Vitamin D-Induced Molecular Mechanisms to Potentiate Cancer Therapy and to Reverse Drug-Resistance in Cancer Cells
Abstract
:1. Introduction
2. Search Strategy
3. Vitamin D: Synthesis and Metabolism
4. Vitamin D Mechanisms of Action
5. Vitamin D and Cancer: Epidemiological Evidence
6. Vitamin D and Cancer: Molecular Mechanisms in In Vivo and In Vitro Studies
6.1. Animal Studies
6.2. In Vitro Studies
6.3. Vitamin D and Cancer: A Focus on Cancer Stem Cells and miRNA In Vitro Studies
7. Vitamin D and Cancer: Molecular Mechanisms of Cancer Drug Resistance
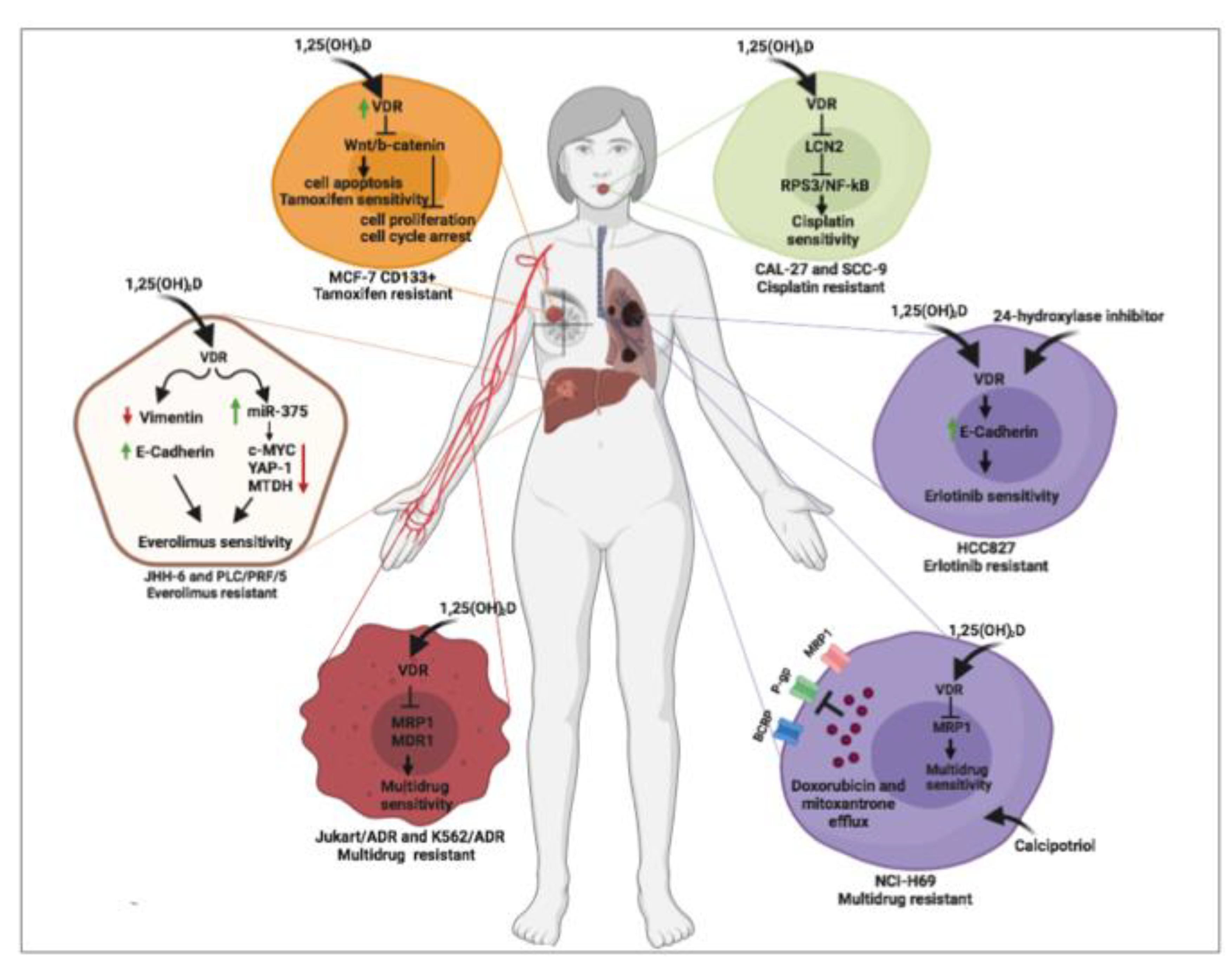
8. Vitamin D and Cancer: Reversal Effects of Vitamin D on Cancer Drug Resistance
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goltzman, D. Functions of vitamin d in bone. Histochem. Cell Biol. 2018, 149, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin d metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Hii, C.S.; Ferrante, A. The non-genomic actions of vitamin d. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and extraskeletal actions of vitamin d: Current evidence and outstanding questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [PubMed] [Green Version]
- Giovannucci, E. Vitamin d and cancer incidence in the harvard cohorts. Ann. Epidemiol. 2009, 19, 84–88. [Google Scholar] [CrossRef]
- Yuan, C.; Sato, K.; Hollis, B.W.; Zhang, S.; Niedzwiecki, D.; Ou, F.S.; Chang, I.W.; O’Neil, B.H.; Innocenti, F.; Lenz, H.J.; et al. Plasma 25-hydroxyvitamin d levels and survival in patients with advanced or metastatic colorectal cancer: Findings from calgb/swog 80405 (alliance). Clin. Cancer Res. 2019, 25, 7497–7505. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Zhang, X.; Chen, Y.; Huo, X.; Deng, S.; Yang, X.; Luo, Y.; Luo, Y.; Lu, X.; Zhang, M.; et al. Alteration of serum 25(oh) vitamin d, vitamin d binding protein, and c-reactive protein levels in acute leukemia patients. Clin. Lab. 2018, 64, 1553–1559. [Google Scholar] [CrossRef]
- Thomas, X.; Chelghoum, Y.; Fanari, N.; Cannas, G. Serum 25-hydroxyvitamin d levels are associated with prognosis in hematological malignancies. Hematology 2011, 16, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yu, W.D.; Trump, D.L.; Johnson, C.S. 1,25d3 enhances antitumor activity of gemcitabine and cisplatin in human bladder cancer models. Cancer 2010, 116, 3294–3303. [Google Scholar] [CrossRef] [Green Version]
- Yonaga, H.; Okada, S.; Akutsu, T.; Ohdaira, H.; Suzuki, Y.; Urashima, M. Effect modification of vitamin d supplementation by histopathological characteristics on survival of patients with digestive tract cancer: Post hoc analysis of the amaterasu randomized clinical trial. Nutrients 2019, 11, 2547. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Schwartz, Z.; Boyan, B.D. 24r,25-dihydroxyvitamin d3 modulates tumorigenicity in breast cancer in an estrogen receptor-dependent manner. Steroids 2019, 150, 108447. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Nimeiri, H.S.; McCleary, N.J.; Abrams, T.A.; Yurgelun, M.B.; Cleary, J.M.; Rubinson, D.A.; Schrag, D.; Miksad, R.; Bullock, A.J.; et al. Effect of high-dose vs standard-dose vitamin d3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: The sunshine randomized clinical trial. JAMA 2019, 321, 1370–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urashima, M.; Ohdaira, H.; Akutsu, T.; Okada, S.; Yoshida, M.; Kitajima, M.; Suzuki, Y. Effect of vitamin d supplementation on relapse-free survival among patients with digestive tract cancers: The amaterasu randomized clinical trial. JAMA 2019, 321, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Akiba, T.; Morikawa, T.; Odaka, M.; Nakada, T.; Kamiya, N.; Yamashita, M.; Yabe, M.; Inagaki, T.; Asano, H.; Mori, S.; et al. Vitamin d supplementation and survival of patients with non-small cell lung cancer: A randomized, double-blind, placebo-controlled trial. Clin. Cancer Res. 2018, 24, 4089–4097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleet, J.C.; DeSmet, M.; Johnson, R.; Li, Y. Vitamin d and cancer: A review of molecular mechanisms. Biochem. J. 2012, 441, 61–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergeev, I.N. Vitamin d and cellular Ca2+ signaling in breast cancer. Anticancer Res. 2012, 32, 299–302. [Google Scholar]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of vitamin d beyond the skeletal function: A review of the molecular and clinical studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef] [Green Version]
- Min, D.; Lv, X.B.; Wang, X.; Zhang, B.; Meng, W.; Yu, F.; Hu, H. Downregulation of mir-302c and mir-520c by 1,25(oh)2d3 treatment enhances the susceptibility of tumour cells to natural killer cell-mediated cytotoxicity. Br. J. Cancer 2013, 109, 723–730. [Google Scholar] [CrossRef] [Green Version]
- So, J.Y.; Suh, N. Targeting cancer stem cells in solid tumors by vitamin d. J. Steroid Biochem. Mol. Biol. 2015, 148, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Zeichner, S.B.; Koru-Sengul, T.; Shah, N.; Liu, Q.; Markward, N.J.; Montero, A.J.; Gluck, S.; Silva, O.; Ahn, E.R. Improved clinical outcomes associated with vitamin d supplementation during adjuvant chemotherapy in patients with her2+ nonmetastatic breast cancer. Clin. Breast Cancer 2015, 15, e1–e11. [Google Scholar] [CrossRef]
- Wietrzyk, J.; Nevozhay, D.; Filip, B.; Milczarek, M.; Kutner, A. The antitumor effect of lowered doses of cytostatics combined with new analogs of vitamin d in mice. Anticancer Res. 2007, 27, 3387–3398. [Google Scholar] [PubMed]
- Podgorska, E.; Drzal, A.; Matuszak, Z.; Swakon, J.; Slominski, A.; Elas, M.; Urbanska, K. Calcitriol and calcidiol can sensitize melanoma cells to low(-)let proton beam irradiation. Int. J. Mol. Sci. 2018, 19, 2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Zhang, Y.; Li, H.; Zhou, Y.; Zhang, Q.; Chen, R.; Jin, T.; Hu, K.; Li, S.; Wang, Y.; et al. Vitamin d promotes the cisplatin sensitivity of oral squamous cell carcinoma by inhibiting lcn2-modulated nf-kappab pathway activation through rps3. Cell Death Dis. 2019, 10, 936. [Google Scholar] [CrossRef]
- Zheng, W.; Duan, B.; Zhang, Q.; Ouyang, L.; Peng, W.; Qian, F.; Wang, Y.; Huang, S. Vitamin d-induced vitamin d receptor expression induces tamoxifen sensitivity in mcf-7 stem cells via suppression of wnt/beta-catenin signaling. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Provvisiero, D.P.; Negri, M.; de Angelis, C.; Di Gennaro, G.; Patalano, R.; Simeoli, C.; Papa, F.; Ferrigno, R.; Auriemma, R.S.; De Martino, M.C.; et al. Vitamin d reverts resistance to the mtor inhibitor everolimus in hepatocellular carcinoma through the activation of a mir-375/oncogenes circuit. Sci. Rep. 2019, 9, 11695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.; Nuriding, H. Reversal effect of vitamin d on different multidrug-resistant cells. Genet. Mol. Res. 2014, 13, 6239–6247. [Google Scholar] [CrossRef]
- Tan, K.W.; Sampson, A.; Osa-Andrews, B.; Iram, S.H. Calcitriol and calcipotriol modulate transport activity of abc transporters and exhibit selective cytotoxicity in mrp1-overexpressing cells. Drug Metab. Dispos. Biol. Fate Chem. 2018, 46, 1856–1866. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Shaurova, T.; Shoemaker, S.; Petkovich, M.; Hershberger, P.A.; Wu, Y. Tumor-targeted nanoparticles deliver a vitamin d-based drug payload for the treatment of egfr tyrosine kinase inhibitor-resistant lung cancer. Mol. Pharm. 2018, 15, 3216–3226. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Kostenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin d deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 1–16. [Google Scholar] [CrossRef]
- Van Schoor, N.M.; Lips, P. Worldwide vitamin d status. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 671–680. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and vitamin d: A global perspective for health. Derm.-Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.G.; Ochalek, J.T.; Kaufmann, M.; Jones, G.; Deluca, H.F. Cyp2r1 is a major, but not exclusive, contributor to 25-hydroxyvitamin d production in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 15650–15655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usui, E.; Noshiro, M.; Okuda, K. Molecular cloning of cdna for vitamin d3 25-hydroxylase from rat liver mitochondria. FEBS Lett. 1990, 262, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Cali, J.J.; Russell, D.W. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome p-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J. Biol. Chem. 1991, 266, 7774–7778. [Google Scholar]
- Ichikawa, F.; Sato, K.; Nanjo, M.; Nishii, Y.; Shinki, T.; Takahashi, N.; Suda, T. Mouse primary osteoblasts express vitamin d3 25-hydroxylase mrna and convert 1 alpha-hydroxyvitamin d3 into 1 alpha,25-dihydroxyvitamin d3. Bone 1995, 16, 129–135. [Google Scholar] [CrossRef]
- Andersson, S.; Davis, D.L.; Dahlback, H.; Jornvall, H.; Russell, D.W. Cloning, structure, and expression of the mitochondrial cytochrome p-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J. Biol. Chem. 1989, 264, 8222–8229. [Google Scholar]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar]
- Fu, G.K.; Lin, D.; Zhang, M.Y.; Bikle, D.D.; Shackleton, C.H.; Miller, W.L.; Portale, A.A. Cloning of human 25-hydroxyvitamin d-1 alpha-hydroxylase and mutations causing vitamin d-dependent rickets type 1. Mol. Endocrinol. 1997, 11, 1961–1970. [Google Scholar]
- Stoffels, K.; Overbergh, L.; Giulietti, A.; Verlinden, L.; Bouillon, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin-d3-1alpha-hydroxylase in human monocytes. J. Bone Miner. Res. 2006, 21, 37–47. [Google Scholar] [CrossRef]
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-hydroxyvitamin d-24-hydroxylase (cyp24a1): Its important role in the degradation of vitamin d. Arch. Biochem. Biophys. 2012, 523, 9–18. [Google Scholar] [CrossRef]
- Kumar, R.; Thompson, J.R. The regulation of parathyroid hormone secretion and synthesis. J. Am. Soc. Nephrol. 2011, 22, 216–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tebben, P.J.; Singh, R.J.; Kumar, R. Vitamin d-mediated hypercalcemia: Mechanisms, diagnosis, and treatment. Endocr. Rev. 2016, 37, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Horvath, H.C.; Lakatos, P.; Kosa, J.P.; Bacsi, K.; Borka, K.; Bises, G.; Nittke, T.; Hershberger, P.A.; Speer, G.; Kallay, E. The candidate oncogene cyp24a1: A potential biomarker for colorectal tumorigenesis. J. Histochem. Cytochem. 2010, 58, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Tannour-Louet, M.; Lewis, S.K.; Louet, J.F.; Stewart, J.; Addai, J.B.; Sahin, A.; Vangapandu, H.V.; Lewis, A.L.; Dittmar, K.; Pautler, R.G.; et al. Increased expression of cyp24a1 correlates with advanced stages of prostate cancer and can cause resistance to vitamin d3-based therapies. FASEB J. 2014, 28, 364–372. [Google Scholar] [CrossRef]
- Osanai, M.; Lee, G.H. Cyp24a1-induced vitamin d insufficiency promotes breast cancer growth. Oncol. Rep. 2016, 36, 2755–2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin d signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Haussler, C.A.; Hsieh, J.C.; Thompson, P.D.; Selznick, S.H.; Dominguez, C.E.; Jurutka, P.W. The nuclear vitamin d receptor: Biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998, 13, 325–349. [Google Scholar] [CrossRef]
- de Angelis, C.; Galdiero, M.; Pivonello, C.; Garifalos, F.; Menafra, D.; Cariati, F.; Salzano, C.; Galdiero, G.; Piscopo, M.; Vece, A.; et al. The role of vitamin d in male fertility: A focus on the testis. Rev. Endocr. Metab. Disord. 2017, 18, 285–305. [Google Scholar] [CrossRef]
- Dawson, M.I.; Xia, Z. The retinoid x receptors and their ligands. Biochim. Biophys. Acta 2012, 1821, 21–56. [Google Scholar] [CrossRef] [Green Version]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin d: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Shaffer, P.L.; Gewirth, D.T. Structural basis of vdr-DNA interactions on direct repeat response elements. EMBO J. 2002, 21, 2242–2252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitrakopoulou, V.I.; Tsilidis, K.K.; Haycock, P.C.; Dimou, N.L.; Al-Dabhani, K.; Martin, R.M.; Lewis, S.J.; Gunter, M.J.; Mondul, A.; Shui, I.M.; et al. Circulating vitamin d concentration and risk of seven cancers: Mendelian randomisation study. BMJ 2017, 359, j4761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capiati, D.A.; Vazquez, G.; Tellez Inon, M.T.; Boland, R.L. Role of protein kinase c in 1,25(oh)(2)-vitamin d(3) modulation of intracellular calcium during development of skeletal muscle cells in culture. J. Cell. Biochem. 2000, 77, 200–212. [Google Scholar] [CrossRef]
- Zheng, Y.; Trivedi, T.; Lin, R.C.; Fong-Yee, C.; Nolte, R.; Manibo, J.; Chen, Y.; Hossain, M.; Horas, K.; Dunstan, C.; et al. Loss of the vitamin d receptor in human breast and prostate cancers strongly induces cell apoptosis through downregulation of wnt/beta-catenin signaling. Bone Res. 2017, 5, 17023. [Google Scholar] [CrossRef]
- Anderson, M.G.; Nakane, M.; Ruan, X.; Kroeger, P.E.; Wu-Wong, J.R. Expression of vdr and cyp24a1 mrna in human tumors. Cancer Chemother. Pharmacol. 2006, 57, 234–240. [Google Scholar] [CrossRef]
- Lee, J.E.; Li, H.; Chan, A.T.; Hollis, B.W.; Lee, I.M.; Stampfer, M.J.; Wu, K.; Giovannucci, E.; Ma, J. Circulating levels of vitamin d and colon and rectal cancer: The physicians’ health study and a meta-analysis of prospective studies. Cancer Prev. Res. 2011, 4, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.Y.; Yao, Q.; Zhuo, Z.; Ma, Z.; Chen, G. Circulating vitamin d level and mortality in prostate cancer patients: A dose-response meta-analysis. Endocr. Connect. 2018, 7, R294–R303. [Google Scholar] [CrossRef] [Green Version]
- Neuhouser, M.L.; Manson, J.E.; Millen, A.; Pettinger, M.; Margolis, K.; Jacobs, E.T.; Shikany, J.M.; Vitolins, M.; Adams-Campbell, L.; Liu, S.; et al. The influence of health and lifestyle characteristics on the relation of serum 25-hydroxyvitamin d with risk of colorectal and breast cancer in postmenopausal women. Am. J. Epidemiol. 2012, 175, 673–684. [Google Scholar] [CrossRef]
- Deschasaux, M.; Souberbielle, J.C.; Latino-Martel, P.; Sutton, A.; Charnaux, N.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; Le Clerc, S.; Kesse-Guyot, E.; et al. A prospective study of plasma 25-hydroxyvitamin d concentration and prostate cancer risk. Br. J. Nutr. 2016, 115, 305–314. [Google Scholar] [CrossRef]
- Markotic, A.; Langer, S.; Kelava, T.; Vucic, K.; Turcic, P.; Tokic, T.; Stefancic, L.; Radetic, E.; Farrington, S.; Timofeeva, M.; et al. Higher post-operative serum vitamin d level is associated with better survival outcome in colorectal cancer patients. Nutr. Cancer 2019, 71, 1078–1085. [Google Scholar] [CrossRef]
- Mezawa, H.; Sugiura, T.; Watanabe, M.; Norizoe, C.; Takahashi, D.; Shimojima, A.; Tamez, S.; Tsutsumi, Y.; Yanaga, K.; Urashima, M. Serum vitamin d levels and survival of patients with colorectal cancer: Post-hoc analysis of a prospective cohort study. BMC Cancer 2010, 10, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandstedt, J.; Almquist, M.; Ulmert, D.; Manjer, J.; Malm, J. Vitamin d, pth, and calcium and tumor aggressiveness in prostate cancer: A prospective nested case-control study. Cancer Causes Control. 2016, 27, 69–80. [Google Scholar] [CrossRef]
- Gilbert, R.; Metcalfe, C.; Fraser, W.D.; Lewis, S.; Donovan, J.; Hamdy, F.; Neal, D.E.; Lane, J.A.; Martin, R.M.; Tilling, K. Associations of circulating 25-hydroxyvitamin d, 1,25-dihydroxyvitamin d, and vitamin d pathway genes with prostate-specific antigen progression in men with localized prostate cancer undergoing active monitoring. Eur. J. Cancer Prev. 2013, 22, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Thanasitthichai, S.; Prasitthipayong, A.; Boonmark, K.; Purisa, W.; Guayraksa, K. Negative impact of 25-hydroxyvitamin d deficiency on breast cancer survival. Asian Pac. J. Cancer Prev. 2019, 20, 3101–3106. [Google Scholar] [CrossRef] [PubMed]
- Villasenor, A.; Ballard-Barbash, R.; Ambs, A.; Bernstein, L.; Baumgartner, K.; Baumgartner, R.; Ulrich, C.M.; Hollis, B.W.; McTiernan, A.; Neuhouser, M.L. Associations of serum 25-hydroxyvitamin d with overall and breast cancer-specific mortality in a multiethnic cohort of breast cancer survivors. Cancer Causes Control. 2013, 24, 759–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesa, K.M.; Segal, N.H.; Cronin, A.M.; Sjoberg, D.D.; Jacobs, G.N.; Coleton, M.I.; Fleisher, M.; Dnistrian, A.M.; Saltz, L.B.; Cassileth, B.R. Serum 25-hydroxy vitamin d and survival in advanced colorectal cancer: A retrospective analysis. Nutr. Cancer 2015, 67, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Skender, S.; Bohm, J.; Schrotz-King, P.; Chang-Claude, J.; Siegel, E.M.; Steindorf, K.; Owen, R.W.; Ose, J.; Hoffmeister, M.; Brenner, H.; et al. Plasma 25-hydroxyvitamin d3 levels in colorectal cancer patients and associations with physical activity. Nutr. Cancer 2017, 69, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Fakih, M.G.; Andrews, C.; McMahon, J.; Muindi, J.R. A prospective clinical trial of cholecalciferol 2000 iu/day in colorectal cancer patients: Evidence of a chemotherapy-response interaction. Anticancer Res. 2012, 32, 1333–1338. [Google Scholar]
- Wu, K.; Feskanich, D.; Fuchs, C.S.; Chan, A.T.; Willett, W.C.; Hollis, B.W.; Pollak, M.N.; Giovannucci, E. Interactions between plasma levels of 25-hydroxyvitamin d, insulin-like growth factor (igf)-1 and c-peptide with risk of colorectal cancer. PLoS ONE 2011, 6, e28520. [Google Scholar] [CrossRef]
- Zhang, L.; Zou, H.; Zhao, Y.; Hu, C.; Atanda, A.; Qin, X.; Jia, P.; Jiang, Y.; Qi, Z. Association between blood circulating vitamin d and colorectal cancer risk in asian countries: A systematic review and dose-response meta-analysis. BMJ Open 2019, 9, e030513. [Google Scholar] [CrossRef] [Green Version]
- Fedirko, V.; Mandle, H.B.; Zhu, W.; Hughes, D.J.; Siddiq, A.; Ferrari, P.; Romieu, I.; Riboli, E.; Bueno-de-Mesquita, B.; van Duijnhoven, F.J.B.; et al. Vitamin d-related genes, blood vitamin d levels and colorectal cancer risk in western european populations. Nutrients 2019, 11, 1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vayrynen, J.P.; Mutt, S.J.; Herzig, K.H.; Vayrynen, S.A.; Kantola, T.; Karhu, T.; Karttunen, T.J.; Klintrup, K.; Makela, J.; Makinen, M.J.; et al. Decreased preoperative serum 25-hydroxyvitamin d levels in colorectal cancer are associated with systemic inflammation and serrated morphology. Sci. Rep. 2016, 6, 36519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, A.; Nettey, O.S.; Gogana, P.; Sheikh, U.; Macias, V.; Kajdacsy-Balla, A.; Sharifi, R.; Kittles, R.A.; Murphy, A.B. Physiologic serum 1,25 dihydroxyvitamin d is inversely associated with prostatic ki67 staining in a diverse sample of radical prostatectomy patients. Cancer Causes Control. 2019, 30, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Sawada, N.; Inoue, M.; Iwasaki, M.; Yamaji, T.; Shimazu, T.; Sasazuki, S.; Tsugane, S. Plasma 25-hydroxy vitamin d and subsequent prostate cancer risk in a nested case-control study in japan: The jphc study. Eur. J. Clin. Nutr. 2017, 71, 132–136. [Google Scholar] [CrossRef]
- Yaturu, S.; Zdunek, S.; Youngberg, B. Vitamin d levels in subjects with prostate cancer compared to age-matched controls. Prostate Cancer 2012, 2012, 524206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, S.K.; Kolb, S.; Fu, R.; Horst, R.; Feng, Z.; Stanford, J.L. Circulating levels of 25-hydroxyvitamin d and prostate cancer prognosis. Cancer Epidemiol. 2013, 37, 666–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, R.; Metcalfe, C.; Fraser, W.D.; Donovan, J.; Hamdy, F.; Neal, D.E.; Lane, J.A.; Martin, R.M. Associations of circulating retinol, vitamin e, and 1,25-dihydroxyvitamin d with prostate cancer diagnosis, stage, and grade. Cancer Causes Control. 2012, 23, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Trukova, K.; Popiel, B.; Lammersfeld, C.; Vashi, P.G. The association between pre-treatment serum 25-hydroxyvitamin d and survival in newly diagnosed stage iv prostate cancer. PLoS ONE 2015, 10, e0119690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramakrishnan, S.; Steck, S.E.; Arab, L.; Zhang, H.; Bensen, J.T.; Fontham, E.T.H.; Johnson, C.S.; Mohler, J.L.; Smith, G.J.; Su, L.J.; et al. Association among plasma 1,25(oh)2 d, ratio of 1,25(oh)2 d to 25(oh)d, and prostate cancer aggressiveness. Prostate 2019, 79, 1117–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Hu, P.; Xie, D.; Qin, Y.; Wang, F.; Wang, H. Meta-analysis of vitamin d, calcium and the prevention of breast cancer. Breast Cancer Res. Treat. 2010, 121, 469–477. [Google Scholar] [CrossRef]
- Mohr, S.B.; Gorham, E.D.; Alcaraz, J.E.; Kane, C.J.; Macera, C.A.; Parsons, J.K.; Wingard, D.L.; Garland, C.F. Serum 25-hydroxyvitamin d and prevention of breast cancer: Pooled analysis. Anticancer Res. 2011, 31, 2939–2948. [Google Scholar] [PubMed]
- Estebanez, N.; Gomez-Acebo, I.; Palazuelos, C.; Llorca, J.; Dierssen-Sotos, T. Vitamin d exposure and risk of breast cancer: A meta-analysis. Sci. Rep. 2018, 8, 9039. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Lappe, J.M.; Heaney, R.P. Serum 25-hydroxyvitamin d concentrations >/=40 ng/ml are associated with >65% lower cancer risk: Pooled analysis of randomized trial and prospective cohort study. PLoS ONE 2016, 11, e0152441. [Google Scholar] [CrossRef] [Green Version]
- Ordonez-Mena, J.M.; Schottker, B.; Fedirko, V.; Jenab, M.; Olsen, A.; Halkjaer, J.; Kampman, E.; de Groot, L.; Jansen, E.; Bueno-de-Mesquita, H.B.; et al. Pre-diagnostic vitamin d concentrations and cancer risks in older individuals: An analysis of cohorts participating in the chances consortium. Eur. J. Epidemiol. 2016, 31, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.R.M.; de Sousa Almeida-Filho, B.; De Luca Vespoli, H.; Schmitt, E.B.; Nahas-Neto, J.; Nahas, E.A.P. Low pretreatment serum concentration of vitamin d at breast cancer diagnosis in postmenopausal women. Menopause 2019, 26, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.R.; Hankinson, S.E.; Bertone-Johnson, E.R.; Ding, E.L. Plasma vitamin d levels, menopause, and risk of breast cancer: Dose-response meta-analysis of prospective studies. Medicine 2013, 92, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Je, Y. Vitamin d intake, blood 25(oh)d levels, and breast cancer risk or mortality: A meta-analysis. Br. J. Cancer 2014, 110, 2772–2784. [Google Scholar] [CrossRef] [Green Version]
- Gandini, S.; Boniol, M.; Haukka, J.; Byrnes, G.; Cox, B.; Sneyd, M.J.; Mullie, P.; Autier, P. Meta-analysis of observational studies of serum 25-hydroxyvitamin d levels and colorectal, breast and prostate cancer and colorectal adenoma. Int. J. Cancer 2011, 128, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Kwan, M.L.; Ergas, I.J.; Roh, J.M.; Cheng, T.D.; Hong, C.C.; McCann, S.E.; Tang, L.; Davis, W.; Liu, S.; et al. Association of serum level of vitamin d at diagnosis with breast cancer survival: A case-cohort analysis in the pathways study. JAMA Oncol. 2017, 3, 351–357. [Google Scholar] [CrossRef]
- Vaughan-Shaw, P.G.; O’Sullivan, F.; Farrington, S.M.; Theodoratou, E.; Campbell, H.; Dunlop, M.G.; Zgaga, L. The impact of vitamin d pathway genetic variation and circulating 25-hydroxyvitamin d on cancer outcome: Systematic review and meta-analysis. Br. J. Cancer 2017, 116, 1092–1110. [Google Scholar] [CrossRef]
- Skaaby, T.; Husemoen, L.L.; Thuesen, B.H.; Pisinger, C.; Jorgensen, T.; Roswall, N.; Larsen, S.C.; Linneberg, A. Prospective population-based study of the association between serum 25-hydroxyvitamin-d levels and the incidence of specific types of cancer. Cancer Epidemiol. Biomark. 2014, 23, 1220–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Lee, D.H.; Jeon, J.Y.; Ryu, J.; Kim, S.; Kim, J.Y.; Park, H.S.; Kim, S.I.; Park, B.W. Serum 25-hydroxyvitamin d deficiency and increased risk of breast cancer among korean women: A case-control study. Breast Cancer Res. Treat. 2015, 152, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Bilinski, K.; Boyages, J. Association between 25-hydroxyvitamin d concentration and breast cancer risk in an australian population: An observational case-control study. Breast Cancer Res. Treat. 2013, 137, 599–607. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Almeida-Filho, B.; De Luca Vespoli, H.; Pessoa, E.C.; Machado, M.; Nahas-Neto, J.; Nahas, E.A.P. Vitamin d deficiency is associated with poor breast cancer prognostic features in postmenopausal women. J. Steroid Biochem. Mol. Biol. 2017, 174, 284–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matusiak, D.; Benya, R.V. Cyp27a1 and cyp24 expression as a function of malignant transformation in the colon. J. Histochem. Cytochem. 2007, 55, 1257–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corder, E.H.; Guess, H.A.; Hulka, B.S.; Friedman, G.D.; Sadler, M.; Vollmer, R.T.; Lobaugh, B.; Drezner, M.K.; Vogelman, J.H.; Orentreich, N. Vitamin d and prostate cancer: A prediagnostic study with stored sera. Cancer Epidemiol. Biomark. 1993, 2, 467–472. [Google Scholar]
- Xu, Y.; Shao, X.; Yao, Y.; Xu, L.; Chang, L.; Jiang, Z.; Lin, Z. Positive association between circulating 25-hydroxyvitamin d levels and prostate cancer risk: New findings from an updated meta-analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1465–1477. [Google Scholar] [CrossRef]
- Nunes, S.B.; de Matos Oliveira, F.; Neves, A.F.; Araujo, G.R.; Marangoni, K.; Goulart, L.R.; Araujo, T.G. Association of vitamin d receptor variants with clinical parameters in prostate cancer. SpringerPlus 2016, 5, 364. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Stopsack, K.H.; Allott, E.H.; Gerke, T.; Giovannucci, E.L.; Mucci, L.A.; Kantoff, P.W. Intratumoral sterol-27-hydroxylase (cyp27a1) expression in relation to cholesterol synthesis and vitamin d signaling and its association with lethal prostate cancer. Cancer Epidemiol. Biomark. 2019, 28, 1052–1058. [Google Scholar] [CrossRef] [Green Version]
- Trummer, O.; Langsenlehner, U.; Krenn-Pilko, S.; Pieber, T.R.; Obermayer-Pietsch, B.; Gerger, A.; Renner, W.; Langsenlehner, T. Vitamin d and prostate cancer prognosis: A mendelian randomization study. World J. Urol. 2016, 34, 607–611. [Google Scholar] [CrossRef]
- Weinstein, S.J.; Mondul, A.M.; Kopp, W.; Rager, H.; Virtamo, J.; Albanes, D. Circulating 25-hydroxyvitamin d, vitamin d-binding protein and risk of prostate cancer. Int. J. Cancer 2013, 132, 2940–2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidegger, I.; Ofer, P.; Doppler, W.; Rotter, V.; Klocker, H.; Massoner, P. Diverse functions of igf/insulin signaling in malignant and noncancerous prostate cells: Proliferation in cancer cells and differentiation in noncancerous cells. Endocrinology 2012, 153, 4633–4643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, F.L.; Goodman, P.J.; Tangen, C.; Torkko, K.C.; Schenk, J.M.; Song, X.; Pollak, M.; Thompson, I.M.; Neuhouser, M.L. Interactions of the insulin-like growth factor axis and vitamin d in prostate cancer risk in the prostate cancer prevention trial. Nutrients 2017, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Klein, P.; Grossbard, M.L. Vitamin d and breast cancer. Oncol. 2012, 17, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Welsh, J. Vitamin d and breast cancer: Past and present. J. Steroid Biochem. Mol. Biol. 2018, 177, 15–20. [Google Scholar] [CrossRef]
- Lopes, N.; Sousa, B.; Martins, D.; Gomes, M.; Vieira, D.; Veronese, L.A.; Milanezi, F.; Paredes, J.; Costa, J.L.; Schmitt, F. Alterations in vitamin d signalling and metabolic pathways in breast cancer progression: A study of vdr, cyp27b1 and cyp24a1 expression in benign and malignant breast lesions. BMC Cancer 2010, 10, 483. [Google Scholar] [CrossRef] [Green Version]
- Fuhrman, B.J.; Freedman, D.M.; Bhatti, P.; Doody, M.M.; Fu, Y.P.; Chang, S.C.; Linet, M.S.; Sigurdson, A.J. Sunlight, polymorphisms of vitamin d-related genes and risk of breast cancer. Anticancer Res. 2013, 33, 543–551. [Google Scholar]
- Perna, L.; Butterbach, K.; Haug, U.; Schottker, B.; Muller, H.; Arndt, V.; Holleczek, B.; Burwinkel, B.; Brenner, H. Vitamin d receptor genotype rs731236 (taq1) and breast cancer prognosis. Cancer Epidemiol. Biomark. 2013, 22, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Gnagnarella, P.; Pasquali, E.; Serrano, D.; Raimondi, S.; Disalvatore, D.; Gandini, S. Vitamin d receptor polymorphism foki and cancer risk: A comprehensive meta-analysis. Carcinogenesis 2014, 35, 1913–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gee, J.; Bailey, H.; Kim, K.; Kolesar, J.; Havighurst, T.; Tutsch, K.D.; See, W.; Cohen, M.B.; Street, N.; Levan, L.; et al. Phase ii open label, multi-center clinical trial of modulation of intermediate endpoint biomarkers by 1alpha-hydroxyvitamin d2 in patients with clinically localized prostate cancer and high grade pin. Prostate 2013, 73, 970–978. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin d deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesselink, E.; Bours, M.J.L.; de Wilt, J.H.W.; Aquarius, M.; Breukink, S.O.; Hansson, B.; Keulen, E.T.P.; Kok, D.E.; van den Ouweland, J.; van Roekel, E.H.; et al. Chemotherapy and vitamin d supplement use are determinants of serum 25-hydroxyvitamin d levels during the first six months after colorectal cancer diagnosis. J. Steroid Biochem. Mol. Biol. 2020, 199, 105577. [Google Scholar] [CrossRef] [PubMed]
- Antunac Golubic, Z.; Barsic, I.; Librenjak, N.; Plestina, S. Vitamin d supplementation and survival in metastatic colorectal cancer. Nutr. Cancer 2018, 70, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Shahvazi, S.; Soltani, S.; Ahmadi, S.M.; de Souza, R.J.; Salehi-Abargouei, A. The effect of vitamin d supplementation on prostate cancer: A systematic review and meta-analysis of clinical trials. Horm. Metab. Res. 2019, 51, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, D.; Trudel, D.; Van der Kwast, T.; Nonn, L.; Giangreco, A.A.; Li, D.; Dias, A.; Cardoza, M.; Laszlo, S.; Hersey, K.; et al. Randomized clinical trial of vitamin d3 doses on prostatic vitamin d metabolite levels and ki67 labeling in prostate cancer patients. J. Clin. Endocrinol. Metab. 2013, 98, 1498–1507. [Google Scholar] [CrossRef] [Green Version]
- Scher, H.I.; Jia, X.; Chi, K.; de Wit, R.; Berry, W.R.; Albers, P.; Henick, B.; Waterhouse, D.; Ruether, D.J.; Rosen, P.J.; et al. Randomized, open-label phase iii trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J. Clin. Oncol. 2011, 29, 2191–2198. [Google Scholar] [CrossRef]
- Chadha, M.K.; Tian, L.; Mashtare, T.; Payne, V.; Silliman, C.; Levine, E.; Wong, M.; Johnson, C.; Trump, D.L. Phase 2 trial of weekly intravenous 1,25 dihydroxy cholecalciferol (calcitriol) in combination with dexamethasone for castration-resistant prostate cancer. Cancer 2010, 116, 2132–2139. [Google Scholar] [CrossRef]
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnell, S.L. Effect of vitamin d and calcium supplementation on cancer incidence in older women: A randomized clinical trial. JAMA 2017, 317, 1234–1243. [Google Scholar] [CrossRef]
- Hummel, D.M.; Thiem, U.; Hobaus, J.; Mesteri, I.; Gober, L.; Stremnitzer, C.; Graca, J.; Obermayer-Pietsch, B.; Kallay, E. Prevention of preneoplastic lesions by dietary vitamin d in a mouse model of colorectal carcinogenesis. J. Steroid Biochem. Mol. Biol. 2013, 136, 284–288. [Google Scholar] [CrossRef] [Green Version]
- Hobaus, J.; Tennakoon, S.; Heffeter, P.; Groeschel, C.; Aggarwal, A.; Hummel, D.M.; Thiem, U.; Marculescu, R.; Berger, W.; Kallay, E. Impact of cyp24a1 overexpression on growth of colorectal tumour xenografts in mice fed with vitamin d and soy. Int. J. Cancer 2016, 138, 440–450. [Google Scholar] [CrossRef] [Green Version]
- Elimrani, I.; Koenekoop, J.; Dionne, S.; Marcil, V.; Delvin, E.; Levy, E.; Seidman, E.G. Vitamin d reduces colitis- and inflammation-associated colorectal cancer in mice independent of nod2. Nutr. Cancer 2017, 69, 276–288. [Google Scholar] [CrossRef] [PubMed]
- El-Shemi, A.G.; Refaat, B.; Kensara, O.A.; Mohamed, A.M.; Idris, S.; Ahmad, J. Paricalcitol enhances the chemopreventive efficacy of 5-fluorouracil on an intermediate-term model of azoxymethane-induced colorectal tumors in rats. Cancer Prev. Res. 2016, 9, 491–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milczarek, M.; Psurski, M.; Kutner, A.; Wietrzyk, J. Vitamin d analogs enhance the anticancer activity of 5-fluorouracil in an in vivo mouse colon cancer model. BMC Cancer 2013, 13, 294. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Wang, Q.L.; Liu, X.; Dong, S.H.; Li, H.X.; Li, C.Y.; Guo, L.S.; Gao, J.M.; Berger, N.A.; Li, L.; et al. Combined use of vitamin d3 and metformin exhibits synergistic chemopreventive effects on colorectal neoplasia in rats and mice. Cancer Prev. Res. 2015, 8, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.Y.; Swami, S.; Krishnan, A.V.; Feldman, D. Combination of calcitriol and dietary soy exhibits enhanced anticancer activity and increased hypercalcemic toxicity in a mouse xenograft model of prostate cancer. Prostate 2012, 72, 1628–1637. [Google Scholar] [CrossRef] [Green Version]
- Swami, S.; Krishnan, A.V.; Wang, J.Y.; Jensen, K.; Horst, R.; Albertelli, M.A.; Feldman, D. Dietary vitamin d(3) and 1,25-dihydroxyvitamin d(3) (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology 2012, 153, 2576–2587. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, S.; Karasik, E.; Gillard, B.; Williams, J.; Winchester, T.; Moser, M.T.; Smiraglia, D.J.; Foster, B.A. Lsd1 dual function in mediating epigenetic corruption of the vitamin d signaling in prostate cancer. Clin. Epigenet. 2017, 9, 82. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.; Rabbani, Z.; Mouraviev, V.; Tsivian, M.; Caso, J.; Satoh, T.; Baba, S.; Vujaskovic, Z.; Baust, J.M.; Baust, J.G.; et al. Role of vitamin d(3) as a sensitizer to cryoablation in a murine prostate cancer model: Preliminary in vivo study. Urology 2010, 76, 764. e14–764. e20. [Google Scholar] [CrossRef]
- Santucci, K.L.; Snyder, K.K.; Baust, J.M.; Van Buskirk, R.G.; Mouraviev, V.; Polascik, T.J.; Gage, A.A.; Baust, J.G. Use of 1,25alpha dihydroxyvitamin d3 as a cryosensitizing agent in a murine prostate cancer model. Prostate Cancer Prostatic Dis. 2011, 14, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Rossdeutscher, L.; Li, J.; Luco, A.L.; Fadhil, I.; Ochietti, B.; Camirand, A.; Huang, D.C.; Reinhardt, T.A.; Muller, W.; Kremer, R. Chemoprevention activity of 25-hydroxyvitamin d in the mmtv-pymt mouse model of breast cancer. Cancer Prev. Res. 2015, 8, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Rollakanti, K.R.; Anand, S.; Maytin, E.V. Vitamin d enhances the efficacy of photodynamic therapy in a murine model of breast cancer. Cancer Med. 2015, 4, 633–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, A.V.; Swami, S.; Feldman, D. Equivalent anticancer activities of dietary vitamin d and calcitriol in an animal model of breast cancer: Importance of mammary cyp27b1 for treatment and prevention. J. Steroid Biochem. Mol. Biol. 2013, 136, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larriba, M.J.; Ordonez-Moran, P.; Chicote, I.; Martin-Fernandez, G.; Puig, I.; Munoz, A.; Palmer, H.G. Vitamin d receptor deficiency enhances wnt/beta-catenin signaling and tumor burden in colon cancer. PLoS ONE 2011, 6, e23524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, L.; Diaz-Munoz, M.; Garcia-Gaytan, A.C.; Mendez, I. Mechanistic effects of calcitriol in cancer biology. Nutrients 2015, 7, 5020–5050. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, V.; Falzon, M. Restoration of the anti-proliferative and anti-migratory effects of 1,25-dihydroxyvitamin d by silibinin in vitamin d-resistant colon cancer cells. Cancer Lett. 2015, 362, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Bi, X.; Shi, Q.; Zhang, H.; Bao, Y.; Hu, D.; Pohl, N.; Fang, W.; Dong, H.; Xia, X.; Fan, D.; et al. C-jun nh2-teminal kinase 1 interacts with vitamin d receptor and affects vitamin d-mediated inhibition of cancer cell proliferation. J. Steroid Biochem. Mol. Biol. 2016, 163, 164–172. [Google Scholar] [CrossRef]
- Martinez-Reza, I.; Diaz, L.; Barrera, D.; Segovia-Mendoza, M.; Pedraza-Sanchez, S.; Soca-Chafre, G.; Larrea, F.; Garcia-Becerra, R. Calcitriol inhibits the proliferation of triple-negative breast cancer cells through a mechanism involving the proinflammatory cytokines il-1beta and tnf-alpha. J. Immunol. Res. 2019, 2019, 6384278. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, M.; Reichert, K.; Woeste, A.; Polack, S.; Fischer, D.; Hoellen, F.; Rody, A.; Koster, F.; Thill, M. Effects of combined treatment with vitamin d and cox2 inhibitors on breast cancer cell lines. Anticancer Res. 2018, 38, 1201–1207. [Google Scholar]
- Segovia-Mendoza, M.; Diaz, L.; Gonzalez-Gonzalez, M.E.; Martinez-Reza, I.; Garcia-Quiroz, J.; Prado-Garcia, H.; Ibarra-Sanchez, M.J.; Esparza-Lopez, J.; Larrea, F.; Garcia-Becerra, R. Calcitriol and its analogues enhance the antiproliferative activity of gefitinib in breast cancer cells. J. Steroid Biochem. Mol. Biol. 2015, 148, 122–131. [Google Scholar] [CrossRef]
- Segovia-Mendoza, M.; Diaz, L.; Prado-Garcia, H.; Reginato, M.J.; Larrea, F.; Garcia-Becerra, R. The addition of calcitriol or its synthetic analog eb1089 to lapatinib and neratinib treatment inhibits cell growth and promotes apoptosis in breast cancer cells. Am. J. Cancer Res. 2017, 7, 1486–1500. [Google Scholar]
- Pickholtz, I.; Saadyan, S.; Keshet, G.I.; Wang, V.S.; Cohen, R.; Bouwman, P.; Jonkers, J.; Byers, S.W.; Papa, M.Z.; Yarden, R.I. Cooperation between brca1 and vitamin d is critical for histone acetylation of the p21waf1 promoter and growth inhibition of breast cancer cells and cancer stem-like cells. Oncotarget 2014, 5, 11827–11846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundqvist, J.; Yde, C.W.; Lykkesfeldt, A.E. 1alpha,25-dihydroxyvitamin d3 inhibits cell growth and nfkappab signaling in tamoxifen-resistant breast cancer cells. Steroids 2014, 85, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, J.M.; Binek, A.; Ahrends, T.; Nowacka, J.D.; Szydlowska, A.; Turczyk, L.; Wasiewicz, T.; Wierzbicki, P.M.; Sadej, R.; Tuckey, R.C.; et al. Differential antitumor effects of vitamin d analogues on colorectal carcinoma in culture. Int. J. Oncol. 2015, 47, 1084–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razak, S.; Afsar, T.; Almajwal, A.; Alam, I.; Jahan, S. Growth inhibition and apoptosis in colorectal cancer cells induced by vitamin d-nanoemulsion (nvd): Involvement of wnt/beta-catenin and other signal transduction pathways. Cell Biosci. 2019, 9, 15. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, C.; Cong, L.; Wu, N.; Wang, X.; Hao, M.; Liu, T.; Wang, L.; Liu, Y.; Cong, X. Cyp24a1 inhibition facilitates the antiproliferative effect of 1,25(oh)2d3 through downregulation of the wnt/beta-catenin pathway and methylation-mediated regulation of cyp24a1 in colorectal cancer cells. Dna Cell Biol. 2018, 37, 742–749. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, P.; Gao, Y.; Ta, N.; Zhang, Y.; Cai, J.; Zhao, Y.; Liu, S.; Zheng, J. Meg3 activated by vitamin d inhibits colorectal cancer cells proliferation and migration via regulating clusterin. EBioMedicine 2018, 30, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Li, H.X.; Gao, J.M.; Liang, J.Q.; Xi, J.M.; Fu, M.; Wu, Y.J. Vitamin d3 potentiates the growth inhibitory effects of metformin in du145 human prostate cancer cells mediated by ampk/mtor signalling pathway. Clin. Exp. Pharmacol. Physiol. 2015, 42, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Chiang, K.C.; Chen, S.C.; Yeh, C.N.; Pang, J.H.; Shen, S.C.; Hsu, J.T.; Liu, Y.Y.; Chen, L.W.; Kuo, S.F.; Takano, M.; et al. Mart-10, a less calcemic vitamin d analog, is more potent than 1alpha,25-dihydroxyvitamin d3 in inhibiting the metastatic potential of mcf-7 breast cancer cells in vitro. J. Steroid Biochem. Mol. Biol. 2014, 139, 54–60. [Google Scholar] [CrossRef]
- Yang, J.; Ikezoe, T.; Nishioka, C.; Ni, L.; Koeffler, H.P.; Yokoyama, A. Inhibition of mtorc1 by rad001 (everolimus) potentiates the effects of 1,25-dihydroxyvitamin d(3) to induce growth arrest and differentiation of aml cells in vitro and in vivo. Exp. Hematol. 2010, 38, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Jiang, R.; Yang, Y.; Ding, S.; Deng, H. 1,25-dihydroxyvitamin d3 inhibits growth of the breast cancer cell line mcf-7 and downregulates cytochrome p4501b1 through the cox-2/pge2 pathway. Oncol. Rep. 2012, 28, 2131–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, K.C.; Yeh, C.N.; Chen, S.C.; Shen, S.C.; Hsu, J.T.; Yeh, T.S.; Pang, J.H.; Su, L.J.; Takano, M.; Kittaka, A.; et al. Mart-10, a new generation of vitamin d analog, is more potent than 1alpha,25-dihydroxyvitamin d(3) in inhibiting cell proliferation and inducing apoptosis in er+ mcf-7 breast cancer cells. Evid.-Based Complement. Altern. Med. Ecam 2012, 2012, 310872. [Google Scholar]
- Yang, J.; Zhu, S.; Lin, G.; Song, C.; He, Z. Vitamin d enhances omega-3 polyunsaturated fatty acids-induced apoptosis in breast cancer cells. Cell Biol. Int. 2017, 41, 890–897. [Google Scholar] [CrossRef]
- Guo, L.S.; Li, H.X.; Li, C.Y.; Zhang, S.Y.; Chen, J.; Wang, Q.L.; Gao, J.M.; Liang, J.Q.; Gao, M.T.; Wu, Y.J. Synergistic antitumor activity of vitamin d3 combined with metformin in human breast carcinoma mda-mb-231 cells involves m-tor related signaling pathways. Die Pharm. 2015, 70, 117–122. [Google Scholar]
- Abu El Maaty, M.A.; Strassburger, W.; Qaiser, T.; Dabiri, Y.; Wolfl, S. Differences in p53 status significantly influence the cellular response and cell survival to 1,25-dihydroxyvitamin d3-metformin cotreatment in colorectal cancer cells. Mol. Carcinog. 2017, 56, 2486–2498. [Google Scholar] [CrossRef] [PubMed]
- Hoyer-Hansen, M.; Bastholm, L.; Mathiasen, I.S.; Elling, F.; Jaattela, M. Vitamin d analog eb1089 triggers dramatic lysosomal changes and beclin 1-mediated autophagic cell death. Cell Death Differ. 2005, 12, 1297–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blutt, S.E.; McDonnell, T.J.; Polek, T.C.; Weigel, N.L. Calcitriol-induced apoptosis in lncap cells is blocked by overexpression of bcl-2. Endocrinology 2000, 141, 10–17. [Google Scholar] [CrossRef]
- Axanova, L.S.; Chen, Y.Q.; McCoy, T.; Sui, G.; Cramer, S.D. 1,25-dihydroxyvitamin d(3) and pi3k/akt inhibitors synergistically inhibit growth and induce senescence in prostate cancer cells. Prostate 2010, 70, 1658–1671. [Google Scholar] [CrossRef] [Green Version]
- Saramaki, A.; Banwell, C.M.; Campbell, M.J.; Carlberg, C. Regulation of the human p21(waf1/cip1) gene promoter via multiple binding sites for p53 and the vitamin d3 receptor. Nucleic Acids Res. 2006, 34, 543–554. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.C.; Chen, J.Y.; Hung, W.C. Vitamin d3 receptor/sp1 complex is required for the induction of p27kip1 expression by vitamin d3. Oncogene 2004, 23, 4856–4861. [Google Scholar] [CrossRef] [Green Version]
- Simboli-Campbell, M.; Narvaez, C.J.; van Weelden, K.; Tenniswood, M.; Welsh, J. Comparative effects of 1,25(oh)2d3 and eb1089 on cell cycle kinetics and apoptosis in mcf-7 breast cancer cells. Breast Cancer Res. Treat. 1997, 42, 31–41. [Google Scholar] [CrossRef]
- Shang, S.; Hua, F.; Hu, Z.W. The regulation of beta-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 2017, 8, 33972–33989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohan, J.N.; Weigel, N.L. 1alpha,25-dihydroxyvitamin d3 reduces c-myc expression, inhibiting proliferation and causing g1 accumulation in c4-2 prostate cancer cells. Endocrinology 2009, 150, 2046–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, M.B.; Goetsch, P.D.; Pike, J.W. Vdr/rxr and tcf4/beta-catenin cistromes in colonic cells of colorectal tumor origin: Impact on c-fos and c-myc gene expression. Mol. Endocrinol. 2012, 26, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Gooz, M. Adam-17: The enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 146–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcidiacono, M.V.; Yang, J.; Fernandez, E.; Dusso, A. The induction of c/ebpbeta contributes to vitamin d inhibition of adam17 expression and parathyroid hyperplasia in kidney disease. Nephrol. Dial. Transplant. 2015, 30, 423–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doberstein, K.; Steinmeyer, N.; Hartmetz, A.K.; Eberhardt, W.; Mittelbronn, M.; Harter, P.N.; Juengel, E.; Blaheta, R.; Pfeilschifter, J.; Gutwein, P. Microrna-145 targets the metalloprotease adam17 and is suppressed in renal cell carcinoma patients. Neoplasia 2013, 15, 218–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allavena, P.; Garlanda, C.; Borrello, M.G.; Sica, A.; Mantovani, A. Pathways connecting inflammation and cancer. Curr. Opin. Genet. Dev. 2008, 18, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, L.; Xu, H.J.; Li, Y.; Hu, C.M.; Yang, J.Y.; Sun, M.Y. The anti-inflammatory effects of vitamin d in tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonn, L.; Peng, L.; Feldman, D.; Peehl, D.M. Inhibition of p38 by vitamin d reduces interleukin-6 production in normal prostate cells via mitogen-activated protein kinase phosphatase 5: Implications for prostate cancer prevention by vitamin d. Cancer Res. 2006, 66, 4516–4524. [Google Scholar] [CrossRef] [Green Version]
- Thill, M.; Fischer, D.; Kelling, K.; Hoellen, F.; Dittmer, C.; Hornemann, A.; Salehin, D.; Diedrich, K.; Friedrich, M.; Becker, S. Expression of vitamin d receptor (vdr), cyclooxygenase-2 (cox-2) and 15-hydroxyprostaglandin dehydrogenase (15-pgdh) in benign and malignant ovarian tissue and 25-hydroxycholecalciferol (25(oh2)d3) and prostaglandin e2 (pge2) serum level in ovarian cancer patients. J. Steroid Biochem. Mol. Biol. 2010, 121, 387–390. [Google Scholar]
- Sun, J.; Kong, J.; Duan, Y.; Szeto, F.L.; Liao, A.; Madara, J.L.; Li, Y.C. Increased nf-kappab activity in fibroblasts lacking the vitamin d receptor. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E315–E322. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Mustafi, R.; Cerda, S.; Chumsangsri, A.; Xia, Y.R.; Li, Y.C.; Bissonnette, M. Lithocholic acid down-regulation of nf-kappab activity through vitamin d receptor in colonic cancer cells. J. Steroid Biochem. Mol. Biol. 2008, 111, 37–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen-Lahav, M.; Shany, S.; Tobvin, D.; Chaimovitz, C.; Douvdevani, A. Vitamin d decreases nfkappab activity by increasing ikappabalpha levels. Nephrol. Dial. Transplant. 2006, 21, 889–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tse, A.K.; Zhu, G.Y.; Wan, C.K.; Shen, X.L.; Yu, Z.L.; Fong, W.F. 1alpha,25-dihydroxyvitamin d3 inhibits transcriptional potential of nuclear factor kappa b in breast cancer cells. Mol. Immunol. 2010, 47, 1728–1738. [Google Scholar] [CrossRef]
- So, J.Y.; Lee, H.J.; Smolarek, A.K.; Paul, S.; Wang, C.X.; Maehr, H.; Uskokovic, M.; Zheng, X.; Conney, A.H.; Cai, L.; et al. A novel gemini vitamin d analog represses the expression of a stem cell marker cd44 in breast cancer. Mol. Pharmacol. 2011, 79, 360–367. [Google Scholar] [CrossRef]
- Wahler, J.; So, J.Y.; Cheng, L.C.; Maehr, H.; Uskokovic, M.; Suh, N. Vitamin d compounds reduce mammosphere formation and decrease expression of putative stem cell markers in breast cancer. J. Steroid Biochem. Mol. Biol. 2015, 148, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreepadmanabh, M.; Toley, B.J. Investigations into the cancer stem cell niche using in-vitro 3-d tumor models and microfluidics. Biotechnol. Adv. 2018, 36, 1094–1110. [Google Scholar] [CrossRef]
- Fernandez-Barral, A.; Costales-Carrera, A.; Buira, S.P.; Jung, P.; Ferrer-Mayorga, G.; Larriba, M.J.; Bustamante-Madrid, P.; Dominguez, O.; Real, F.X.; Guerra-Pastrian, L.; et al. Vitamin d differentially regulates colon stem cells in patient-derived normal and tumor organoids. FEBS J. 2020, 287, 53–72. [Google Scholar] [CrossRef] [Green Version]
- Kotlarz, A.; Przybyszewska, M.; Swoboda, P.; Neska, J.; Miloszewska, J.; Grygorowicz, M.A.; Kutner, A.; Markowicz, S. Imatinib inhibits the regrowth of human colon cancer cells after treatment with 5-fu and cooperates with vitamin d analogue pri-2191 in the downregulation of expression of stemness-related genes in 5-fu refractory cells. J. Steroid Biochem. Mol. Biol. 2019, 189, 48–62. [Google Scholar] [CrossRef]
- Maund, S.L.; Barclay, W.W.; Hover, L.D.; Axanova, L.S.; Sui, G.; Hipp, J.D.; Fleet, J.C.; Thorburn, A.; Cramer, S.D. Interleukin-1alpha mediates the antiproliferative effects of 1,25-dihydroxyvitamin d3 in prostate progenitor/stem cells. Cancer Res. 2011, 71, 5276–5286. [Google Scholar] [CrossRef] [Green Version]
- Zeljic, K.; Supic, G.; Magic, Z. New insights into vitamin d anticancer properties: Focus on mirna modulation. Mol. Genet. Genom. 2017, 292, 511–524. [Google Scholar] [CrossRef]
- Chen, S.; Bu, D.; Ma, Y.; Zhu, J.; Chen, G.; Sun, L.; Zuo, S.; Li, T.; Pan, Y.; Wang, X.; et al. H19 overexpression induces resistance to 1,25(oh)2d3 by targeting vdr through mir-675-5p in colon cancer cells. Neoplasia 2017, 19, 226–236. [Google Scholar] [CrossRef]
- Padi, S.K.; Zhang, Q.; Rustum, Y.M.; Morrison, C.; Guo, B. Microrna-627 mediates the epigenetic mechanisms of vitamin d to suppress proliferation of human colorectal cancer cells and growth of xenograft tumors in mice. Gastroenterology 2013, 145, 437–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giangreco, A.A.; Vaishnav, A.; Wagner, D.; Finelli, A.; Fleshner, N.; Van der Kwast, T.; Vieth, R.; Nonn, L. Tumor suppressor micrornas, mir-100 and -125b, are regulated by 1,25-dihydroxyvitamin d in primary prostate cells and in patient tissue. Cancer Prev. Res. 2013, 6, 483–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.K.; Long, M.D.; Battaglia, S.; Hu, Q.; Liu, S.; Sucheston-Campbell, L.E.; Campbell, M.J. Vdr regulation of microrna differs across prostate cell models suggesting extremely flexible control of transcription. Epigenetics 2015, 10, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohri, T.; Nakajima, M.; Takagi, S.; Komagata, S.; Yokoi, T. Microrna regulates human vitamin d receptor. Int. J. Cancer 2009, 125, 1328–1333. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Liu, K.; Shen, Q.; Li, Q.; Hao, J.; Han, F.; Jiang, R.W. Reversal of multidrug resistance in cancer by multi-functional flavonoids. Front. Oncol. 2019, 9, 487. [Google Scholar] [CrossRef] [Green Version]
- Casals, E.; Gusta, M.F.; Cobaleda-Siles, M.; Garcia-Sanz, A.; Puntes, V.F. Cancer resistance to treatment and antiresistance tools offered by multimodal multifunctional nanoparticles. Cancer Nanotechnol. 2017, 8, 7. [Google Scholar] [CrossRef]
- Bonanno, L.; Favaretto, A.; Rosell, R. Platinum drugs and DNA repair mechanisms in lung cancer. Anticancer Res. 2014, 34, 493–501. [Google Scholar]
- Neel, D.S.; Bivona, T.G. Resistance is futile: Overcoming resistance to targeted therapies in lung adenocarcinoma. NPJ Precis. Oncol. 2017, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microrna genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pervin, S.; Hewison, M.; Braga, M.; Tran, L.; Chun, R.; Karam, A.; Chaudhuri, G.; Norris, K.; Singh, R. Down-regulation of vitamin d receptor in mammospheres: Implications for vitamin d resistance in breast cancer and potential for combination therapy. PLoS ONE 2013, 8, e53287. [Google Scholar] [CrossRef]
- Vanoirbeek, E.; Krishnan, A.; Eelen, G.; Verlinden, L.; Bouillon, R.; Feldman, D.; Verstuyf, A. The anti-cancer and anti-inflammatory actions of 1,25(oh)(2)d(3). Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 593–604. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negri, M.; Gentile, A.; de Angelis, C.; Montò, T.; Patalano, R.; Colao, A.; Pivonello, R.; Pivonello, C. Vitamin D-Induced Molecular Mechanisms to Potentiate Cancer Therapy and to Reverse Drug-Resistance in Cancer Cells. Nutrients 2020, 12, 1798. https://doi.org/10.3390/nu12061798
Negri M, Gentile A, de Angelis C, Montò T, Patalano R, Colao A, Pivonello R, Pivonello C. Vitamin D-Induced Molecular Mechanisms to Potentiate Cancer Therapy and to Reverse Drug-Resistance in Cancer Cells. Nutrients. 2020; 12(6):1798. https://doi.org/10.3390/nu12061798
Chicago/Turabian StyleNegri, Mariarosaria, Annalisa Gentile, Cristina de Angelis, Tatiana Montò, Roberta Patalano, Annamaria Colao, Rosario Pivonello, and Claudia Pivonello. 2020. "Vitamin D-Induced Molecular Mechanisms to Potentiate Cancer Therapy and to Reverse Drug-Resistance in Cancer Cells" Nutrients 12, no. 6: 1798. https://doi.org/10.3390/nu12061798
APA StyleNegri, M., Gentile, A., de Angelis, C., Montò, T., Patalano, R., Colao, A., Pivonello, R., & Pivonello, C. (2020). Vitamin D-Induced Molecular Mechanisms to Potentiate Cancer Therapy and to Reverse Drug-Resistance in Cancer Cells. Nutrients, 12(6), 1798. https://doi.org/10.3390/nu12061798







