Amino Acids and Developmental Origins of Hypertension
Abstract
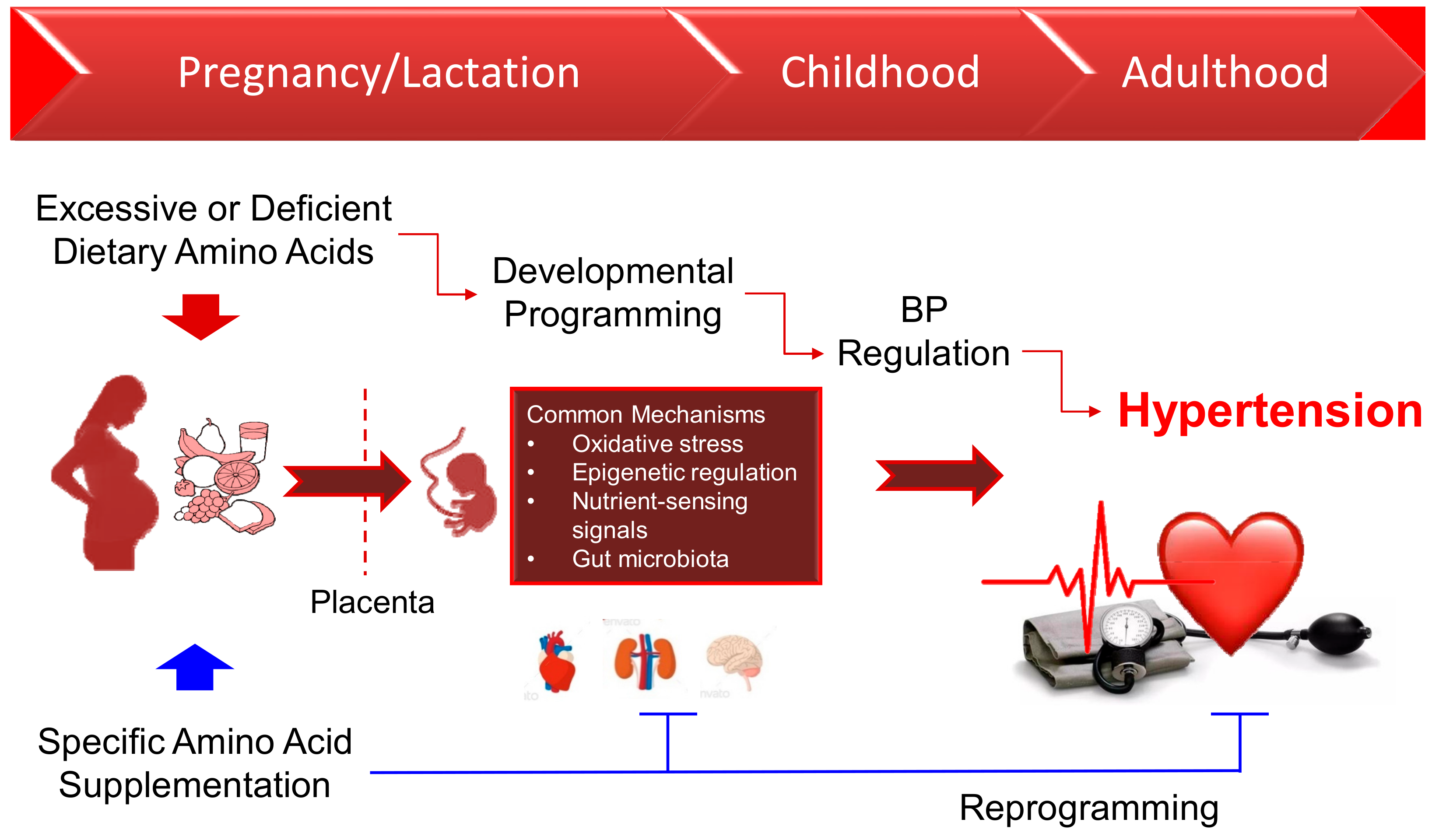
:1. Introduction
2. Amino Acid Requirements during Pregnancy and Fetal Development
2.1. Amino Acid Requirements in Pregnancy
2.2. Amino Acid Transport in the Placenta
2.3. Amino Acids and Fetal Development
3. Amino Acids and Hypertension
3.1. The Role of Amino Acids in the Regulation of BP
3.2. Dietary Amino Acids and Established Hypertension
4. Insight from Animal Models Targeting Amino Acids to Prevent Hypertension of Developmental Origin
4.1. Arginine
4.2. Taurine
4.3. Citrulline
4.4. Cysteine
4.5. Others
5. Common Mechanisms in the Developmental Programming of Hypertension
5.1. Oxidative Stress
5.2. Epigenetic Regulation
5.3. Nutrient-Sensing Signals
5.4. Gut Microbiota
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADMA | Asymmetric dimethylarginine |
| AMPK | Cyclic adenosine monophosphate-activated protein kinase |
| BCAA | Branched chain amino acid |
| DOHaD | Developmental origins of health and disease |
| EAR | Estimated Average Requirement |
| FHH | Fawn-hooded hypertensive |
| GR | Glucocorticoid receptor |
| H2S | Hydrogen sulfide |
| IUGR | Intrauterine growth restriction |
| L-NAME | NG-nitro–L-arginine methyl ester |
| mTOR | The mechanistic target of rapamycin |
| NAC | N-acetylcysteine |
| NO | Nitric oxide |
| PPAR | Peroxisome proliferator-activated receptor |
| PGC-1α | PPARγ coactivator-1α |
| RDA | Recommended Dietary Allowance |
| SDMA | Symmetric dimethylarginine |
| SHR | Spontaneously hypertensive rat |
| SIRT | Silent information regulator transcript |
References
- Luyckx, V.A.; Bertram, J.F.; Brenner, B.M.; Fall, C.; Hoy, W.E.; Ozanne, S.E.; Vikse, B.E. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013, 382, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Bagby, S.P. Maternal nutrition, low nephron number, and hypertension in later life: Pathways of nutritional programming. J. Nutr. 2007, 137, 1066–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, F.; Monuteaux, M.C.; van Elburg, R.M.; Gillman, M.W.; Belfort, M.B. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 2012, 59, 226–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paauw, N.D.; van Rijn, B.B.; Lely, A.T.; Joles, J.A. Pregnancy as a critical window for blood pressure regulation in mother and child: Programming and reprogramming. Acta Physiol. (Oxf) 2017, 219, 241–259. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, K.M.; Barker, D.J. Fetal programming and adult health. Public Health Nutr. 2001, 4, 611–624. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. The Double-Edged Sword Effects of Maternal Nutrition in the Developmental Programming of Hypertension. Nutrients 2018, 10, E1917. [Google Scholar] [CrossRef] [Green Version]
- Hanson, M. The birth and future health of DOHaD. J. Dev. Orig. Health Dis. 2015, 6, 434–437. [Google Scholar] [CrossRef]
- Tain, Y.L.; Joles, J.A. Reprogramming: A preventive strategy in hypertension focusing on the kidney. Int. J. Mol. Sci. 2015, 17, E23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noyan-Ashraf, M.H.; Wu, L.; Wang, R.; Juurlink, B.H. Dietary approaches to positively influence fetal determinants of adult health. FASEB J. 2006, 20, 371–373. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr. 2000, 71, 1218S–1225S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elango, R.; Ball, R.O. Protein and amino acid requirements during pregnancy. Adv. Nutr. 2016, 7, 839S–844S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes: Energy, carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Teunissen-Beekman, K.F.; van Baak, M.A. The role of dietary protein in blood pressure regulation. Curr. Opin. Lipidol. 2013, 24, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Fontana, M.; Giusti, A.M.; Pinto, A.; Iannucci, G.; Lenzi, A.; Donini, L.M. Amino Acids and Hypertension in Adults. Nutrients 2019, 11, E1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.N.; Tain, Y.L. Impact of Arginine Nutrition and Metabolism during Pregnancy on Offspring Outcomes. Nutrients 2019, 11, E1452. [Google Scholar] [CrossRef] [Green Version]
- Felig, P.; Kim, Y.J.; Lynch, V.; Hendler, R. Amino acid metabolism during starvation in human pregnancy. J. Clin. Inv. 1972, 51, 1195–1202. [Google Scholar] [CrossRef]
- Schoengold, D.M.; DeFiore, R.H.; Parlett, R.C. Free amino acids in plasma throughout pregnancy. Am. J. Obstet. Gynecol. 1978, 131, 490–499. [Google Scholar] [CrossRef]
- Payne, M.; Stephens, T.; Lim, K.; Ball, R.O.; Pencharz, P.B.; Elango, R. Lysine Requirements of Healthy Pregnant Women are Higher During Late Stages of Gestation Compared to Early Gestation. J. Nutr. 2018, 148, 94–99. [Google Scholar] [CrossRef]
- Ennis, M.A.; Rasmussen, B.F.; Lim, K.; Ball, R.O.; Pencharz, P.B.; Courtney-Martin, G.; Elango, R. Dietary phenylalanine requirements during early and late gestation in healthy pregnant women. Am. J. Clin. Nutr. 2020, 111, 351–359. [Google Scholar] [CrossRef]
- Soltesz, G.; Harris, D.; Mackenzie, I.Z.; Aynsley-Green, A. The metabolic and endocrine milieu of the human fetus and mother at 18-21 weeks of gestation. I. Plasma amino acid concentrations. Pediatr. Res. 1985, 19, 91–93. [Google Scholar] [CrossRef] [Green Version]
- Cleal, J.K.; Lewis, R.M. The mechanisms and regulation of placental amino acid transport to the human foetus. J. Neuroendocrinol. 2008, 20, 419–426. [Google Scholar] [CrossRef]
- Battaglia, F.C.; Regnault, T.R. Placental transport and metabolism of amino acids. Placenta 2001, 22, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Cleal, J.K.; Lofthouse, E.M.; Sengers, B.G.; Lewis, R.M. A systems perspective on placental amino acid transport. J. Physiol. 2018, 596, 5511–5522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeltzli, S.D.; Kelley, L.K.; Moe, A.J.; Smith, C.H. Anionic amino acid transport systems in isolated basal plasma membrane of human placenta. Am. J. Physiol. 1990, 259, C47–C55. [Google Scholar] [CrossRef]
- Kanai, Y.; Clemencon, B.; Simonin, A.; Leuenberger, M.; Lochner, M.; Weisstanner, M.; Hediger, M.A. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol. Asp. Med. 2013, 34, 108–120. [Google Scholar] [CrossRef]
- Cleal, J.K.; Glazier, J.D.; Ntani, G.; Crozier, S.R.; Day, P.E.; Harvey, N.C.; Robinson, S.M.; Cooper, C.; Godfrey, K.M.; Hanson, M.A.; et al. Facilitated transporters mediate net efflux of amino acids to the fetus across the basal membrane of the placental syncytiotrophoblast. J. Physiol. 2011, 589, 987–997. [Google Scholar] [CrossRef]
- Palacin, M.; Kanai, Y. The ancillary proteins of HATs: SLC3 family of amino acid transporters. Pflugers. Arch. 2004, 447, 490–494. [Google Scholar] [CrossRef]
- Roos, S.; Powell, T.L.; Jansson, T. Human placental taurine transporter in uncomplicated and IUGR pregnancies: Cellular localization, protein expression, and regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R886–R893. [Google Scholar] [CrossRef]
- Speake, P.F.; Glazier, J.D.; Ayuk, P.T.; Reade, M.; Sibley, C.P.; D’Souza, S.W. L-Arginine transport across the basal plasma membrane of the syncytiotrophoblast of the human placenta from normal and preeclamptic pregnancies. J. Clin. Endocrinol. Metab. 2003, 88, 4287–4292. [Google Scholar] [CrossRef] [Green Version]
- Widdows, K.L.; Panitchob, N.; Crocker, I.P.; Please, C.P.; Hanson, M.A.; Sibley, C.P.; Johnstone, E.D.; Sengers, B.G.; Lewis, R.M.; Glazier, J.D. Integration of computational modeling with membrane transport studies reveals new insights into amino acid exchange transport mechanisms. FASEB J. 2015, 29, 2583–2594. [Google Scholar] [CrossRef] [Green Version]
- Desforges, M.; Mynett, K.J.; Jones, R.L.; Greenwood, S.L.; Westwood, M.; Sibley, C.P.; Glazier, J.D. The SNAT4 isoform of the system A amino acid transporter is functional in human placental microvillous plasma membrane. J. Physiol. 2009, 587, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Day, P.E.; Cleal, J.K.; Lofthouse, E.M.; Goss, V.; Koster, G.; Postle, A.; Jackson, J.M.; Hanson, M.A.; Jackson, A.A.; Lewis, R.M. Partitioning of glutamine synthesised by the isolated perfused human placenta between the maternal and fetal circulations. Placenta 2013, 34, 1223–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodoy, S.; Fotiadis, D.; Stoeger, C.; Kanai, Y.; Palacin, M. The small SLC43 family: Facilitator system l amino acid transporters and the orphan EEG1. Mol. Asp. Med. 2013, 34, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Pinho, M.J.; Serrao, M.P.; Gomes, P.; Hopfer, U.; Jose, P.A.; Soares-da-Silva, P. Over-expression of renal LAT1 and LAT2 and enhanced L-DOPA uptake in SHR immortalized renal proximal tubular cells. Kidney Int. 2004, 66, 216–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakoki, M.; Wang, W.; Mattson, D.L. Cationic amino acid transport in the renal medulla and blood pressure regulation. Hypertension 2002, 39, 287–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goberdhan, D.C.; Wilson, C.; Harris, A.L. Amino acid sensing by mTORC1: Intracellular transporters mark the spot. Cell Metab. 2016, 23, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Betz, C.; Hall, M.N. Where is mTOR and what is it doing there? J. Cell Biol. 2013, 203, 563–574. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Sarbassov, D.D.; Ali, S.M.; King, J.E.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 2002, 110, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Cramer, S.; Beveridge, M.; Kilberg, M.; Novak, D. Physiological importance of system A-mediated amino acid transport to rat fetal development. Am. J. Physiol. Cell Physiol. 2002, 282, C153–C160. [Google Scholar] [CrossRef]
- Avagliano, L.; Garò, C.; Marconi, A.M. Placental amino acids transport in intrauterine growth restriction. J. Pregnancy 2012, 2012, 972562. [Google Scholar] [CrossRef]
- Roos, S.; Kanai, Y.; Prasad, P.D.; Powell, T.L.; Jansson, T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am. J. Physiol. Cell Physiol. 2009, 296, C142–C150. [Google Scholar] [CrossRef] [Green Version]
- Stein, A.D.; Zybert, P.A.; van der Pal-de Bruin, K.; Lumey, L.H. Exposure to famine during gestation, size at birth, and blood pressure at age 59 y: Evidence from the Dutch Famine. Eur. J. Epidemiol. 2006, 21, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.M.; Hall, M.H.; Barker, D.J.; Cross, J.; Shiell, A.W.; Godfrey, K.M. Diet in pregnancy and the offspring’s blood pressure 40 years later. Br. J. Obstet. Gynaecol. 1996, 103, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Shiell, A.W.; Campbell-Brown, M.; Haselden, S.; Robinson, S.; Godfrey, K.M.; Barker, D.J. High-meat, low-carbohydrate diet in pregnancy: Relation to adult blood pressure in the offspring. Hypertension 2001, 38, 1282–1288. [Google Scholar] [CrossRef] [Green Version]
- Kalkhoff, R.K.; Kandaraki, E.; Morrow, P.G.; Mitchell, T.H.; Kelber, S.; Borkowf, H.I. Relationship between neonatal birth weight and maternal plasma amino acid profiles in lean and obese nondiabetic women and in type I diabetic pregnant women. Metabolism 1988, 37, 234–239. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Satterfield, M.C.; Li, X.; Wang, X.; Johnson, G.A.; Burghardt, R.C.; Dai, Z.; Wang, J.; Wu, Z. Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 2013, 45, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Moores, R.R., Jr.; Rietberg, C.C.; Battaglia, F.C.; Fennessey, P.V.; Meschia, G. Metabolism and transport of maternal serine by the ovine placenta: Glycine production and absence of serine transport into the fetus. Pediatr. Res. 1993, 33, 590–594. [Google Scholar] [CrossRef] [Green Version]
- Wagner, I.; Musso, H. New naturally occurring amino acids. Angew. Chem. Int. Ed. Engl. 1983, 22, 816–828. [Google Scholar] [CrossRef]
- Takemoto, Y. Amino acids that centrally influence blood pressure and regional blood flow in conscious rats. J. Amino Acids 2012, 2012, 831759. [Google Scholar] [CrossRef] [Green Version]
- Nitz, K.; Lacy, M.; Atzler, D. Amino Acids and Their Metabolism in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Koeners, M.P.; van Faassen, E.E.; Wesseling, S.; de Sain-van der Velden, M.; Koomans, H.A.; Braam, B.; Joles, J.A. Maternal Supplementation With Citrulline Increases Renal Nitric Oxide in Young Spontaneously Hypertensive Rats and Has Long-Term Antihypertensive Effects. Hypertension 2007, 50, 1077–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiper, J.; Vallance, P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc. Res. 1999, 43, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Hsu, C.N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moali, C.; Boucher, J.L.; Sari, M.A.; Stuehr, D.J.; Mansuy, D. Substrate specificity of NO synthases: Detailed comparison of L-arginine, homo-L-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-L-arginine. Biochemistry 1998, 37, 10453–10460. [Google Scholar] [CrossRef] [PubMed]
- Van Guldener, C.; Nanayakkara, P.W.; Stehouwer, C.D. Homocysteine and blood pressure. Curr. Hypertens. Rep. 2003, 5, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Robaczewska, J.; Kedziora-Kornatowska, K.; Kozakiewicz, M.; Zary-Sikorska, E.; Pawluk, H.; Pawliszak, W.; Kedziora, J. Role of glutathione metabolism and glutathione-related antioxidant defense systems in hypertension. J. Physiol. Pharmacol. 2016, 67, 331–337. [Google Scholar] [PubMed]
- Hsu, C.N.; Tain, Y.L. Hydrogen Sulfide in Hypertension and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2018, 19, E1438. [Google Scholar] [CrossRef] [Green Version]
- Vasdev, S.; Singal, P.; Gill, V. The antihypertensive effect of cysteine. Int. J. Angiol. 2009, 18, 7–21. [Google Scholar] [CrossRef] [Green Version]
- Abebe, W.; Mozaffari, M.S. Role of taurine in the vasculature: An overview of experimental and human studies. Am. J. Cardiovasc. Dis. 2011, 1, 293–311. [Google Scholar]
- Militante, J.D.; Lombardini, J.B. Treatment of hypertension with oral taurine: Experimental and clinical studies. Amino Acids 2002, 23, 381–393. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.Y.; Qin, L.Q.; Zhang, Z.; Zhao, Y.; Wang, J.; Arigoni, F.; Zhang, W. Effect of oral l-arginine supplementation on blood pressure: A meta-analysis of randomized, double-blind, placebo-controlled trials. Am. Heart J. 2011, 162, 959–965. [Google Scholar] [CrossRef]
- Menzel, D.; Haller, H.; Wilhelm, M.; Robenek, H. l-arginine and B vitamins improve endothelial function in subjects with mild to moderate blood pressure elevation. Eur. J. Nutr. 2018, 57, 557–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venho, B.; Voutilainen, S.; Valkonen, V.P.; Virtanen, J.; Lakka, T.A.; Rissanen, T.H.; Ovaskainen, M.L.; Laitinen, M.; Salonen, J.T. Arginine intake, blood pressure, and the incidence of acute coronary events in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2002, 76, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Oomen, C.M.; van Erk, M.J.; Feskens, E.J.; Kok, F.J.; Kromhout, D. Arginine intake and risk of coronary heart disease mortality in elderly men. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2134–2139. [Google Scholar] [CrossRef] [Green Version]
- Mirenayat, M.S.; Moradi, S.; Mohammadi, H.; Rouhani, M.H. Effect of L-Citrulline Supplementation on Blood Pressure: A Systematic Review and Meta-Analysis of Clinical Trials. Curr. Hypertens. Rep. 2018, 20, 98. [Google Scholar] [CrossRef]
- Stamler, J.; Brown, I.J.; Daviglus, M.L.; Chan, Q.; Miura, K.; Okuda, N.; Ueshima, H.; Zhao, L.; Elliott, P. Dietary glycine and blood pressure: The International Study on Macro/Micronutrients and Blood Pressure. Am. J. Clin. Nutr. 2013, 98, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, K.R.; Milton, J.E.; Packard, D.P.; Shuler, L.A.; Short, R.A. Dietary amino acids and blood pressure: A cohort study of patients with cardiovascular disease. Am. J. Kidney Dis. 2012, 59, 803–809. [Google Scholar] [CrossRef]
- Teymoori, F.; Asghari, G.; Mirmiran, P.; Azizim, F. High dietary intake of aromatic amino acids increases risk of hypertension. J. Am. Soc. Hypertens. 2018, 12, 25–33. [Google Scholar] [CrossRef]
- Altorf-van der Kuil, W.; Engberink, M.F.; De Neve, M.; van Rooij, F.J.; Hofman, A.; van’tVeer, P.; Witteman, J.C.; Franco, O.H.; Geleijnse, J.M. Dietary amino acids and the risk of hypertension in a Dutch older population: The Rotterdam Study. Am. J. Clin. Nutr. 2013, 97, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Siomkajło, M.; Rybka, J.; Mierzchała-Pasierb, M.; Gamian, A.; Stankiewicz-Olczyk, J.; Bolanowski, M.; Daroszewski, J. Specific plasma amino acid disturbances associated with metabolic syndrome. Endocrine 2017, 58, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Sun, L.; Gong, Y.; Zhou, Y.; Yang, P.; Ye, Z.; Fu, J.; Huang, A.; Fu, Z.; Yu, W.; et al. Relationship between Branched-Chain Amino Acids, Metabolic Syndrome, and Cardiovascular Risk Profile in a Chinese Population: A Cross-Sectional Study. Int. J. Endocrinol. 2016, 2016, 8173905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, A.; MacGregor, A.; Pallister, T.; Spector, T.; Cassidy, A. Associations between branched chain amino acid intake and biomarkers of adiposity and cardiometabolic health independent of genetic factors: A twin study. Int. J. Cardiol. 2016, 223, 992–998. [Google Scholar] [CrossRef] [Green Version]
- Waldron, M.; Patterson, S.D.; Tallent, J.; Jeffries, O. The Effects of Oral Taurine on Resting Blood Pressure in Humans: A Meta-Analysis. Curr. Hypertens. Rep. 2018, 20, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altorf-van der Kuil, W.; Engberink, M.F.; Brink, E.J.; van Baak, M.A.; Bakker, S.J.; Navis, G.; van’t Veer, P.; Geleijnse, J.M. Dietary protein and blood pressure: A systematic review. PLoS ONE 2010, 5, e12102. [Google Scholar] [CrossRef] [Green Version]
- Krajcovicova-Kudlackova, M.; Babinska, K.; Valachovicova, M. Health benefits and risks of plant proteins. Bratisl. Lek. Listy. 2005, 106, 231–234. [Google Scholar]
- Wheeler, M.L.; Fineberg, S.E.; Fineberg, N.S.; Gibson, R.G.; Hackward, L.L. Animal versus plant protein meals in individuals with type 2 diabetes and microalbuminuria: Effects on renal, glycemic, and lipid parameters. Diabetes Care 2002, 25, 1277–1282. [Google Scholar] [CrossRef] [Green Version]
- Sathishkumar, K.; Elkins, R.; Yallampalli, U.; Yallampalli, C. Protein restriction during pregnancy induces hypertension and impairs endothelium-dependent vascular function in adult female offspring. J. Vasc. Res. 2009, 46, 229–239. [Google Scholar] [CrossRef]
- Woods, L.L.; Ingelfinger, J.R.; Nyengaard, J.R.; Rasch, R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr. Res. 2001, 49, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Cambonie, G.; Comte, B.; Yzydorczyk, C.; Ntimbane, T.; Germain, N.; Lê, N.L.; Pladys, P.; Gauthier, C.; Lahaie, I.; Abran, D.; et al. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1236–R1245. [Google Scholar] [CrossRef]
- Bai, S.Y.; Briggs, D.I.; Vickers, M.H. Increased systolic blood pressure in rat offspring following a maternal low-protein diet is normalized by maternal dietary choline supplementation. J. Dev. Orig. Health Dis. 2012, 3, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Van-Wickle, J.; Goyal, D.; Longo, L.D. Antenatal maternal low protein diet: ACE-2 in the mouse lung and sexually dimorphic programming of hypertension. BMC Physiol. 2015, 15, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMillen, I.C.; Robinson, J.S. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef]
- Herring, C.M.; Bazer, F.W.; Johnson, G.A.; Wu, G. Impacts of maternal dietary protein intake on fetal survival, growth, and development. Exp. Biol. Med. (Maywood) 2018, 243, 525–533. [Google Scholar] [CrossRef]
- Tain, Y.L.; Chan, J.Y.H.; Lee, C.T.; Hsu, C.N. Maternal Melatonin Therapy Attenuates Methyl-Donor Diet-Induced Programmed Hypertension in Male Adult Rat Offspring. Nutrients 2018, 10, E1407. [Google Scholar] [CrossRef] [Green Version]
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef]
- Koeners, M.P.; Braam, B.; van der Giezen, D.M.; Goldschmeding, R.; Joles, J.A. Perinatal micronutrient supplements ameliorate hypertension and proteinuria in adult fawn-hooded hypertensive rats. Am. J. Hypertens. 2010, 23, 802–808. [Google Scholar] [CrossRef]
- Koeners, M.P.; Racasan, S.; Koomans, H.A.; Joles, J.A.; Braam, B. Nitric oxide, superoxide and renal blood flow autoregulation in SHR after perinatal L-arginine and antioxidants. Acta Physiol. 2007, 190, 329–338. [Google Scholar] [CrossRef]
- Racasan, S.; Braam, B.; van der Giezen, D.M.; Goldschmeding, R.; Boer, P.; Koomans, H.A.; Joles, J.A. Perinatal L-arginine and antioxidant supplements reduce adult blood pressure in spontaneously hypertensive rats. Hypertension 2004, 44, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Wesseling, S.; Koeners, M.P.; Kantouh, F.; Joles, J.A.; Braam, B. Consequences of perinatal treatment with L-arginine and antioxidants for the renal transcriptome in spontaneously hypertensive rats. Pflugers. Arch. 2009, 458, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Roysommuti, S.; Lerdweeraphon, W.; Malila, P.; Jirakulsomchok, D.; Wyss, J.M. Perinatal taurine alters arterial pressure control and renal function in adult offspring. Adv. Exp. Med. Biol. 2009, 643, 145–156. [Google Scholar] [PubMed] [Green Version]
- Thaeomor, A.; Teangphuck, P.; Chaisakul, J.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal Taurine Supplementation Prevents Metabolic and Cardiovascular Effects of Maternal Diabetes in Adult Rat Offspring. Adv. Exp. Med. Biol. 2017, 975, 295–305. [Google Scholar] [PubMed]
- Mensegue, M.F.; Burgueño, A.L.; Tellechea, M.L. Perinatal taurine exerts a hypotensive effect in male spontaneously hypertensive rats and down-regulates endothelial oxide nitric synthase in the aortic arch. Clin. Exp. Pharmacol. Physiol. 2020. [Google Scholar] [CrossRef]
- Horie, R.; Yamori, Y.; Nara, Y.; Sawamura, M.; Mano, M. Effects of sulphur amino acids on the development of hypertension and atherosclerosis in stroke-prone spontaneously hypertensive rats. J. Hypertens. Suppl. 1987, 5, S223–S225. [Google Scholar]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Hsu, C.N.; Lee, W.C.; Huang, L.T.; Lee, C.T.; Lin, C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS ONE 2013, 8, e55420. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, C.T.; Huang, L.T. Long-term effects of maternal citrulline supplementation on renal transcriptome prevention of nitric oxide depletion-related programmed hypertension: The impact of gene-nutrient interactions. Int. J. Mol. Sci. 2014, 15, 23255–23268. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Huang, L.T.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal citrulline supplementation prevents prenatal NG-nitro-l-arginine-methyl ester (L-NAME)-induced programmed hypertension in rats. Biol. Reprod. 2015, 92, 7. [Google Scholar] [CrossRef] [Green Version]
- Tai, I.H.; Sheen, J.M.; Lin, Y.J.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Huang, L.T.; Tain, Y.L. Maternal N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and prevents programmed hypertension in male offspring exposed to prenatal dexamethasone and postnatal high-fat diet. Nitric Oxide 2016, 53, 6–12. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N.; Lee, C.Y.; Lin, Y.J.; Tsai, C.C. N-Acetylcysteine prevents programmed hypertension in male rat offspring born to suramin-treated mothers. Biol. Reprod. 2016, 95, 8. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal N(G)-Nitro-L-arginine-methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 2016, 215, 636.e1–e36.e72. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Huang, D.X.; Li, Y.; Dasgupta, C.; Wang, L.; Zhang, L. Antenatal antioxidant prevents nicotine-mediated hypertensive response in rat adult offspring. Biol. Reprod. 2015, 93, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, A.A.; Dunn, R.L.; Marchand, M.C.; Langley-Evans, S.C. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin. Sci. 2002, 103, 633–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, T.; Yura, S.; Tatsumi, K.; Kondoh, E.; Mogami, H.; Fujita, K.; Kakui, K.; Aoe, S.; Itoh, H.; Sagawa, N.; et al. Branched-chain amino acid supplemented diet during maternal food restriction prevents developmental hypertension in adult rat offspring. J. Dev. Orig. Health Dis. 2011, 2, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Luiking, Y.C.; Ten Have, G.A.M.; Wolfe, R.R.; Deutz, N.E.P. Arginine de novo and nitric oxide production in disease states. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1177–E1189. [Google Scholar] [CrossRef] [Green Version]
- Grimble, G.K. Adverse gastrointestinal effects of arginine and related amino acids. J. Nutr. 2007, 137, 1693S–1701S. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues-Krause, J.; Krause, M.; Rocha, I.M.G.D.; Umpierre, D.; Fayh, A.P.T. Association of l-Arginine Supplementation with Markers of Endothelial Function in Patients with Cardiovascular or Metabolic Disorders: A Systematic Review and Meta-Analysis. Nutrients 2018, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Alves, G.M.; Barão, M.A.; Odo, L.N.; Nascimento Gomes, G.; Franco Md Mdo, C.; Nigro, D.; Lucas, S.R.; Laurindo, F.R.; Brandizzi, L.I.; Zaladek Gil, F. L-Arginine effects on blood pressure and renal function of intrauterine restricted rats. Pediatr. Nephrol. 2002, 17, 856–862. [Google Scholar] [CrossRef]
- Carvalho, D.S.; Diniz, M.M.; Haidar, A.A.; Cavanal, M.F.; da Silva Alves, E.; Carpinelli, A.R.; Gil, F.Z.; Hirata, A.E. L-Arginine supplementation improves insulin sensitivity and beta cell function in the offspring of diabetic rats through AKT and PDX-1 activation. Eur. J. Pharmacol. 2016, 791, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Lassala, A.; Bazer, F.W.; Cudd, T.A.; Datta, S.; Keisler, D.H.; Satterfield, M.C.; Spencer, T.E.; Wu, G. Parenteral administration of L-arginine prevents fetal growth restriction in undernourished ewes. J. Nutr. 2010, 140, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Wu, X.; Yin, Y.L.; Liu, Y.Q.; Geng, M.M.; Yang, H.S.; Blachier, F.; Wu, G.Y. Effects of dietary L-arginine or N-carbamylglutamate supplementation during late gestation of sows on the miR-15b/16, miR-221/222, VEGFA and eNOS expression in umbilical vein. Amino Acids 2012, 42, 2111–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucknooghe, T.; Remacle, C.; Reusens, B. Is taurine a functional nutrient? Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Sturman, J.A. Taurine in development. Physiol. Rev. 1993, 73, 119–147. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Tsujino, T.; Watari, Y.; Nonaka, H.; Emoto, N.; Yokoyama, M. Oral taurine supplementation prevents fructose-induced hypertension in rats. Heart Vessels 2004, 19, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Shibata, K.; Matsuda, T.; Furukawa, T. Inhibition of hypertension and salt intake by oral taurine treatment in hypertensive rats. Hypertension 1987, 10, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.J.; Arneja, A.S.; Tappia, P.S.; Dhalla, N.S. The potential health benefits of taurine in cardiovascular disease. Exp. Clin. Cardiol. 2008, 13, 57–65. [Google Scholar]
- Cynober, L.; Moinard, C.; De Bandt, J.P. The 2009 ESPEN Sir David Cuthbertson. Citrulline: A new major signaling molecule or just another player in the pharmaconutrition game? Clin. Nutr. 2010, 29, 545–551. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Boger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Lassala, A.; Bazer, F.W.; Cudd, T.A.; Li, P.; Li, X.; Satterfield, M.C. Intravenous Administration of L-Citrulline to Pregnant Ewes Is More Effective Than L-Arginine for Increasing Arginine Availability in the Fetus. J. Nutr. 2009, 139, 660–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. L-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, H.; Ford, S.P.; Bazer, F.W.; Spencer, T.E.; Nathanielsz, P.W.; Nijland, M.J.; Hess, B.W.; Wu, G. Maternal nutrient restriction reduces concentrations of amino acids and polyamines in ovine maternal and fetal plasma and fetal fluids. Biol. Reprod. 2004, 71, 901–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.N.; Lin, Y.J.; Lu, P.C.; Tain, Y.L. Early supplementation of D-cysteine or L-cysteine prevents hypertension and kidney damage in spontaneously hypertensive rats exposed to high-salt intake. Mol. Nutr. Food Res. 2018, 62, 2. [Google Scholar] [CrossRef]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. Dietary Glycine Is Rate-Limiting for Glutathione Synthesis and May Have Broad Potential for Health Protection. Ochsner. J. 2018, 18, 81–87. [Google Scholar]
- Zhang, Z.Y.; Monleon, D.; Verhamme, P.; Staessen, J.A. Branched-Chain Amino Acids as Critical Switches in Health and Disease. Hypertension 2018, 72, 1012–1022. [Google Scholar] [CrossRef]
- Ojeda, N.B.; Grigore, D.; Alexander, B.T. Developmental programming of hypertension: Insight from animal models of nutritional manipulation. Hypertension 2008, 52, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Hsu, C.N.; Chan, J.Y.; Huang, L.T. Renal Transcriptome analysis of programmed hypertension induced by maternal nutritional insults. Int. J. Mol. Sci. 2015, 16, 17826–17837. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S. Impact of Maternal Diet on the Epigenome during In Utero Life and the Developmental Programming of Diseases in Childhood and Adulthood. Nutrients 2015, 7, 9492–9507. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Hsu, C.N. Interplay between oxidative stress and nutrient sensing signaling in the developmental origins of cardiovascular disease. Int. J. Mol. Sci. 2017, 18, E841. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Regulation of Nitric Oxide Production in the Developmental Programming of Hypertension and Kidney Disease. Int. J. Mol. Sci. 2019, 60, E681. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef]
- Wilcox, C.S. Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R913–R935. [Google Scholar] [CrossRef] [PubMed]
- Franco Mdo, C.; Ponzio, B.F.; Gomes, G.N.; Gil, F.Z.; Tostes, R.; Carvalho, M.H.; Fortes, Z.B. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009, 85, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lin, Y.J.; Sheen, J.M.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Tsai, C.C.; Huang, L.T.; Hsu, C.N. High Fat Diets Sex-Specifically Affect the Renal Transcriptome and Program Obesity, Kidney Injury, and Hypertension in the Offspring. Nutrients 2017, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.N.; Lin, Y.J.; Hou, C.Y.; Tain, Y.L. Maternal Administration of Probiotic or Prebiotic Prevents Male Adult Rat Offspring against Developmental Programming of Hypertension Induced by High Fructose Consumption in Pregnancy and Lactation. Nutrients 2018, 10, 1229. [Google Scholar] [CrossRef] [Green Version]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Lillycrop, K.A.; Slater-Jefferies, J.L.; Hanson, M.A.; Godfrey, K.M.; Jackson, A.A.; Burdge, G.C. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br. J. Nutr. 2007, 97, 1064–1073. [Google Scholar]
- Moisiadis, V.G.; Matthews, S.G. Glucocorticoids and fetal programming part 1: Outcomes. Nat. Rev. Endocrinol. 2014, 10, 391–402. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N.; Chan, J.Y. PPARs link early life nutritional insults to later programmed hypertension and metabolic syndrome. Int. J. Mol. Sci. 2015, 17, 20. [Google Scholar] [CrossRef] [Green Version]
- Bogdarina, I.; Welham, S.; King, P.J.; Burns, S.P.; Clark, A.J. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 2007, 100, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Sugden, M.C.; Caton, P.W.; Holness, M.J. PPAR control: It’s SIRTainly as easy as PGC. J. Endocrinol. 2010, 204, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Cetrullo, S.; D’Adamo, S.; Tantini, B.; Borzi, R.M.; Flamigni, F. mTOR, AMPK, and Sirt1: Key Players in Metabolic Stress Management. Crit. Rev. Eukaryot Gene Expr. 2015, 25, 59–75. [Google Scholar] [CrossRef]
- Meijer, A.J. Amino acids as regulators and components of nonproteinogenic pathways. J. Nutr. 2003, 133, 2057S–2062S. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. AMP-Activated Protein Kinase as a Reprogramming Strategy for Hypertension and Kidney Disease of Developmental Origin. Int. J. Mol. Sci. 2018, 19, 1744. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.M.; Meyer, K.M.; Prince, A.L.; Aagaard, K.M. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes 2016, 7, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.X.; Dai, Z.L.; Zhu, W.Y. Important impacts of intestinal bacteria on utilization of dietary amino acids in pigs. Amino Acids 2014, 46, 2489–2501. [Google Scholar] [CrossRef]
- Grohmann, U.; Bronte, V. Control of immune response by amino acid metabolism. Immunol. Rev. 2010, 236, 243–264. [Google Scholar] [CrossRef]
- Al Khodor, S.; Reichert, B.; Shatat, I.F. The Microbiome and Blood Pressure: Can Microbes Regulate Our Blood Pressure? Front. Pediatr. 2017, 5, 138. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 2018, 31, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Chan, J.Y.H.; Lee, C.T.; Tain, Y.L. Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy. Nutrients 2019, 11, E2908. [Google Scholar] [CrossRef] [Green Version]
- Lankelma, J.M.; Nieuwdorp, M.; de Vos, W.M.; Wiersinga, W.J. The gut microbiota in internal medicine: Implications for health and disease. Neth. J. Med. 2015, 73, 61–68. [Google Scholar]

| Human Gene | Protein | System | Location | Substrate | Ref. |
|---|---|---|---|---|---|
| SLC1A1 | EAAT3 | XAG | MVM, BM | Anionic amino acids | [24,25] |
| SLC1A2 | EAAT2 | XAG | MVM, BM | Anionic amino acids | |
| SLC1A3 | EAAT1 | XAG | MVM, BM | Anionic amino acids | |
| SLC1A6 | EAAT4 | XAG | MVM, BM | Anionic amino acids | |
| SLC1A4 | ASCT1 | ASC | BM | Neutral amino acids | [25,26] |
| SLC1A5 | ASCT2 | ASC | BM | Neutral amino acids | |
| SLC3A1 | rBAT | b0,+ | ? | Cationic and neutral amino acids | [27] |
| SLC3A2 | 4F2hc | L | MVM, BM | Neutral amino acids, BCAAs, and tryptophan | |
| SLC6A6 | TAUT | β | MVM | Taurine | [28] |
| SLC7A1 | CAT1 | y+ | MVM, BM | Cationic amino acids | [29] |
| SLC7A2 | CAT2B | y+ | MVM, BM | Cationic amino acids | |
| SLC7A3P | CAT3 | y+ | MVM, BM | Cationic amino acids | |
| SLC7A5 | LAT1 | L | MVM, BM | Cationic amino acids | [26,30] |
| SLC7A6 | y+LAT2 | y+L | MVM, BM | Cationic amino acids | |
| SLC7A7 | y+LAT1 | y+L | MVM, BM | Cationic amino acids | |
| SLC7A8 | LAT2 | L | MVM, BM | Cationic amino acids | |
| SLC7A10 | ASC1 | ASC | BM | Small neutral amino acids | |
| SLC7A11 | xCT | Xc- | ? | Cysteine and glutamate | |
| SLC16A10 | TAT1 | T | BM | Aromatic amino acids | [26] |
| SLC38A1 | SNAT1 | A | MVM | Neutral amino acids | [31,32] |
| SLC38A2 | SNAT2 | A | MVM | Neutral amino acids | |
| SCL38A3 | SNAT3 | N | MVM | Neutral amino acids | |
| SLC38A4 | SNAT4 | A | MVM | Neutral amino acids | |
| SCL38A5 | SNAT5 | N | MVM | Neutral amino acids | |
| SLC43A1 | LAT3 | L | BM | Neutral amino acids | [26,33] |
| SLC43A2 | LAT4 | L | BM | Neutral amino acids |
| Intervention | Animal Model | Species/Gender | Age at Measure | Ref. |
|---|---|---|---|---|
| Arginine/Taurine | ||||
| Arginine (20 g/L) and taurine (25 g/L) in drinking water plus antioxidants * from day 7 of gestation to postnatal week 4 | Genetic hypertension | FHH/M and F | 9 weeks | [88] |
| Arginine (20 g/L) and taurine (25 g/L) in drinking water plus antioxidants * from day 7 of gestation to postnatal week 8 | Genetic hypertension | SHR/M and F | 24 weeks | [89] |
| Arginine (20 g/L) and taurine (25 g/L) in drinking water plus antioxidants * from day 7 of gestation to postnatal week 8 | Genetic hypertension | SHR/M and F | 36 weeks | [90] |
| Arginine (20 g/L) and taurine (25 g/L) in drinking water plus antioxidants * from day 7 of gestation to postnatal week 4 | Genetic hypertension | SHR/F | 48 weeks | [91] |
| Taurine | ||||
| 3% taurine in drinking water during pregnancy and lactation | High-sugar diet | SD/F | 8 weeks | [92] |
| 3% taurine in drinking water during pregnancy and lactation | Streptozotocin-induced diabetes | Wistar/M and F | 16 weeks | [93] |
| 3% taurine in drinking water during pregnancy and lactation | Genetic hypertension | SHR/M | 22 weeks | [94] |
| 5% taurine in drinking water during pregnancy | Genetic hypertension | SHRSP/M | 3 months | [95] |
| Citrulline | ||||
| 2.5 g/L citrulline in drinking water during pregnancy and lactation | Maternal 50% caloric restriction | SD/M | 12 weeks | [96] |
| 2.5 g/L citrulline in drinking water during pregnancy and lactation | Prenatal dexamethasone exposure | SD/M | 12 weeks | [97] |
| 2.5 g/L citrulline in drinking water during pregnancy and lactation | Streptozotocin-induced diabetes | SD/M | 12 weeks | [98] |
| 2.5 g/L citrulline in drinking water during pregnancy and lactation | Maternal L-NAME exposure | SD/M | 12 weeks | [99,100] |
| 2.5 g/L of water from day 7 of gestation to postnatal week 6 | Genetic hypertension | SHR/M and F | 50 weeks | [52] |
| Cysteine | ||||
| 1% NAC in drinking water during pregnancy and lactation | Prenatal dexamethasone and postnatal high-fat diet | SD/M | 12 weeks | [101] |
| 1% NAC in drinking water during pregnancy and lactation | Suramin-induced pre-eclampsia | SD/M | 12 weeks | [102] |
| 1% NAC in drinking water during pregnancy and lactation | Maternal L-NAME exposure | SD/M | 12 weeks | [103] |
| NAC (500 mg/kg/day) in drinking water from gestational day 4 to postnatal day 10 | Maternal nicotine exposure | SD/M | 8 months | [104] |
| Glycine | ||||
| 3% glycine in chow during pregnancy and lactation | Maternal 9% protein restriction | Wistar/M | 4 weeks | [105] |
| Branched chain amino acids | ||||
| BCAA-supplemented diets in pregnancy | Maternal 70% caloric restriction | SD/M | 16 weeks | [106] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-N.; Tain, Y.-L. Amino Acids and Developmental Origins of Hypertension. Nutrients 2020, 12, 1763. https://doi.org/10.3390/nu12061763
Hsu C-N, Tain Y-L. Amino Acids and Developmental Origins of Hypertension. Nutrients. 2020; 12(6):1763. https://doi.org/10.3390/nu12061763
Chicago/Turabian StyleHsu, Chien-Ning, and You-Lin Tain. 2020. "Amino Acids and Developmental Origins of Hypertension" Nutrients 12, no. 6: 1763. https://doi.org/10.3390/nu12061763
APA StyleHsu, C.-N., & Tain, Y.-L. (2020). Amino Acids and Developmental Origins of Hypertension. Nutrients, 12(6), 1763. https://doi.org/10.3390/nu12061763






