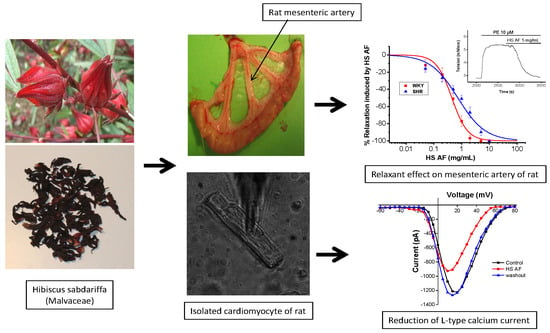
Aqueous Fraction from Hibiscus sabdariffa Relaxes Mesenteric Arteries of Normotensive and Hypertensive Rats through Calcium Current Reduction and Possibly Potassium Channels Modulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. H. Sabdariffa Extraction and Fractionation
2.3. HPLC Analysis
2.4. Animals
2.5. Contractility Experiments on an Isolated Mesenteric Artery
2.6. Ventricular Cardiomyocytes Isolation
2.7. Whole Cell Patch-Clamp Experiments
2.8. Microelectrode Experiments for the Measurement of Membrane Potential
2.9. Data and Statistical Analysis
2.10. Chemicals
3. Results
3.1. Preparation of H. Sabdariffa AF and Characterization
3.2. Effects of H. sabdariffa AF on Rat Mesenteric Artery Ring
3.3. Role of Endothelium and nitric oxide (NO) Pathway
3.4. Involvement of Adrenergic Pathway
3.5. Effect of H. sabdariffa AF on Ionic Currents
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| High-performance liquid chromatography | HPLC |
| Maximum effect | Emax |
| Concentration required to relax the induced tone by 50% | EC50 |
| number of preparations/number of rats | n |
| Krebs-Ringer Bicarbonate Buffer | KRB |
| Hydroxyethyl piperazineethanesulfonic acid | HEPES |
| kraftbrühe | KB |
References
- Ali, B.H.; Al Wabel, N.; Blunden, G. Phytochemical, pharmacological and toxicological aspects of Hibiscus sabdariffa L.: A review. Phytother. Res. PTR 2005, 19, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.F. Fruits of Warm Climates; JF Morton: Miami, FL, USA, 1987; ISBN 0-9610184-1-0. [Google Scholar]
- Ross, I.A. Medicinal Plants of the World: Volume 1: Chemical Constituents, Traditional and Modern Medicinal Uses, 2nd ed.; Humana Press: Totowa, NJ, USA, 2003; ISBN 978-1-58829-281-0. [Google Scholar]
- Haji Faraji, M.; Haji Tarkhani, A. The effect of sour tea (Hibiscus sabdariffa) on essential hypertension. J. Ethnopharmacol. 1999, 65, 231–236. [Google Scholar] [CrossRef]
- Herrera-Arellano, A.; Miranda-Sánchez, J.; Avila-Castro, P.; Herrera-Alvarez, S.; Jiménez-Ferrer, J.E.; Zamilpa, A.; Román-Ramos, R.; Ponce-Monter, H.; Tortoriello, J. Clinical effects produced by a standardized herbal medicinal product of Hibiscus sabdariffa on patients with hypertension. A randomized, double-blind, lisinopril-controlled clinical trial. Planta Med. 2007, 73, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Arellano, A.; Flores-Romero, S.; Chávez-Soto, M.A.; Tortoriello, J. Effectiveness and tolerability of a standardized extract from Hibiscus sabdariffa in patients with mild to moderate hypertension: A controlled and randomized clinical trial. Phytomedicine 2004, 11, 375–382. [Google Scholar] [CrossRef]
- McKay, D.L.; Chen, C.-Y.O.; Saltzman, E.; Blumberg, J.B. Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J. Nutr. 2010, 140, 298–303. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Ahadi, Z.; Barzegar, K. The effect of green tea and sour tea on blood pressure of patients with type 2 diabetes: A randomized clinical trial. J. Diet. Suppl. 2013, 10, 105–115. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Jalali-Khanabadi, B.-A.; Afkhami-Ardekani, M.; Fatehi, F.; Noori-Shadkam, M. The effects of sour tea (Hibiscus sabdariffa) on hypertension in patients with type II diabetes. J. Hum. Hypertens. 2009, 23, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Mojiminiyi, F.B.O.; Dikko, M.; Muhammad, B.Y.; Ojobor, P.D.; Ajagbonna, O.P.; Okolo, R.U.; Igbokwe, U.V.; Mojiminiyi, U.E.; Fagbemi, M.A.; Bello, S.O.; et al. Antihypertensive effect of an aqueous extract of the calyx of Hibiscus sabdariffa. Fitoterapia 2007, 78, 292–297. [Google Scholar] [CrossRef]
- Odigie, I.P.; Ettarh, R.R.; Adigun, S.A. Chronic administration of aqueous extract of Hibiscus sabdariffa attenuates hypertension and reverses cardiac hypertrophy in 2K-1C hypertensive rats. J. Ethnopharmacol. 2003, 86, 181–185. [Google Scholar] [CrossRef]
- Onyenekwe, P.C.; Ajani, E.O.; Ameh, D.A.; Gamaniel, K.S. Antihypertensive effect of roselle (Hibiscus sabdariffa) calyx infusion in spontaneously hypertensive rats and a comparison of its toxicity with that in Wistar rats. Cell Biochem. Funct. 1999, 17, 199–206. [Google Scholar] [CrossRef]
- Adegunloye, B.J.; Omoniyi, J.O.; Owolabi, O.A.; Ajagbonna, O.P.; Sofola, O.A.; Coker, H.A. Mechanisms of the blood pressure lowering effect of the calyx extract of Hibiscus sabdariffa in rats. Afr. J. Med. Med. Sci. 1996, 25, 235–238. [Google Scholar] [PubMed]
- Ajay, M.; Chai, H.J.; Mustafa, A.M.; Gilani, A.H.; Mustafa, M.R. Mechanisms of the anti-hypertensive effect of Hibiscus sabdariffa L. calyces. J. Ethnopharmacol. 2007, 109, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obiefuna, P.C.M.; Owolabi, O.A.; Adegunloye, B.J.; Obiefuna, I.P.; Sofola, O.A. The Petal Extract of Hibiscus sabdariffa Produces Relaxation of Isolated Rat Aorta. Int. J. Pharmacogn. 1994, 32, 69–74. [Google Scholar] [CrossRef]
- Sarr, M.; Ngom, S.; Kane, M.O.; Wele, A.; Diop, D.; Sarr, B.; Gueye, L.; Andriantsitohaina, R.; Diallo, A.S. In vitro vasorelaxation mechanisms of bioactive compounds extracted from Hibiscus sabdariffa on rat thoracic aorta. Nutr. Metab. 2009, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.B.; Salih, W.M.; Mohamed, A.H.; Homeida, A.M. Investigation of the antispasmodic potential of Hibiscus sabdariffa calyces. J. Ethnopharmacol. 1991, 31, 249–257. [Google Scholar] [CrossRef]
- Fouda, A.-M.M.; Daba, M.-H.Y.; Dahab, G.M. Inhibitory effects of aqueous extract of Hibiscus sabdariffa on contractility of the rat bladder and uterus. Can. J. Physiol. Pharmacol. 2007, 85, 1020–1031. [Google Scholar] [CrossRef]
- Salah, A.M.; Gathumbi, J.; Vierling, W. Inhibition of intestinal motility by methanol extracts of Hibiscus sabdariffa L. (Malvaceae) in rats. Phytother. Res. PTR 2002, 16, 283–285. [Google Scholar] [CrossRef]
- Sharaf, A. The pharmacological characteristics of Hibiscus sabdariffa L. Planta Med. 1962, 10, 48–52. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef] [Green Version]
- Zheoat, A.M.; Gray, A.I.; Igoli, J.O.; Ferro, V.A.; Drummond, R.M. Hibiscus acid from Hibiscus sabdariffa (Malvaceae) has a vasorelaxant effect on the rat aorta. Fitoterapia 2019, 134, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K. Structure and Function of Various Vascular Beds. In Textbook of Angiology; Chang, J.B., Ed.; Springer: New York, NY, USA, 2000; ISBN 978-1-4612-1190-7. [Google Scholar]
- Hopkins, A.L.; Lamm, M.G.; Funk, J.L.; Ritenbaugh, C. Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: A comprehensive review of animal and human studies. Fitoterapia 2013, 85, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojeda, D.; Jiménez-Ferrer, E.; Zamilpa, A.; Herrera-Arellano, A.; Tortoriello, J.; Alvarez, L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol. 2010, 127, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Wahabi, H.A.; Alansary, L.A.; Al-Sabban, A.H.; Glasziuo, P. The effectiveness of Hibiscus sabdariffa in the treatment of hypertension: A systematic review. Phytomedicine 2010, 17, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Meneses, A.; Hong, E. Spontaneously hypertensive rats: A potential model to identify drugs for treatment of learning disorders. Hypertens. Dallas Tex 1979 1998, 31, 968–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajid, M.; Khan, M.R.; Ismail, H.; Latif, S.; Rahim, A.A.; Mehboob, R.; Shah, S.A. Antidiabetic and antioxidant potential of Alnus nitida leaves in alloxan induced diabetic rats. J. Ethnopharmacol. 2020, 251, 112544. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Figueroa-Espinoza, M.C.; Barouh, N.; Baréa, B.; Fernandes, A.; de Freitas, V.; Salas, E. Isolation and Characterization of Anthocyanins from Hibiscus sabdariffa Flowers. J. Nat. Prod. 2016, 79, 1709–1718. [Google Scholar] [CrossRef]
- Kubota, Y.; Umegaki, K.; Kagota, S.; Tanaka, N.; Nakamura, K.; Kunitomo, M.; Shinozuka, K. Evaluation of blood pressure measured by tail-cuff methods (without heating) in spontaneously hypertensive rats. Biol. Pharm. Bull. 2006, 29, 1756–1758. [Google Scholar] [CrossRef] [Green Version]
- Bronquard, C.; Maupoil, V.; Arbeille, B.; Fetissof, F.; Findlay, I.; Cosnay, P.; Freslon, J.-L. Contractile and relaxant properties of rat-isolated pulmonary veins related to localization and histology. Fundam. Clin. Pharmacol. 2007, 21, 55–65. [Google Scholar] [CrossRef]
- Pasqualin, C.; Yu, A.; Malécot, C.O.; Gannier, F.; Cognard, C.; Godin-Ribuot, D.; Morand, J.; Bredeloux, P.; Maupoil, V. Structural heterogeneity of the rat pulmonary vein myocardium: Consequences on intracellular calcium dynamics and arrhythmogenic potential. Sci. Rep. 2018, 8, 3244. [Google Scholar] [CrossRef]
- Mulvany, M.J.; Nilsson, H.; Flatman, J.A. Role of membrane potential in the response of rat small mesenteric arteries to exogenous noradrenaline stimulation. J. Physiol. 1982, 332, 363–373. [Google Scholar] [CrossRef]
- Andersen, Ø.M.; Jordheim, M. The Anthocyanins. In Flavonoids: Chemistry, Biochemistry, and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2006; Chapter 10; pp. 471–537. [Google Scholar]
- Feelisch, M.; Kotsonis, P.; Siebe, J.; Clement, B.; Schmidt, H.H. The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3,-a] quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome P-450 enzymes involved in nitric oxide donor bioactivation. Mol. Pharmacol. 1999, 56, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belles, B.; Malécot, C.O.; Hescheler, J.; Trautwein, W. “Run-down” of the Ca current during long whole-cell recordings in guinea pig heart cells: Role of phosphorylation and intracellular calcium. Pflüg. Arch. 1988, 411, 353–360. [Google Scholar] [CrossRef]
- Leloup, A.J.A.; Van Hove, C.E.; De Moudt, S.; De Meyer, G.R.Y.; De Keulenaer, G.W.; Fransen, P. Vascular smooth muscle cell contraction and relaxation in the isolated aorta: A critical regulator of large artery compliance. Physiol. Rep. 2019, 7. [Google Scholar] [CrossRef]
- Mulvany, M.J.; Halpern, W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977, 41, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owolabi, O.A.; Adegunloye, B.J.; Ajagbona, O.P.; Sofola, O.A.; Obiefuna, P.C.M. Mechanism of Relaxant Effect Mediated by an Aqueous Extract of Hibiscus sabdariffa Petals in Isolated Rat Aorta. Int. J. Pharmacogn. 1995, 33, 210–214. [Google Scholar] [CrossRef]
- Micucci, M.; Malaguti, M.; Toschi, T.G.; Di Lecce, G.; Aldini, R.; Angeletti, A.; Chiarini, A.; Budriesi, R.; Hrelia, S. Cardiac and Vascular Synergic Protective Effect of Olea europea L. Leaves and Hibiscus sabdariffa L. Flower Extracts. Oxid. Med. Cell. Longev. 2015, 2015, 318125. [Google Scholar] [CrossRef] [Green Version]
- Bolton, T.B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol. Rev. 1979, 59, 606–718. [Google Scholar] [CrossRef]
- Karaki, H.; Weiss, G.B. Calcium channels in smooth muscle. Gastroenterology 1984, 87, 960–970. [Google Scholar] [CrossRef]
- Lim, Y.-C.; Budin, S.B.; Othman, F.; Latip, J.; Zainalabidin, S. Roselle Polyphenols Exert Potent Negative Inotropic Effects via Modulation of Intracellular Calcium Regulatory Channels in Isolated Rat Heart. Cardiovasc. Toxicol. 2017, 17, 251–259. [Google Scholar] [CrossRef]
- Tykocki, N.R.; Boerman, E.M.; Jackson, W.F. Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles. Compr. Physiol. 2017, 7, 485–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Scholz, E.P.; Zitron, E.; Katus, H.A.; Karle, C.A. Cardiovascular Ion Channels as a Molecular Target of Flavonoids. Cardiovasc. Ther. 2010, 28, e46–e52. [Google Scholar] [CrossRef] [PubMed]
- Torres-Piedra, M.; Figueroa, M.; Hernández-Abreu, O.; Ibarra-Barajas, M.; Navarrete-Vázquez, G.; Estrada-Soto, S. Vasorelaxant effect of flavonoids through calmodulin inhibition: Ex vivo, in vitro, and in silico approaches. Bioorg. Med. Chem. 2011, 19, 542–546. [Google Scholar] [CrossRef]
- Beltrán-Debón, R.; Alonso-Villaverde, C.; Aragonès, G.; Rodríguez-Medina, I.; Rull, A.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Camps, J.; Joven, J. The aqueous extract of Hibiscus sabdariffa calices modulates the production of monocyte chemoattractant protein-1 in humans. Phytomedicine 2010, 17, 186–191. [Google Scholar] [CrossRef]
- Peng, C.-H.; Chyau, C.-C.; Chan, K.-C.; Chan, T.-H.; Wang, C.-J.; Huang, C.-N. Hibiscus sabdariffa polyphenolic extract inhibits hyperglycemia, hyperlipidemia, and glycation-oxidative stress while improving insulin resistance. J. Agric. Food Chem. 2011, 59, 9901–9909. [Google Scholar] [CrossRef]
- Ali, B.H.; Cahliková, L.; Opletal, L.; Karaca, T.; Manoj, P.; Ramkumar, A.; Al Suleimani, Y.M.; Al Za’abi, M.; Nemmar, A.; Chocholousova-Havlikova, L.; et al. Effect of aqueous extract and anthocyanins of calyces of Hibiscus sabdariffa (Malvaceae) in rats with adenine-induced chronic kidney disease. J. Pharm. Pharmacol. 2017, 69, 1219–1229. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsayed, A.M.A.; Zhang, B.L.; Bredeloux, P.; Boudesocque-Delaye, L.; Yu, A.; Peineau, N.; Enguehard-Gueiffier, C.; Ahmed, E.M.; Pasqualin, C.; Maupoil, V. Aqueous Fraction from Hibiscus sabdariffa Relaxes Mesenteric Arteries of Normotensive and Hypertensive Rats through Calcium Current Reduction and Possibly Potassium Channels Modulation. Nutrients 2020, 12, 1782. https://doi.org/10.3390/nu12061782
Alsayed AMA, Zhang BL, Bredeloux P, Boudesocque-Delaye L, Yu A, Peineau N, Enguehard-Gueiffier C, Ahmed EM, Pasqualin C, Maupoil V. Aqueous Fraction from Hibiscus sabdariffa Relaxes Mesenteric Arteries of Normotensive and Hypertensive Rats through Calcium Current Reduction and Possibly Potassium Channels Modulation. Nutrients. 2020; 12(6):1782. https://doi.org/10.3390/nu12061782
Chicago/Turabian StyleAlsayed, Anas M.A., Bei Li Zhang, Pierre Bredeloux, Leslie Boudesocque-Delaye, Angèle Yu, Nicolas Peineau, Cécile Enguehard-Gueiffier, Elhadi M. Ahmed, Côme Pasqualin, and Véronique Maupoil. 2020. "Aqueous Fraction from Hibiscus sabdariffa Relaxes Mesenteric Arteries of Normotensive and Hypertensive Rats through Calcium Current Reduction and Possibly Potassium Channels Modulation" Nutrients 12, no. 6: 1782. https://doi.org/10.3390/nu12061782
APA StyleAlsayed, A. M. A., Zhang, B. L., Bredeloux, P., Boudesocque-Delaye, L., Yu, A., Peineau, N., Enguehard-Gueiffier, C., Ahmed, E. M., Pasqualin, C., & Maupoil, V. (2020). Aqueous Fraction from Hibiscus sabdariffa Relaxes Mesenteric Arteries of Normotensive and Hypertensive Rats through Calcium Current Reduction and Possibly Potassium Channels Modulation. Nutrients, 12(6), 1782. https://doi.org/10.3390/nu12061782