Anemia of Chronic Diseases: Wider Diagnostics—Better Treatment?
Abstract
:1. Introduction
2. Iron Metabolism—General Remarks
3. Iron Absorption and Characterization of Proteins Involved in the Metabolism
4. Regulation of Iron Metabolism at the Cellular and Systemic Level
5. Pathogenesis of Anemia of Chronic Disease
6. Diagnosis of Anemia of Chronic Disease
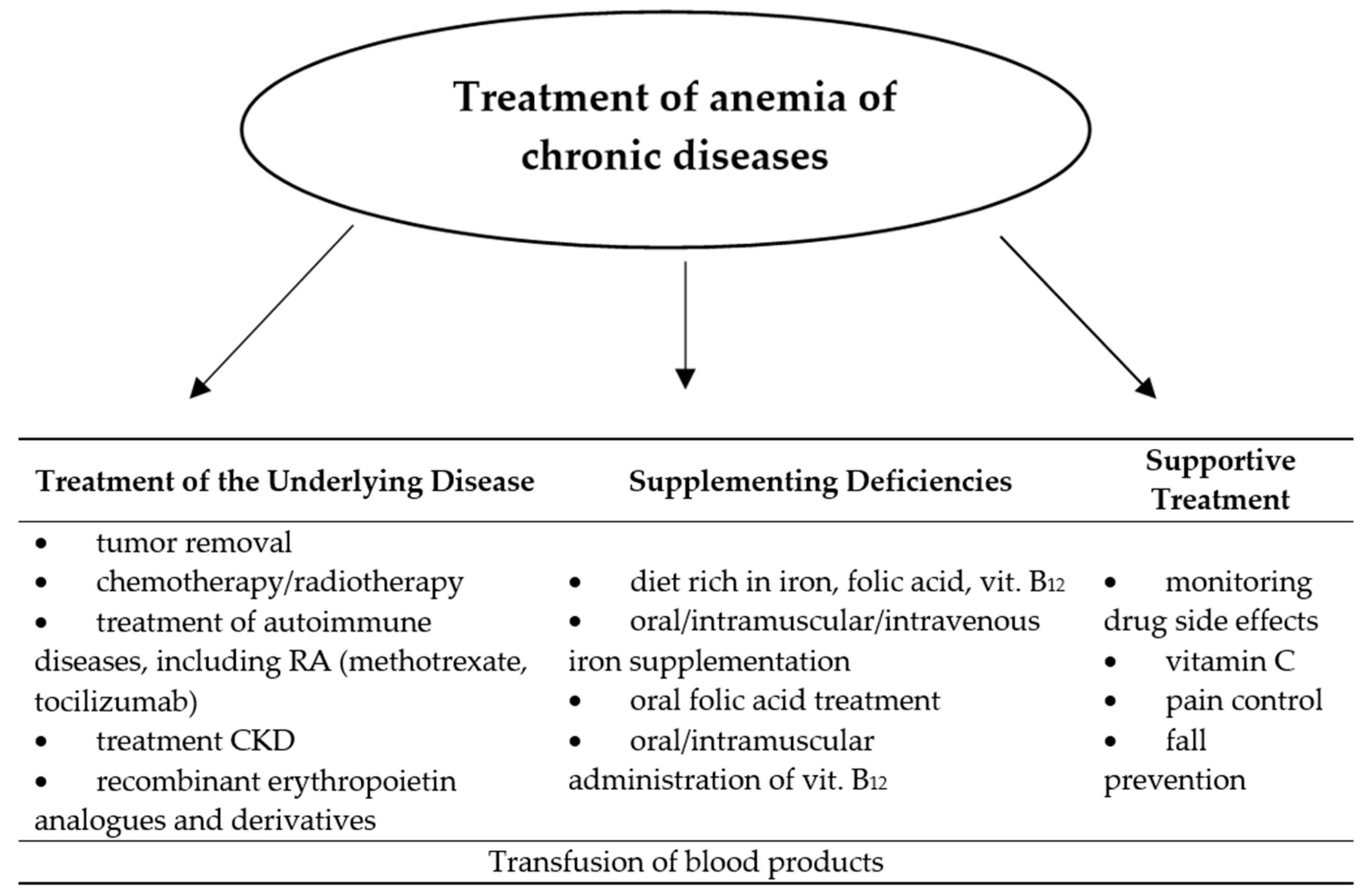
7. Treatment of Anemia of Chronic Diseases
8. The Role of Nutrition and Supplementation of Hematopoietic Factors in the Treatment of Anemia of Chronic Diseases
9. Oral Iron
10. The Impact of Diet and Drugs for the Treatment of Iron Deficiency
11. Parenteral Iron
12. Folic Acid
13. Vitamin B12
14. Treatment Considerations
15. Conclusions
- Anemia of chronic diseases is still a type of anemia that is difficult to treat. This is the result of the complex pathomechanism of this disease and the ability of the underlying disease to make a multifactorial, pathological modulation of the erythropoiesis process.
- Improper therapeutic management may result from diagnostic errors, inappropriate treatment of the underlying disease and underestimation of the benefits of hematopoietic factors supplementation.
- Administration of recombinant erythropoietin analogues in patients during chemotherapy allows us to reduce the necessity of transfusion of blood products, although the indications for their use are very limited.
- The supplementation of hematopoietic factors should be implemented simultaneously with the diagnosis of the underlying disease and last until its cure or longer, with the exception of recombinant erythropoietin analogues and derivatives.
- Proper nutrition and prevention of food deficiencies remains the primary form of preventing any type of anemia, including anemia of chronic diseases.
- Better knowledge of proteins and mechanisms involved in the formation of anemia of chronic diseases associated with, among others, malignant neoplasms gives a great chance of creating molecularly targeted drugs for the treatment of these diseases. Potentially the most effective is the inhibition of hepcidin production and activity, therapy with transferrin conjugates with anti-cancer drugs, and increasing iron absorption from the gastrointestinal tract and synthesis of erythropoietin using PHD inhibitors and HIF-2 α stabilizers.
Author Contributions
Funding
Conflicts of Interest
References
- Kaushansky, K. Lineage-specific hematopoietic growth factors. N. Engl. J. Med. 2006, 354, 2034–2045. [Google Scholar] [CrossRef]
- Arcasoy, M.O. The non-haematopoietic biological effects of erythropoietin. Br. J. Haematol. 2008, 141, 14–31. [Google Scholar] [CrossRef]
- Maxwell, P.; Melendez-Rodríguez, F.; Matchett, K.B.; Aragones, J.; Ben-Califa, N.; Jaekel, H.; Hengst, L.; Lindner, H.; Bernardini, A.; Brockmeier, U.; et al. Novel antibodies directed against the human erythropoietin receptor: Creating a basis for clinical implementation. Br. J. Haematol 2015, 168, 429–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Imam, M.U.; Zhang, S.; Ma, J.; Wang, H.; Wang, F. Antioxidants Mediate Both Iron Homeostasis and Oxidative Stress. Nutrients 2017, 9, 641. [Google Scholar] [CrossRef] [PubMed]
- Yun-Jung, B.; Jee-Young, Y.; Chung-Ja, S.; Hyun-Sook, K.; Mi-Kyung, S. Dietary intake and serum levels of iron in relation to oxidative stress in breast cancer patient. J. Clin. Biochem. Nutr. 2009, 45, 355–360. [Google Scholar]
- Chiou, B.; Connor, J.R. Emerging and Dynamic Biomedical Uses of Ferritin. Pharmaceuticals (Basel) 2018, 11, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dignass, A.; Farrag, K.; Stein, J. Limitations of Serum Ferritin in Diagnosing Iron Deficiency in Inflammatory Conditions. Int. J. Chronic Dis. 2018, 2018, 9394060. [Google Scholar] [CrossRef] [Green Version]
- Santana-Codina, N.; Mancias, J.D. The Role of NCOA4-Mediated Ferritinophagy in Health and Disease. Pharmaceiticals (Basel) 2018, 11, 114. [Google Scholar] [CrossRef] [Green Version]
- Bellelli, R.; Federico, G.; Matte, A.; Colecchia, D.; Iolascon, A.; Chiariello, M.; Santoro, M.; De Franceschi, L.; Carlomagno, F. NCOA4 Deficiency Impairs Systemic Iron Homeostasis. Cell Rep. 2016, 14, 411–421. [Google Scholar] [CrossRef] [Green Version]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A.; et al. An Iron-Regulated Ferric Reductase Associated with the Absorption of Dietary Iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Hirsch, J.I.; Moore, E.W. Evidence that bile salts are important for iron absorption. Am. J. Physiol. 1994, 266, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Hirsch, J.I.; Moore, E.W. High-affinity Binding Is Essential for Enhancement of Intestinal Fe2+ and Ca2+ Uptake by Bile Salts. Gastroenterology 1992, 102, 1997–2005. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Hepcidin and Iron homeostasis. Biochim. Biophys. Acta 2012, 1823, 1434–1443. [Google Scholar] [CrossRef] [Green Version]
- Jordan, J.B.; Poppe, L.; Haniu, M.; Arvedson, T.; Syed, R.; Li, V.; Kohno, H.; Kim, H.; Schnier, P.D.; Harvey, T.S.; et al. Hepcidin Revisited, Disulfide Connectivity, Dynamics, and Structure. J. Biol. Chem. 2009, 284, 24155–24167. [Google Scholar] [CrossRef] [Green Version]
- Tussing-Humphreys, L.; Pustacioglu, C.; Nemeth, E.; Braunschweig, C. Rethinking Iron Regulation and Assessment in Iron Deficiency, Anemia of Chronic Disease, and Obesity: Introducing Hepcidin. J. Acad. Nutr. Diet. 2012, 112, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Means, R.T., Jr. Hepcidin and Anaemia. Blood Rev. 2004, 18, 219–225. [Google Scholar] [CrossRef]
- Sophie, W.-A.; Gérard, W.; Christoph, G.; Andreas, B.; Beat, M.F.; Bernard, F.; Jean-Daniel, T. Physiology of Iron Metabolism. Transfus. Med. Hemother. 2014, 41, 213–221. [Google Scholar]
- Gkouvatsos, K.; Papanikolaou, G.; Pantopoulos, K. Regulation of Iron Transport and the Role of Transferrin. Biochim. Biophys. Acta 2012, 1820, 188–202. [Google Scholar] [CrossRef]
- Maccio, A.; Madeddu, C. Management of Anemia of Inflammation in the Elderly. Anemia 2012, 2012, 1–20. [Google Scholar] [CrossRef]
- Scheers, N. Regulatory Effects of Cu, Zn, and Ca on Fe Absorption: The Intricate Play between Nutrient Transporters. Nutrients 2013, 5, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.R.; Kalinowski, D.S.; Lau, S.; Jansson, P.J.; Lovejoy, D.B. Cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents. Biochim. Biophys. Acta 2009, 1790, 702–717. [Google Scholar] [CrossRef] [PubMed]
- Gomme, P.T.; McCann, K.B.; Bertolini, J. Transferrin: Structure, function and potential therapeutic actions. Drug Disc. Tod. 2005, 10, 267–273. [Google Scholar] [CrossRef]
- Renassia, C.; Peyssonnaux, C. New insights into the links between hypoxia and iron homeostasis. Curr. Opin. Hematol. 2019, 26, 125–130. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jiang, B.H.; Chin, B.Y.; Iyer, N.V.; Alam, J.; Semenza, G.L.; Choi, A.M. Hypoxia-inducible factor-1 Mediates Transcriptional Activation of the Heme oxygenase-1 Gene in Response to Hypoxia. J. Biol. Chem. 1997, 272, 5375–5381. [Google Scholar] [CrossRef] [Green Version]
- Tacchini, L.; Bianchi, L.; Bernelli-Zazzera, A.; Cairo, G. Transferrin Receptor Induction by Hypoxia. HIF-1-mediated Transcriptional Activation and Cell-Specific Post-Transcriptional Regulation. J. Biol. Chem. 1999, 274, 24142–24146. [Google Scholar] [CrossRef] [Green Version]
- Rankin, E.B.; Biju, M.P.; Liu, Q.; Unger, T.L.; Rha, J.; Johnson, R.S.; Simon, M.C.; Keith, B.; Haase, V.H. Hypoxia-inducible factor-2 (HIF-2) Regulates Hepatic Erythropoietin In Vivo. J. Clin. Invest. 2007, 117, 1068–1077. [Google Scholar] [CrossRef]
- Mastrogiannaki, M.; Matak, P.; Peyssonnaux, C. The gut in iron homeostasis: Role of HIF-2 under normal and pathological conditions. Blood 2013, 122, 885–892. [Google Scholar] [CrossRef] [Green Version]
- del Vecchio, L.; Locatelli, F. Investigational Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors (HIF-PHI) for the Treatment of Anemia Associated with Chronic Kidney Disease. Expert Opin. Investig. Drugs 2018, 27, 613–621. [Google Scholar] [CrossRef]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tas, F.; Eralp, Y.; Basaran, M.; Sakar, B.; Alici, S.; Argon, A.; Bulutlar, G.; Camlica, H.; Aydiner, A.; Topuz, E. Anemia in oncology practice: Relation to diseases and their therapies. Am. J. Clin. Oncol. 2002, 25, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Grogan, M.; Thomas, G.M.; Melamed, I.; Wong, F.L.; Pearcey, R.G.; Joseph, P.K.; Portelance, L.; Crook, J.; Jones, K.D. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer 1999, 86, 1528–1536. [Google Scholar] [CrossRef]
- Caro, J.J.; Salas, M.; Ward, A.; Goss, G. Anemia as an independent prognostic factor for survival in patients with cancer: A systemic, quantitative review. Cancer 2001, 91, 2214–2221. [Google Scholar] [CrossRef]
- Lee, W.R.; Berkey, B.; Marcial, V.; Fu, K.K.; Cooper, J.S.; Vikram, B.; Coia, L.R.; Rotman, M.; Ortiz, H. Anemia is associated with decreased survival and increased locoregional failure in patients with locally advanced head and neck carcinoma: A secondary analysis of RTOG 85-27. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 1069–1075. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Vilani, L. Serum hepcidin: A novel diagnostic tool in disorders of iron metabolism. Haematologica 2009, 94, 1631–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, N.; Dudas, J.; Ramadori, G. Changes of gene expression of iron regulatory proteins during turpentine oil-induced acute-phase response in the rat. Lab. Investig. 2007, 87, 713–725. [Google Scholar] [CrossRef] [Green Version]
- Jelkmann, W. Proinflammatory cytokines lowering erythropoietin production. J. Interferon Cyt. Res. 1998, 18, 555–559. [Google Scholar] [CrossRef]
- Faquin, W.C.; Schneider, T.J.; Goldberg, M.A. Effect of inflammatory cytokines on hypoxia-induced erythropoietin production. Blood 1992, 79, 1987–1994. [Google Scholar] [CrossRef] [Green Version]
- Lai, S.Y.; Grandis, J.R. Understanding the presence and function of erythropoietin receptors on cancer cells. J. Clin. Oncol. 2006, 24, 4675–4676. [Google Scholar] [CrossRef]
- Acs, G.; Acs, P.; Beckwith, S.M.; Pitts, R.L.; Clements, E.; Wong, K.; Verma, A. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001, 61, 3561–3565. [Google Scholar] [PubMed]
- Yasuda, Y.; Fujita, Y.; Masuda, S.; Musha, T.; Ueda, K.; Tanaka, H.; Fujita, H.; Matsuo, T.; Nagao, M.; Sasaki, R.; et al. Erythropoietin is involved in growth and angiogenesis in malignant tumours of female reproductive organs. Carcinogenesis 2002, 23, 1797–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Eijk, L.T.; Kroot, J.C.; Tromp, M.; van der Hoeven, J.G.; Swinkels, D.W.; Pickkers, P. Inflammation—Induced hepcidin-25 is associated with the development of anemia in septic patients: An observational study. Crit. Care 2011, 15, R9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Mast, Q.; Syafruddin, D.; Keijmel, S.; Riekerink, T.O.; Deky, O.; Asih, P.B.; Swinkels, D.W.; van der Ven, A.J. Increased serum hepcidin and alterations in blood iron parameters associated with asymptomatic P. falciparum and P. vivax malaria. Haematologica 2010, 95, 1068–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.; Reyes, E.; Ofman, J. Prevalence and outcomes of anemia in inflammatory bowel disease: A systemic review of the literature. J. Med. 2004, 116, 44S–49S. [Google Scholar] [CrossRef]
- Müller, H.M.; Horina, J.H.; Kniepeiss, D.; Tripolt, M.B.; Stadelbauer, V.; Schweiger, M.; Tscheliessnigg, K.H. Characteristics and clinical relevance of chronic anemia in adult heart transplant recipient. Clin. Transpl. 2001, 15, 343–348. [Google Scholar] [CrossRef]
- Cullis, J.O. Diagnosis and management of anemia of chronic disease: Current status. Br. J. Haematol. 2011, 154, 289–300. [Google Scholar] [CrossRef]
- Madu, A.J.; Ughasoro, M.D. Anaemia of Chronic Disease: An In-Depth Review. Med. Princ. Pract. 2017, 26, 1–9. [Google Scholar] [CrossRef]
- Jander, A.; Wierciński, R.; Bałasz-Chmielewska, I.; Miklaszewska, M.; Zachwieja, K.; Borzecka, H.; Zachwieja, J.; Olszak-Szot, I.; Kubicki, D.; Ziółkowska, H.; et al. Anaemia treatment in chronically dialysed children: A multicentre nationwide observational study. Scand. J. Urol. Nephrol. 2012, 46, 375–380. [Google Scholar] [CrossRef]
- Winczura, P.; Jassem, J. Recombinant human erythropoietin in treatment of the cancer patients with anemia: Hopes and threats. Oncol. Clin. Pract. 2007, 3, 198–204. [Google Scholar]
- Davis, S.L.; Littlewood, T.J. The investigation and treatment of secondary anaemia. Blood Rev. 2012, 26, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.D.; Holdford, D.A.; Brophy, D.F.; Harpe, S.E.; Mays, D.; Gehr, T.W. Utilization Patterns of IV Iron and Erythropoiesis Stimulating Agents in Anemic Chronic Kidney Disease Patients: A Multihospital Study. Anaemia 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Styszyński, A. Anemia of inflammation in older persons. Part II. Impact of age-dependent diseases, diagnostic and therapeutic options. Geriatria 2013, 7, 1–8. [Google Scholar]
- Hashizume, M.; Uchiyama, Y.; Horai, N.; Tomosugi, N.; Mihara, M. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, improved anemia in monkey arthritis by suppressing IL-6-induced hepcidin production. Rheumatol. Int. 2010, 30, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Fujii, T.; Hamaguchi, M.; Furu, M.; Ito, H.; Terao, C.; Yamamoto, K.; Yamamoto, W.; Matsuo, T.; Mori, M.; et al. Increase of hemoglobin levels by anti-IL-6 receptor antibody (tocilizumab) in rheumatoid arthritis. PLoS ONE 2014, 9, e98202. [Google Scholar] [CrossRef] [PubMed]
- Sasu, B.J.; Cooke, K.S.; Arvedson, T.L.; Plewa, C.; Ellison, A.R.; Sheng, J.; Winters, A.; Juan, T.; Li, H.; Begley, C.G.; et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation- induced anaemia. Blood 2010, 115, 3616–3624. [Google Scholar] [CrossRef]
- Zaritsky, J.; Young, B.; Gales, B.; Wang, H.J.; Rastogi, A.; Westerman, M.; Nemeth, E.; Ganz, T.; Salusky, I.B. Reduction of serum hepcidin by hemodialysis in pediatric and adult patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 1010–1014. [Google Scholar] [CrossRef]
- Egrie, J.C.; Strickland, T.W.; Lane, J.; Aoki, K.; Cohen, A.M.; Smalling, R.; Trail, G.; Lin, F.K.; Browne, J.K.; Hines, D.K. Characterization and biological effects of recombinant human erythropoietin. Immunobiology 1986, 172, 213–224. [Google Scholar] [CrossRef]
- Egrie, J.C.; Browne, J.K. Development ad characterization of darbepoetin alfa. Oncology 2002, 16, 13–22. [Google Scholar]
- Kapka-Skrzypczak, L.; Niedźwiecka, J.; Skrzypczak, M.; Wojtyła, A. Folic acid—effects of deficiency and justification for supplementation. Med. Ogólna i Nauki o Zdrowiu. 2012, 18, 65–69. [Google Scholar]
- Kośmider, A.; Czaczyk, K. Witamina B12—budowa, biosynteza, funkcje i metody oznaczania. Żyw. N. Tech. Jak. 2010, 5, 17–32. [Google Scholar]
- Charytan, C.; Quinibi, W.; Bailie, G.R. Comparison of intravenous iron sucrose to oral iron in the treatment of anemic patients with chronic kidney disease not on dialysis. Nephron. Clin. Pract. 2005, 100, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Salovaara, S.; Sandberg, A.-S.; Andlid, T. Organic acids influence iron uptake in the human epithelial cell line caco-2. J. Agric. Food Chem. 2002, 50, 6233–6238. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Raffatellu, M.; George, M.D.; Akiyama, Y.; Hornsby, M.J.; Nuccio, S.; Paixao, T.A.; Butler, B.P.; Chu, H.; Santos, R.L.; Berger, T.; et al. Lipocalin-2 Resistance Confers an Advantage to Salmonella Enterica Serotype Typhimurium for Growth and Survival in the Inflamed Intestine. Cell Host Microbe 2009, 5, 476–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constante, M.; Fragoso, G.; Lupien-Meilleur, J.; Calvé, A.; Santos, M.M. Iron Supplements Modulate Colon Microbiota Composition and Potentiate the Protective Effects of Probiotics in Dextran Sodium Sulfate-induced Colitis. Inflamm. Bowel Dis. 2017, 23, 753–766. [Google Scholar] [CrossRef]
- Chieppa, M.; Giannelli, G. Immune Cells and Microbiota Response to Iron Starvation. Front Med. (Lausanne) 2018, 5, 109. [Google Scholar] [CrossRef] [Green Version]
- Myśliwiec, M. Oral iron supplementation in renal anemia. For. Nefrol. 2012, 5, 195–203. [Google Scholar]
- Quinibi, W.Y.; Martinez, C.; Smith, M.; Benjamin, J.; Mangione, A.; Roger, S.D. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol. Dial. Transplant. 2011, 26, 1599–1607. [Google Scholar] [CrossRef] [Green Version]
- Macdougall, I.C. Iron supplementation in the non-dialysis chronic kidney disease (ND-CKD) patient: Oral or intravenous? Curr. Med. Res. Opin. 2010, 26, 473–482. [Google Scholar] [CrossRef]
- Macdougall, I.C. Iron supplementation in nephrology and oncology: What do we have in common? Oncologist 2011, 16, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D. Folic acid fortification: The good, the bad, and the puzzle of vitamin B-12. Am. J. Clin. Nutr. 2007, 85, 3–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poli, M.; Girelli, D.; Campostrini, N.; Maccarinelli, F.; Finazzi, D.; Luscieti, S.; Nai, A.; Arosio, P. Heparin: A potent inhibitor of hepcidin expression in vitro and in vivo. Blood 2011, 117, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Andriopoulos, B., Jr.; Corradini, E.; Xia, Y.; Faasse, S.A.; Chen, S.; Grgurevic, L.; Knutson, M.D.; Pietrangelo, A.; Vukicevic, S.; Lin, H.Y.; et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat. Gen. 2009, 41, 482–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, M.K.; Rahman, M.U.; Frederick, B.; Birbara, C.A.; de Vries, D.; Toedter, G.; Wu, X.; Chen, D.; Ranganath, V.K.; Westerman, M.E.; et al. Effect of subcutaneous and intravenous golimumab on inflammatory biomarkers in patients with rheumatoid arthritis: Results of a phase 1, randomized, open-label trial. Rheumatology 2013, 52, 1214–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayliss, T.J.; Smith, J.T.; Schuster, M.; Dragnev, K.H.; Rigas, J.R. A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Exp. Opin. Biol. Ther. 2011, 11, 1663–1668. [Google Scholar] [CrossRef]
- Fung, E.; Sugianto, P.; Hsu, J.; Damoiseaux, R.; Ganz, T.; Nemeth, E. High-throughput screening of small molecules identifies hepcidin antagonists. Mol. Pharmacol. 2013, 83, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratz, F.; Beyer, U.; Roth, T.; Tarasova, N.; Collery, P.; Lechenault, F.; Cazabat, A.; Schumacher, P.; Unger, C.; Falken, U. Transferrin conjugates of doxorubicin: Synthesis, characterization, cellular uptake, and in vitro efficacy. J. Pharm. Sci. 1998, 87, 338–346. [Google Scholar] [CrossRef]
- Xu, X.; Persson, H.L.; Richardson, D.R. Molecular pharmacology of the interaction of anthracyclines with iron. Mol. Pharmacol. 2005, 68, 261–271. [Google Scholar] [CrossRef]
- Joharapurkar, A.A.; Pandya, V.B.; Patel, V.J.; Ranjit, C.; Desai, M.R.J. Prolyl Hydroxylase Inhibitors: A Breakthrough in the Therapy of Anemia Associated with Chronic Diseases. J. Med. Chem. 2018, 61, 6964–6982. [Google Scholar] [CrossRef]

| Feature | Iron Deficiency Anemia | Anemia of Chronic Disease |
|---|---|---|
| the presence of other chronic diseases | rarely | often |
| average baseline hemoglobinconcentration | ≥9 g/dL | ≤9 g/dL |
| serum iron concentration | decreased | significantly decreased |
| MCV/MCH | decreased | normal |
| serum ferritin concentration | low | increased |
| serum hepcidin concentration | low | high |
| percentage of reticulocytes in the blood serum | high | low |
| serum folic acid concentration | normal | decreased |
| serum vitamin B12 concentration | normal | decreased |
| serum creatinine concentration | normal | increased |
| serum erythropoietin concentration | increased | decreased |
| Type of Hematopoietic Factor | Route of Administration | Advantages | Disadvantages |
|---|---|---|---|
| iron | oral | high safety of use, absence of non-transferrin bound iron (NTBI) in the blood. | limited effectiveness, poor absorption, interaction with other drugs, nausea, vomiting, constipation, diarrhea, itching, rash, erythema |
| intramuscular | quick correction of deficiency, less frequent dosing, longer effect, less frequent gastrointestinal ailments, an alternative for swallowing disorders | the need for hospital administration, dysgeusia, headache and dizziness, palpitations, shortness of breath, bleeding, abscess, skin necrosis at the injection site | |
| intravenous | quick correction of deficiency, less frequent dosing, longer effect, less frequent gastrointestinal ailments, an alternative for swallowing disorders | the need for hospital administration, possible anaphylactic reaction, possible development of infection or exacerbation of sepsis, risk of iron overload, a sharp increase or decrease in blood pressure | |
| vitamin B12 | oral | good tolerability, low risk of overdose, rather as maintenance treatment | poorly absorbed from the gastrointestinal tract (1% of the dose), difficulties in compensating for deficiency |
| intramuscular | the method of choice in supplementing the large deficiency, longer effect, less frequent dosing | pain at the injection site, rarely anaphylactic shock and death, and hypersensitivity reactions, pruritus, rash, transient diarrhea | |
| folic acid | oral | the method of choice, good tolerance, well absorbed from the gastrointestinal tract, low risk of overdose | allergic skin reactions, gastrointestinal disorders, nausea, vomiting, sleep disturbance, depression or agitation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiciński, M.; Liczner, G.; Cadelski, K.; Kołnierzak, T.; Nowaczewska, M.; Malinowski, B. Anemia of Chronic Diseases: Wider Diagnostics—Better Treatment? Nutrients 2020, 12, 1784. https://doi.org/10.3390/nu12061784
Wiciński M, Liczner G, Cadelski K, Kołnierzak T, Nowaczewska M, Malinowski B. Anemia of Chronic Diseases: Wider Diagnostics—Better Treatment? Nutrients. 2020; 12(6):1784. https://doi.org/10.3390/nu12061784
Chicago/Turabian StyleWiciński, Michał, Grzegorz Liczner, Karol Cadelski, Tadeusz Kołnierzak, Magdalena Nowaczewska, and Bartosz Malinowski. 2020. "Anemia of Chronic Diseases: Wider Diagnostics—Better Treatment?" Nutrients 12, no. 6: 1784. https://doi.org/10.3390/nu12061784
APA StyleWiciński, M., Liczner, G., Cadelski, K., Kołnierzak, T., Nowaczewska, M., & Malinowski, B. (2020). Anemia of Chronic Diseases: Wider Diagnostics—Better Treatment? Nutrients, 12(6), 1784. https://doi.org/10.3390/nu12061784






