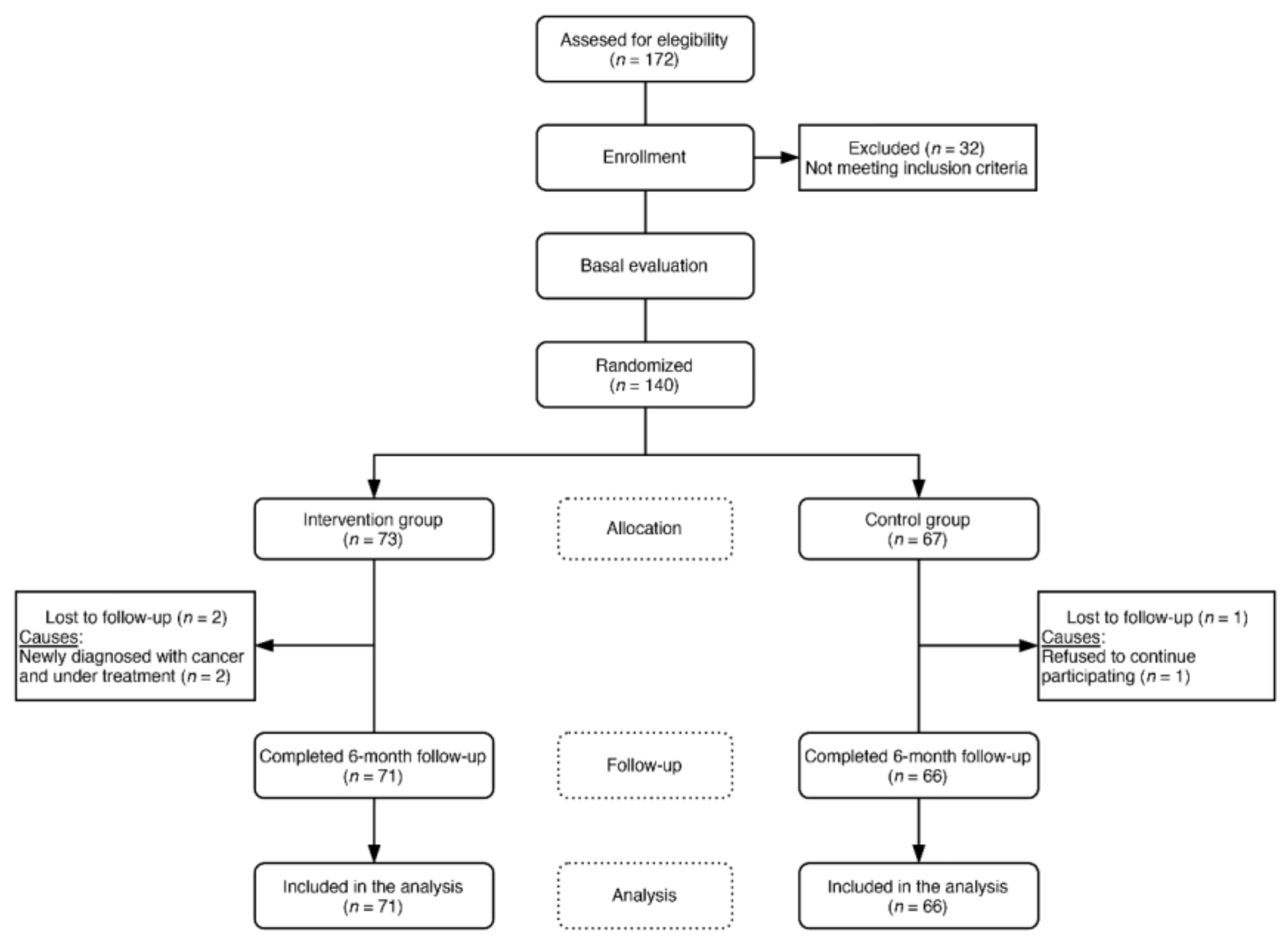
2.4. Procedures and Randomization
2.5. Intervention
2.6. Main Outcomes
2.6.1. Blood Pressure Measurements
2.6.2. Evaluation of Vascular Structure and Function
2.6.3. Laboratory Variable Assessment
2.6.4. Other Measurements
- Body Weight and Body Mass Index
- 2.
- Clinical and Sociodemographic Variables
- 3.
- Adherence to the Intervention
- 4.
- Evaluation of Chocolate Consumption and Habitual Diet
2.7. Data Collection Procedure, Data Management, and Monitoring
2.8. Ethical Considerations
2.9. Statistical Analyses
3. Results
3.1. Baseline Characteristics of the Study Groups
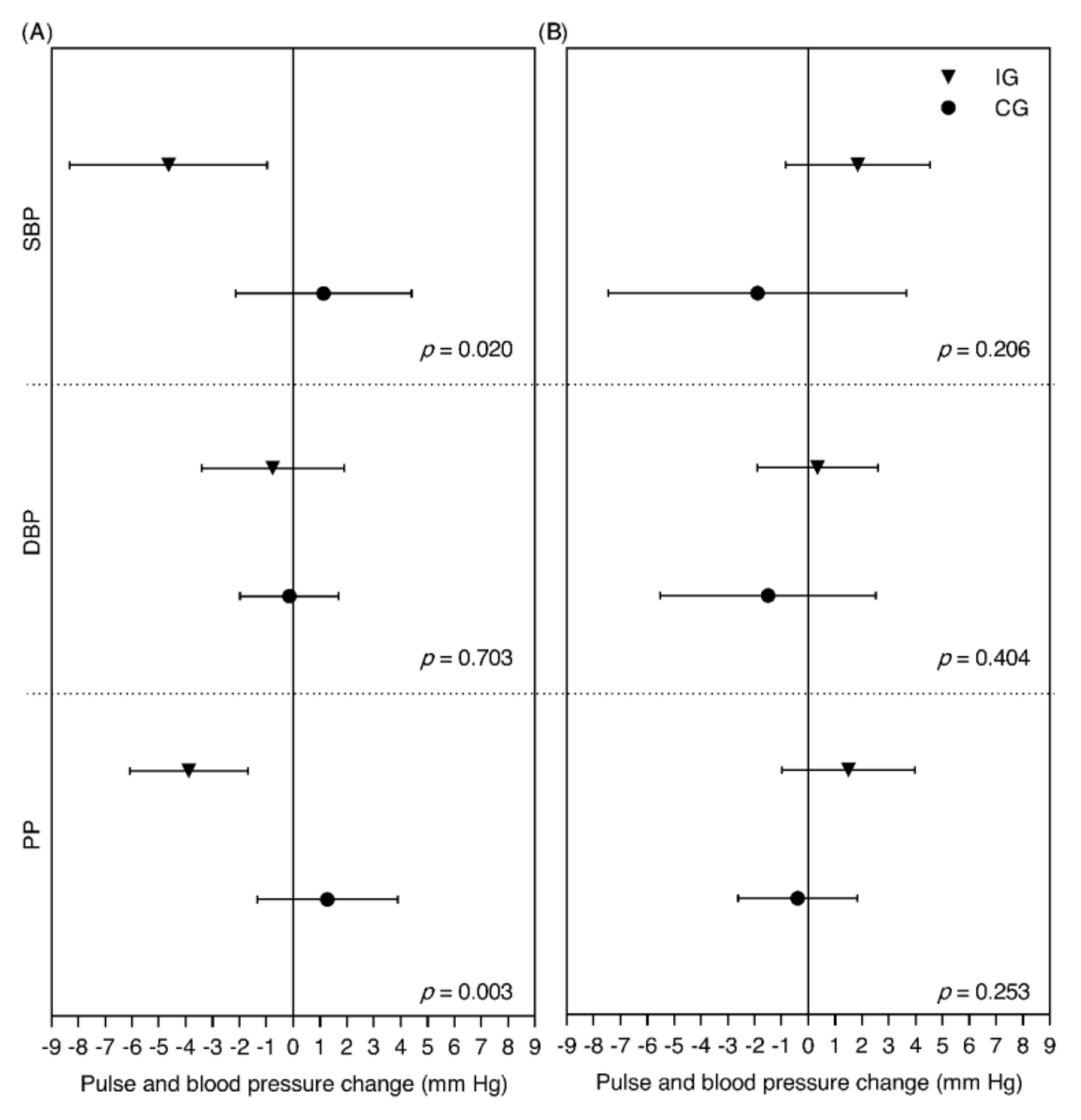
3.2. Cardiovascular Risk Factors and Blood Pressure
3.3. Arterial Stiffness Parameters and Vascular Function
4. Discussion
4.1. Main Findings
4.2. Discussion of Blood Pressure Results
4.3. Discussion of the Results of Other Cardiovascular Risk Factors
4.4. Discussion of the Results of Arterial Stiffness Parameters and Vascular Function
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Zaydun, G.; Tomiyama, H.; Hashimoto, H.; Arai, T.; Koji, Y.; Yambe, M.; Motobe, K.; Hori, S.; Yamashina, A. Menopause is an independent factor augmenting the age-related increase in arterial stiffness in the early postmenopausal phase. Atherosclerosis 2006, 184, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Pallazola, V.A.; Davis, D.M.; Whelton, S.P.; Cardoso, R.; Latina, J.M.; Michos, E.D.; Sarkar, S.; Blumenthal, R.S.; Arnett, D.K.; Stone, N.J.; et al. A Clinician’s Guide to Healthy Eating for Cardiovascular Disease Prevention. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Lei, L.; Zhou, Y.; Ye, F.; Zhao, G. Dietary Flavonoids and the Risk of Colorectal Cancer: An Updated Meta-Analysis of Epidemiological Studies. Nutrients 2018, 10, 950. [Google Scholar] [CrossRef] [PubMed]
- Geleijnse, J.M.; Launer, L.J.; Van der Kuip, D.A.M.; Hofman, A.; Witteman, J.C.M. Inverse association of tea and flavonoid intakes with incident myocardial infarction: The Rotterdam Study. Am. J. Clin. Nutr. 2002, 75, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.Y.; Gonthier, M.-P.; Rémésy, C.; Mila, I.; Lapierre, C.; Lazarus, S.A.; Williamson, G.; Scalbert, A. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am. J. Clin. Nutr. 2003, 77, 912–918. [Google Scholar] [CrossRef]
- Jenzer, H.; Sadeghi-Reeves, L. Nutrigenomics-Associated Impacts of Nutrients on Genes and Enzymes with Special Consideration of Aromatase. Front. Nutr. 2020, 7, 37. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Meyskens, F.L. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol. Nutr. Food Res. 2016, 60, 1264–1274. [Google Scholar] [CrossRef]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar]
- Garcia, J.P.; Santana, A.; Baruqui, D.L.; Suraci, N. The Cardiovascular effects of chocolate. Rev. Cardiovasc. Med. 2018, 19, 123–127. [Google Scholar] [PubMed]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, D.; de Graaf, Y.; van Kerckhoven, R.; Draijer, R. Effect of tea theaflavins and catechins on microvascular function. Nutrients 2014, 6, 5772–5785. [Google Scholar] [CrossRef] [PubMed]
- Samavat, H.; Newman, A.R.; Wang, R.; Yuan, J.-M.; Wu, A.H.; Kurzer, M.S. Effects of green tea catechin extract on serum lipids in postmenopausal women: A randomized, placebo-controlled clinical trial. Am. J. Clin. Nutr. 2016, 104, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef]
- Okamoto, T.; Kobayashi, R.; Natsume, M.; Nakazato, K. Habitual cocoa intake reduces arterial stiffness in postmenopausal women regardless of intake frequency: A randomized parallel-group study. Clin. Interv. Aging 2016, 11, 1645–1652. [Google Scholar] [CrossRef]
- Marsh, C.E.; Carter, H.H.; Guelfi, K.J.; Smith, K.J.; Pike, K.E.; Naylor, L.H.; Green, D.J. Brachial and Cerebrovascular Functions Are Enhanced in Postmenopausal Women after Ingestion of Chocolate with a High Concentration of Cocoa. J. Nutr. 2017, 147, 1686–1692. [Google Scholar] [CrossRef]
- West, S.G.; McIntyre, M.D.; Piotrowski, M.J.; Poupin, N.; Miller, D.L.; Preston, A.G.; Wagner, P.; Groves, L.F.; Skulas-Ray, A.C. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. Br. J. Nutr. 2014, 111, 653–661. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Necozione, S.; di Giosia, P.; Barnabei, R.; Allegaert, L.; Bernaert, H.; Ferri, C. Cocoa consumption dose-dependently improves flow-mediated dilation and arterial stiffness decreasing blood pressure in healthy individuals. J. Hypertens. 2015, 33, 294–303. [Google Scholar] [CrossRef]
- Epidat: Program for Epidemiological Data Analysis, Version 4.2; Consellería de Sanidade: Xunta de Galicia, Spain; Pan American Organization Health (PAHO-WHO), CES University: Medellín, Colombia, 2016.
- O’Brien, E.; Asmar, R.; Beilin, L.; Imai, Y.; Mancia, G.; Mengden, T.; Myers, M.; Padfield, P.; Palatini, P.; Parati, G.; et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J. Hypertens. 2005, 23, 697–701. [Google Scholar] [CrossRef]
- Shirai, K.; Hiruta, N.; Song, M.; Kurosu, T.; Suzuki, J.; Tomaru, T.; Miyashita, Y.; Saiki, A.; Takahashi, M.; Suzuki, K.; et al. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: Theory, evidence and perspectives. J. Atheroscler. Thromb. 2011, 18, 924–938. [Google Scholar] [CrossRef]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.C.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society. J. Vasc. Interv. Radiol. 2006, 17, 1383–1397, quiz 1398. [Google Scholar] [CrossRef]
- Garcia-Ortiz, L.; Recio-Rodriguez, J.I.; Agudo-Conde, C.; Maderuelo-Fernandez, J.A.; Patino-Alonso, M.C.; de Cabo-Laso, A.; Rodriguez-Martin, C.; Gonzalez-Sanchez, J.; Rodriguez-Sanchez, E.; Gomez-Marcos, M.A. Noninvasive validation of central and peripheral augmentation index estimated by a novel wrist-worn tonometer. J. Hypertens. 2018, 36, 2204–2214. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, I.B.; Mohammad, N.H.; Tyrrell, S.; Hall, I.R.; Webb, D.J.; Paul, V.E.; Levy, T.; Cockcroft, J.R. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am. J. Hypertens. 2002, 15, 24–30. [Google Scholar] [CrossRef]
- Munir, S.; Guilcher, A.; Kamalesh, T.; Clapp, B.; Redwood, S.; Marber, M.; Chowienczyk, P. Peripheral augmentation index defines the relationship between central and peripheral pulse pressure. Hypertension (Dallas Tex. 1979) 2008, 51, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvado, J.; Rubio, M.A.; Barbany, M.; Moreno, B. [SEEDO 2007 Consensus for the evaluation of overweight and obesity and the establishment of therapeutic intervention criteria]. Med. Clin. 2007, 128, 184–196, quiz 1 p following 200. [Google Scholar]
- Recio-Rodriguez, J.I.; Rodriguez-Martin, C.; Gonzalez-Sanchez, J.; Rodriguez-Sanchez, E.; Martin-Borras, C.; Martinez-Vizcaino, V.; Arietaleanizbeaskoa, M.S.; Magdalena-Gonzalez, O.; Fernandez-Alonso, C.; Maderuelo-Fernandez, J.A.; et al. EVIDENT Smartphone App, a New Method for the Dietary Record: Comparison With a Food Frequency Questionnaire. JMIR mHealth uHealth 2019, 7, e11463. [Google Scholar] [CrossRef]
- Garcia-Yu, I.A.; Garcia-Ortiz, L.; Gomez-Marcos, M.A.; Alonso-Dominguez, R.; Gonzalez-Sanchez, J.; Mora-Simon, S.; Gonzalez-Manzano, S.; Rodriguez-Sanchez, E.; Maderuelo-Fernandez, J.A.; Recio-Rodriguez, J.I. Vascular and cognitive effects of cocoa-rich chocolate in postmenopausal women: A study protocol for a randomised clinical trial. BMJ Open 2018, 8, e024095. [Google Scholar] [CrossRef]
- Vlachopoulos, C.V.; Alexopoulos, N.A.; Aznaouridis, K.A.; Ioakeimidis, N.C.; Dima, I.A.; Dagre, A.; Vasiliadou, C.; Stefanadi, E.C.; Stefanadis, C.I. Relation of habitual cocoa consumption to aortic stiffness and wave reflections, and to central hemodynamics in healthy individuals. Am. J. Cardiol. 2007, 99, 1473–1475. [Google Scholar] [CrossRef]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Ayoobi, N.; Jafarirad, S.; Haghighizadeh, M.H.; Jahanshahi, A. Protective Effect of Dark Chocolate on Cardiovascular Disease Factors and Body Composition in Type 2 Diabetes: A Parallel, Randomized, Clinical Trial. Iran. Red Crescent Med. J. 2017, 19, e21644. [Google Scholar] [CrossRef]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The Cocoa, Cognition, and Aging (CoCoA) Study—A randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Crews, W.D., Jr.; Harrison, D.W.; Wright, J.W. A double-blind, placebo-controlled, randomized trial of the effects of dark chocolate and cocoa on variables associated with neuropsychological functioning and cardiovascular health: Clinical findings from a sample of healthy, cognitively intact older adu. Am. J. Clin. Nutr. 2008, 87, 872–880. [Google Scholar]
- Dicks, L.; Kirch, N.; Gronwald, D.; Wernken, K.; Zimmermann, B.F.; Helfrich, H.-P.; Ellinger, S. Regular Intake of a Usual Serving Size of Flavanol-Rich Cocoa Powder Does Not Affect Cardiometabolic Parameters in Stably Treated Patients with Type 2 Diabetes and Hypertension-A Double-Blinded, Randomized, Placebo-Controlled Trial. Nutrients 2018, 10, 1435. [Google Scholar] [CrossRef]
- Scientific Opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2809.
| Compounds | Quantity, mg/10 g |
|---|---|
| Protocatechuic acid | 0.58 |
| Procyanidin dimer (B3) | 1.76 |
| Catechin | 10.4 |
| Procyanidin dimer (B2) | 14.4 |
| Epicatechin | 26.1 |
| Procyanidin trimer (C1) | 8.53 |
| Procyanidin A hexoside | 3.54 |
| Quercetin glucoside | 0.02 |
| Quercetin arabinoside | 0.03 |
| Variables | Intervention Group (n = 73) | Control Group (n = 67) |
|---|---|---|
| Age, y | 57.1 ± 3.5 | 57.5 ± 3.8 |
| Civil status, n (%) | ||
| Married/cohabitant | 48 (65.8) | 47 (70.1) |
| Separated/divorced | 8 (11.0) | 7 (10.4) |
| Single | 15 (20.5) | 9 (13.4) |
| Widow | 2 (2.7) | 4 (6.0) |
| Education level, n (%) | ||
| Elementary education | 16 (21.9) | 12 (17.9) |
| Middle-High school | 22 (30.1) | 29 (43.3) |
| Bachelor | 17 (23.3) | 11 (16.4) |
| Postgraduate | 18 (24.7) | 15 (22.4) |
| Time from menopause onset, y | 6.9 ± 4.6 | 6.9 ± 3.6 |
| Untreated hypertension, n (%) | 1 (1.4) | 0 (0.0) |
| Untreated dyslipidemia, n (%) | 8 (11.0) | 10 (14.9) |
| Gestational diabetes, n (%) | 3 (4.1) | 1 (1.5) |
| Thyroid hormone treatment, n (%) | 13 (17.8) | 10 (14.9) |
| Current smoker, n (%) | 12 (16.4) | 9 (13.4) |
| Alcohol consumption, g/week | 23.1 ± 29.4 | 30.6 ± 48.1 |
| Energy, kcal/day | 1720 ± 357 | 1780 ± 402 |
| Carbohydrates, g/day | 168 ± 45.1 | 173 ± 50.2 |
| Proteins, g/day | 76.7 ± 16.7 | 78.2 ± 18.9 |
| Fiber, g/day | 24.0 ± 7.5 | 25.9 ± 9.6 |
| Fats, g/day | 77.4 ± 20.7 | 79.8 ± 20.1 |
| Saturated fats, g/day | 25.1 ± 7.6 | 25.3 ± 7.5 |
| Physical activity, MET–h/week | 31.2 ± 36.8 | 25.7 ± 20.0 |
| Chocolate intake, g/week | 68.6 ± 71.1 | 69.1 ± 74.2 |
| >70% cocoa chocolate intake, g/week | 19.9 ± 36.2 | 15.6 ± 33.2 |
| Intervention Group (n = 71) | Control Group (n = 66) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Baseline | 6 Months | Change | p2 | Baseline | 6 Months | Change | p2 | Intergroup Difference (IG-CG) 3 | p3 | Adjusted Intergroup Difference (IG-CG) 4 | p4 |
| Body weight, kg | 65.1 ± 10.3 | 64.9 ± 10.3 | −0.2 ± 2.2 | 0.438 | 64.9 ± 8.6 | 64.5 ± 8.9 | −0.4 ± 2.7 | 0.272 | 0.16 (−0.67, 0.98) | 0.708 | 0.16 (−0.66, 0.99) | 0.696 |
| BMI, kg/m2 | 25.7 ± 3.8 | 25.6 ± 3.7 | −0.1 ± 0.9 | 0.502 | 25.6 ± 3.1 | 25.4 ± 3.2 | −0.1 ± 1.0 | 0.250 | 0.07 (−0.25, 0.40) | 0.652 | 0.08 (−0.24, 0.40) | 0.627 |
| Glucose, mg/dL | 86.4 ± 8.8 | 86.6 ± 10.0 | 0.2 ± 7.9 | 0.820 | 86.2 ± 8.5 | 87.2 ± 8.7 | 1.0 ± 7.4 | 0.270 | −0.80 (−3.39, 1.79) | 0.542 | −0.74 (−3.19, 1.70) | 0.549 |
| Insulin, mg/dL | 8.2 ± 3.4 | 8.3 ± 5.1 | 0.1 ± 5.1 | 0.869 | 7.5 ± 2.9 | 7.8 ± 3.6 | 0.3 ± 3.0 | 0.434 | −0.19 (−1.63, 1.24) | 0.791 | 0.11 (−1.27, 1.48) | 0.879 |
| TC, mg/dL | 211 ± 28.5 | 212 ± 34.6 | 1.3 ± 23.4 | 0.635 | 204 ± 26.6 | 205 ± 30.2 | 0.9 ± 18.5 | 0.700 | 0.44 (−6.71, 7.60) | 0.902 | 1.12 (−6.06, 8.30) | 0.758 |
| HDL cholesterol, mg/dL | 68.2 ± 17.3 | 67.0 ± 15.9 | −1.2 ± 14.0 | 0.479 | 65.8 ± 13.2 | 65.0 ± 12.9 | −0.9 ± 7.5 | 0.356 | −0.32 (−4.16, 3.52) | 0.870 | 0.45 (−3.00, 3.91) | 0.795 |
| LDL cholesterol, mg/dL | 128 ± 26.4 | 130 ± 29.1 | 2.7 ± 16.9 | 0.175 | 122 ± 26.9 | 124 ± 29.3 | 1.6 ± 17.7 | 0.468 | 1.15 (−4.69, 7.00) | 0.696 | 1.73 (−4.07, 7.54) | 0.556 |
| Triglycerides, mg/dL | 83.4 ± 30.6 | 83.1 ± 34.7 | −0.3 ± 27.4 | 0.938 | 80.0 ± 34.3 | 80.3 ± 28.5 | 0.2 ± 24.1 | 0.935 | −0.49 (−9.24, 8.22) | 0.911 | 0.63 (−7.35, 8.60) | 0.876 |
| HOMA-IR | 1.8 ± 0.8 | 1.8 ± 1.4 | 0.1 ± 1.4 | 0.676 | 1.6 ± 0.7 | 1.7 ± 0.9 | 0.1 ± 0.7 | 0.292 | −0.02 (−0.42, 0.36) | 0.896 | 0.03 (−0.35, 0.41) | 0.874 |
| Creatinine, mg/L | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.0 ± 0.1 | 0.918 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.0 ± 0.1 | 0.242 | 0.02 (−0.02, 0.05) | 0.336 | 0.03 (0.00, 0.05) | 0.084 |
| SBP, mm Hg | 108 ± 16.4 | 106 ± 14.1 | −1.8 ± 10.2 | 0.152 | 108 ± 15.0 | 108 ± 14.4 | −0.2 ± 12.0 | 0.919 | −1.62 (−5.39, 2.16) | 0.398 | −1.45 (−4.79, 1.88) | 0.391 |
| DBP, mm Hg | 72.6 ± 10.7 | 72.4 ± 10.0 | −0.3 ± 7.3 | 0.757 | 72.2 ± 10.3 | 71.4 ± 10.1 | −0.7 ± 7.9 | 0.463 | 0.45 (−2.13, 3.03) | 0.732 | 0.59 (−1.76, 2.94) | 0.622 |
| HR, bpm | 66.5 ± 7.6 | 66.3 ± 8.0 | −0.2 ± 6.0 | 0.804 | 66.7 ± 8.6 | 65.7 ± 7.6 | −1.0 ± 7.6 | 0.298 | 0.80 (−1.51, 3.11) | 0.495 | 0.74 (−1.31, 2.79) | 0.479 |
| PP, mm Hg | 35.6 ± 9.5 | 34.1 ± 7.4 | −1.5 ± 7.2 | 0.088 | 35.6 ± 7.6 | 36.1 ± 7.7 | 0.6 ± 7.1 | 0.518 | −2.07 (−4.50, 0.37) | 0.095 | −2.05 (−4.08, −0.02) | 0.048 |
| MAP, mm Hg | 84.5 ± 12.1 | 83.7 ± 11.0 | −0.8 ± 7.7 | 0.403 | 84.0 ± 11.5 | 83.5 ± 11.2 | −0.5 ± 8.9 | 0.629 | −0.24 (−3.05, 2.56) | 0.865 | −0.10 (−2.63, 2.44) | 0.939 |
| Intervention Group (n = 71) | Control Group (n = 66) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Baseline | 6 Months | Change | p2 | Baseline | 6 Months | Change | p2 | Intergroup Difference (IG-CG) 3 | p3 | Adjusted Intergroup Difference (IG-CG) 4 | p4 |
| CAVI | 7.55 ± 0.91 | 7.78 ± 0.86 | 0.23 ± 0.67 | 0.005 | 7.70 ± 0.83 | 7.74 ± 0.87 | 0.04 ± 0.63 | 0.580 | 0.18 (−0.03, 0.40) | 0.100 | 0.14 (−0.06, 0.34) | 0.175 |
| ABI | 1.09 ± 0.07 | 1.11 ± 0.07 | 0.02 ± 0.07 | 0.064 | 1.10 ± 0.07 | 1.10 ± 0.07 | 0.01 ± 0.08 | 0.523 | 0.01 (−0.02, 0.03) | 0.477 | 0.01 (−0.01, 0.03) | 0.480 |
| baPWV, m/s | 12.1 ± 1.53 | 12.3 ± 1.55 | 0.14 ± 0.92 | 0.220 | 12.3 ± 1.53 | 12.3 ± 1.68 | -0.08 ± 1.01 | 0.538 | 0.21 (−0.11, 0.54) | 0.200 | 0.18 (−0.14, 0.50) | 0.263 |
| CAIx | 41.6 ± 20.5 | 47.3 ± 29.1 | 5.77 ± 35.06 | 0.196 | 43.2 ± 19.4 | 47.9 ± 18.9 | 4.79 ± 22.3 | 0.092 | 0.98 (−9.38, 11.3) | 0.852 | −0.34 (−8.96, 8.27) | 0.937 |
| CAIx75 | 31.2 ± 15.4 | 35.5 ± 21.8 | 4.33 ± 26.3 | 0.196 | 32.4 ± 14.6 | 36.0 ± 14.2 | 3.59 ± 16.7 | 0.092 | 0.73 (−7.03, 8.50) | 0.852 | −0.26 (−6.72, 6.20) | 0.937 |
| PAIx | 100.8 ± 16.1 | 103.5 ± 19.1 | 2.70 ± 26.0 | 0.407 | 101.6 ± 16.2 | 102.6 ± 18.0 | 0.97 ± 22.9 | 0.740 | 1.73 (−6.91, 10.4) | 0.693 | 0.87 (−5.69, 7.42) | 0.794 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Yu, I.A.; Garcia-Ortiz, L.; Gomez-Marcos, M.A.; Rodriguez-Sanchez, E.; Agudo-Conde, C.; Gonzalez-Sanchez, J.; Maderuelo-Fernandez, J.A.; Recio-Rodriguez, J.I. Effects of Cocoa-Rich Chocolate on Blood Pressure, Cardiovascular Risk Factors, and Arterial Stiffness in Postmenopausal Women: A Randomized Clinical Trial. Nutrients 2020, 12, 1758. https://doi.org/10.3390/nu12061758
Garcia-Yu IA, Garcia-Ortiz L, Gomez-Marcos MA, Rodriguez-Sanchez E, Agudo-Conde C, Gonzalez-Sanchez J, Maderuelo-Fernandez JA, Recio-Rodriguez JI. Effects of Cocoa-Rich Chocolate on Blood Pressure, Cardiovascular Risk Factors, and Arterial Stiffness in Postmenopausal Women: A Randomized Clinical Trial. Nutrients. 2020; 12(6):1758. https://doi.org/10.3390/nu12061758
Chicago/Turabian StyleGarcia-Yu, Irene A., Luis Garcia-Ortiz, Manuel A. Gomez-Marcos, Emiliano Rodriguez-Sanchez, Cristina Agudo-Conde, Jesus Gonzalez-Sanchez, Jose A. Maderuelo-Fernandez, and Jose I. Recio-Rodriguez. 2020. "Effects of Cocoa-Rich Chocolate on Blood Pressure, Cardiovascular Risk Factors, and Arterial Stiffness in Postmenopausal Women: A Randomized Clinical Trial" Nutrients 12, no. 6: 1758. https://doi.org/10.3390/nu12061758
APA StyleGarcia-Yu, I. A., Garcia-Ortiz, L., Gomez-Marcos, M. A., Rodriguez-Sanchez, E., Agudo-Conde, C., Gonzalez-Sanchez, J., Maderuelo-Fernandez, J. A., & Recio-Rodriguez, J. I. (2020). Effects of Cocoa-Rich Chocolate on Blood Pressure, Cardiovascular Risk Factors, and Arterial Stiffness in Postmenopausal Women: A Randomized Clinical Trial. Nutrients, 12(6), 1758. https://doi.org/10.3390/nu12061758






