The Impact of Protein Supplementation on Exercise-Induced Muscle Damage, Soreness and Fatigue Following Prolonged Walking Exercise in Vital Older Adults: A Randomized Double-Blind Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
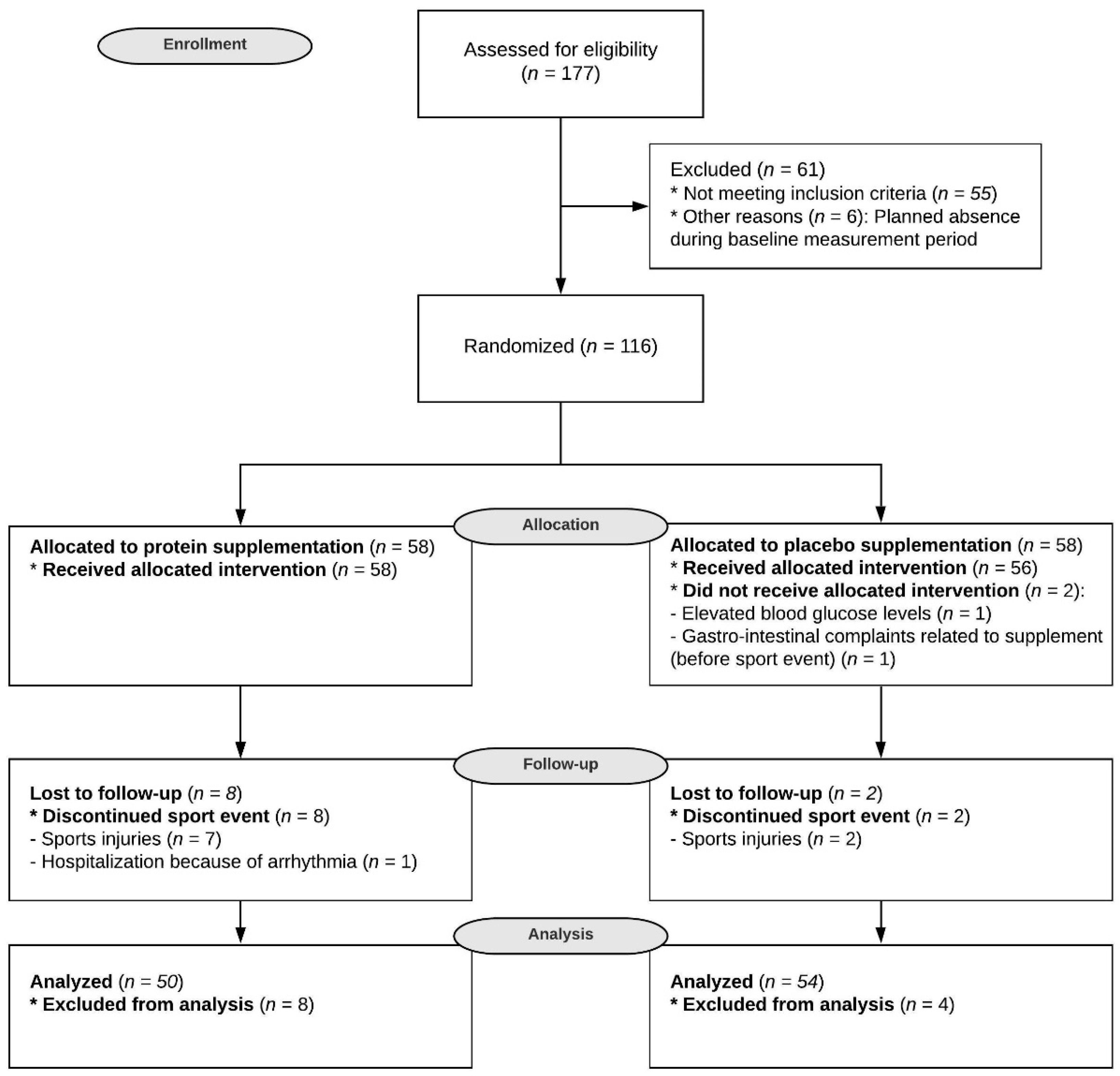
2.1. Subjects
2.2. Study Design
2.3. Protein Intervention
2.4. Measurements
2.5. Statistical Analysis
3. Results
3.1. Subjects
3.2. Dietary Intake
3.3. Muscle Soreness and Fatigue
3.4. Blood Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Maggi, S.P.; Williams, B.D.; Tipton, K.; Wolfe, R.R. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am. J. Physiol. Metab. 1995, 268, E514–E520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifford, T. Nutritional and Pharmacological Interventions to Expedite Recovery Following Muscle-Damaging Exercise in Older Adults: A Narrative Review of the Literature. J. Aging Phys. Act. 2019, 27, 914–928. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, T.; Philp, A.; Watt, P.W. A single protein meal increases recovery of muscle function following an acute eccentric exercise bout. Appl. Physiol. Nutr. Metab. 2008, 33, 483–488. [Google Scholar] [CrossRef]
- Coombes, J.S.; McNaughton, L.R. Effects of branched-chain amino acid supplementation on serum creatine kinase and lactate dehydrogenase after prolonged exercise. J. Sports Med. Phys. Fit. 2000, 40. [Google Scholar]
- Greer, B.K.; Woodard, J.; White, J.P.; Arguello, E.M.; Haymes, E.M. Branched-chain amino acid supplementation and indicators of muscle damage after endurance exercise. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 595–607. [Google Scholar] [CrossRef]
- Huang, W.-C.; Chang, Y.-C.; Chen, Y.-M.; Hsu, Y.-J.; Huang, C.-C.; Kan, N.-W.; Chen, S.-S. Whey Protein Improves Marathon-Induced Injury and Exercise Performance in Elite Track Runners. Int. J. Med Sci. 2017, 14, 648–654. [Google Scholar] [CrossRef] [Green Version]
- Valentine, R.J.; Saunders, M.J.; Todd, M.K.; Laurent, T.G.S. Influence of carbohydrate-protein beverage on cycling endurance and indices of muscle disruption. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 363–378. [Google Scholar] [CrossRef]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ’anabolic resistance’ of ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Burd, N.A.; Gorissen, S.; van Loon, L. Anabolic Resistance of Muscle Protein Synthesis with Aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef]
- Bauer, J.M.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.M.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Casperson, S.L.; Sheffield-Moore, M.; Hewlings, S.J.; Paddon-Jones, D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin. Nutr. 2012, 31, 512–519. [Google Scholar] [CrossRef] [Green Version]
- Farnfield, M.M.; Breen, L.; Carey, K.A.; Garnham, A.; Cameron-Smith, D. Activation of mTOR signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion. Appl. Physiol. Nutr. Metab. 2012, 37, 21–30. [Google Scholar] [CrossRef]
- Cawood, A.L.; Elia, M.; Stratton, R.J. Systematic review, and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res. Rev. 2012, 11, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.-K.; Fielding, R.A.; Martin, F.C.; Michel, J.-P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Finger, D.; Goltz, F.R.; Umpierre, D.; Meyer, E.; Rosa, L.H.T.; Schneider, C. Effects of Protein Supplementation in Older Adults Undergoing Resistance Training: A Systematic Review and Meta-Analysis. Sports Med. 2014, 45, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Chen, G.-C.; Wang, Y.; Zhang, Z.; Dai, X.; Szeto, I.M.Y.; Qin, L. Effects of milk proteins supplementation in older adults undergoing resistance training: A meta-analysis of randomized control trials. J. Nutr. Heal. Aging 2017, 22, 237–245. [Google Scholar] [CrossRef]
- Tieland, M.; Dirks, M.L.; van der Zwaluw, N.; Verdijk, L.B.; van de Rest, O.; de Groot, L.C.; van Loon, L. Protein Supplementation Increases Muscle Mass Gain During Prolonged Resistance-Type Exercise Training in Frail Elderly People: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Med Dir. Assoc. 2012, 13, 713–719. [Google Scholar] [CrossRef]
- Haaf, D.T.; Eijsvogels, T.M.; Bongers, C.C.W.G.; Horstman, A.; Timmers, S.; de Groot, L.C.; Hopman, M.T.E. Protein supplementation improves lean body mass in physically active older adults: A randomized placebo-controlled trial. J. Cachex Sarcopenia Muscle 2019, 10, 298–310. [Google Scholar] [CrossRef]
- The Four Days Marches—The Walk of the World. Available online: https://www.4daagse.nl/en (accessed on 15 May 2019).
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Crispim, S.P.; de Vries, J.H.M.; Geelen, A.; Souverein, O.; Hulshof, P.J.M.; Lafay, L.; Rousseau, A.-S.; Lillegaard, I.T.L.; Andersen, L.F.; Huybrechts, I.; et al. Two non-consecutive 24 h recalls using EPIC-Soft software are sufficiently valid for comparing protein and potassium intake between five European centres—Results from the European Food Consumption Validation (EFCOVAL) study. Br. J. Nutr. 2010, 105, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Rijksinstituut voor Volksgezondheid en Milieu (RIVM). Diet and Cognitive Decline at Middle Age: The Role of Antioxidants; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Hjermstad, M.J.; Fayers, P.; Haugen, D.R.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S. Studies Comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for Assessment of Pain Intensity in Adults: A Systematic Literature Review. J. Pain Symptom Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.M.; Stewart, R.E.; Köke, A.J.A.; Oosterwijk, R.F.A.; Swaan, J.L.; Schreurs, K.M.G.; Preuper, H.R.S. Cut-Off Points for Mild, Moderate, and Severe Pain on the Numeric Rating Scale for Pain in Patients with Chronic Musculoskeletal Pain: Variability and Influence of Sex and Catastrophizing. Front. Psychol. 2016, 7, 1055. [Google Scholar] [CrossRef] [Green Version]
- Schumann, G.; Klauke, R. New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: Preliminary upper reference limits obtained in hospitalized subjects. Clin. Chim. Acta 2003, 327, 69–79. [Google Scholar] [CrossRef]
- Totsuka, M.; Nakaji, S.; Suzuki, K.; Sugawara, K.; Sato, K. Break point of serum creatine kinase release after endurance exercise. J. Appl. Physiol. 2002, 93, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Baumert, P.; Lake, M.J.; Stewart, C.; Drust, B.; Erskine, R.M. Genetic variation, and exercise-induced muscle damage: Implications for athletic performance, injury, and ageing. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 116, 1595–1625. [Google Scholar] [CrossRef] [Green Version]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-Kinase- and Exercise-Related Muscle Damage Implications for Muscle Performance and Recovery. J. Nutr. Metab. 2012, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Tiidus, P.M.; Ianuzzo, C.D. Effects of intensity and duration of muscular exercise on delayed soreness and serum enzyme activities. Med. Sci. Sports Exerc. 1983, 15, 461–465. [Google Scholar] [CrossRef]
- Maeo, S.; Ochi, Y.; Yamamoto, M.; Kanehisa, H.; Nosaka, K. Effect of a prior bout of preconditioning exercise on muscle damage from downhill walking. Appl. Physiol. Nutr. Metab. 2015, 40, 274–279. [Google Scholar] [CrossRef]
- Maeo, S.; Yamamoto, M.; Kanehisa, H. Downhill walking training with and without exercise-induced muscle damage similarly increase knee extensor strength. J. Sports Sci. 2016, 34, 2018–2026. [Google Scholar] [CrossRef]
- Quinn, T.J.; Manley, M.J. The impact of a long training run on muscle damage and running economy in runners training for a marathon. J. Exerc. Sci. Fit. 2012, 10, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Easthope, C.S.; Hausswirth, C.; Louis, J.; Lepers, R.; Vercruyssen, F.; Brisswalter, J. Effects of a trail running competition on muscular performance and efficiency in well-trained young and master athletes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 110, 1107–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, F.R.D.; Libardi, C.A.; Nosaka, K.; Vechin, F.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Comparison in responses to maximal eccentric exercise between elbow flexors and knee extensors of older adults. J. Sci. Med. Sport 2014, 17, 91–95. [Google Scholar] [CrossRef]
- Chen, T.C.; Tseng, W.-C.; Huang, G.-L.; Chen, H.-L.; Tseng, K.-W.; Nosaka, K. Low-intensity eccentric contractions attenuate muscle damage induced by subsequent maximal eccentric exercise of the knee extensors in the elderly. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 113, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, M.A.; Hammouda, O.; Matran, R.; Robin, S.; Fabre, C. Changes in Oxidative Stress Markers and Biological Markers of Muscle Injury with Aging at Rest and in Response to an Exhaustive Exercise. PLoS ONE 2014, 9, e90420. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Tipton, K.; Klein, S.; Wolfe, R.R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. Content 1997, 273, E122–E129. [Google Scholar] [CrossRef]
- Witard, O.C.; McGlory, C.; Hamilton, D.L.; Phillips, S.M. Growing older with health and vitality: A nexus of physical activity, exercise, and nutrition. Biogerontology 2016, 17, 529–546. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.M. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med. 2014, 44, S71–S77. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, L.E.; Villareal, D.T. Physical Exercise as Therapy for Frailty. Nestle Nutr. Inst. Work. Ser. 2015, 83, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.R. Keeping Older Muscle “Young” through Dietary Protein and Physical Activity. Adv. Nutr. 2014, 5, 599S–607S. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.M.; Tipton, K.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. Content 1997, 273, E99–E107. [Google Scholar] [CrossRef] [PubMed]
- Doering, T.M.; Reaburn, P.; Borges, N.R.; Cox, G.; Jenkins, D. The Effect of Higher Than Recommended Protein Feedings Post-Exercise on Recovery Following Downhill Running in Master’s Triathletes. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Maeo, S.; Yamamoto, M.; Kanehisa, H.; Nosaka, K. Prevention of downhill walking-induced muscle damage by non-damaging downhill walking. PLoS ONE 2017, 12, e0173909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, P.D.; Eijsvogels, T.M. New Physical Activity Guidelines: A Call to Activity for Clinicians and Patients. JAMA 2018, 320, 1983–1984. [Google Scholar] [CrossRef] [PubMed]
- Damas, F.; Libardi, C.A.; Ugrinowitsch, C. The development of skeletal muscle hypertrophy through resistance training: The role of muscle damage and muscle protein synthesis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 118, 485–500. [Google Scholar] [CrossRef] [PubMed]


| Nutrient | Protein | Placebo |
|---|---|---|
| Energy, kcal/100 mL | 38 | 39 |
| Protein, g/100 mL | 6.2 | 0.21 |
| Fat, g/100 mL | 0.21 | 1.03 |
| Carbohydrates, g/100 mL | 2.9 | 7.22 |
| Of which lactose, g/100 mL | 2.9 | - |
| Total Group n = 104 | Protein n = 50 | Placebo n = 54 | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 69 (67–73) | 69 (67–72) | 69 (67–73) | 0.96 * |
| Male, n (%) | 84 (81) | 40 (80) | 44 (82) | 0.85 ‡ |
| Body composition | ||||
| Body weight, kg | 82.2 ± 10.4 | 83.4 ± 10.3 | 81.1 ± 10.4 | 0.27 § |
| BMI, kg/m2 | 26.5 ± 2.5 | 26.7 ± 2.6 | 26.3 ± 2.4 | 0.40 § |
| Waist–hip ratio | 0.93 ± 0.07 | 0.93 ± 0.08 | 0.92 ± 0.07 | 0.92 § |
| Diet | ||||
| Energy intake, kcal | 1948 ± 514 | 1932 ± 505 | 1962 ± 527 | 0.77 § |
| Protein intake, g/kg/d | 0.94 ± 0.25 | 0.92 ± 0.27 | 0.97 ± 0.23 | 0.31 § |
| Protein intake at breakfast, g | 12.0 ± 6.1 | 11.0 ± 4.9 | 13.1 ± 6.9 | 0.08 § |
| Protein intake at lunch, g | 19.5 ± 10.0 | 18.7 ± 10.9 | 20.3 ± 9.2 | 0.42 § |
| Protein intake at dinner, g | 35.7 ± 14.5 | 33.3 ± 15.6 | 37.9 ± 13.2 | 0.11 § |
| Animal protein, % | 61.6 ± 10.4 | 61.7 ± 9.6 | 61.4 ± 11.3 | 0.88 § |
| Plant protein, % | 38.0 ± 10.6 | 37.6 ± 9.9 | 38.5 ± 11.2 | 0.68 § |
| Protein, en% | 16.4 ± 3.1 | 16.3 ± 3.3 | 16.5 ± 3.0 | 0.72 § |
| Fat intake, en% | 36.1 ± 6.7 | 35.5 ± 7.1 | 36.7 ± 6.4 | 0.37 § |
| Carbohydrate intake, en% | 41.9 ± 7.7 | 42.9 ± 7.8 | 40.9 ± 7.5 | 0.17 § |
| Walking exercise | ||||
| 12 week pre-event training, km | 385 (257–516) | 396 (288–539) | 341 (244–505) | 0.41 * |
| Distance per day | 0.58 ‡ | |||
| 30 km, n (%) | 72 (71) | 32 (67) | 40 (74) | |
| 40 km, n (%) | 25 (25) | 14 (29) | 11 (20) | |
| 50 km, n (%) | 5 (5) | 2 (4) | 3 (6) | |
| Mean distance per day, km | 33 ± 6 | 32 ± 9 | 33 ± 6 | 0.60 § |
| Heart rate (bpm) | 104 ± 18 | 102 ± 17 | 106 ± 19 | 0.28 § |
| Exercise intensity (% from HRmax) | 67 ± 12 | 66 ± 11 | 68 ± 13 | 0.30 § |
| Baseline | Day 1 | Day 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein n = 50 | Placebo n = 54 | p Value | Protein n = 50 | Placebo n = 54 | p Value | Protein n = 50 | Placebo n = 54 | p Value | |
| Calves | 1.16 ± 0.47 | 1.15 ± 0.57 | 0.50 | 1.53 ± 0.87 | 1.69 ± 1.19 | 0.72 | 1.66 ± 1.36 | 1.64 ± 1.34 | 0.89 |
| Thighs | 1.10 ± 0.36 | 1.11 ± 0.51 | 0.67 | 1.70 ± 1.17 | 1.71 ± 1.54 | 0.51 | 1.83 ± 1.47 | 1.68 ± 1.08 | 0.95 |
| Glutes | 1.24 ± 0.63 | 1.13 ± 0.74 | 0.08 | 1.43 ± 1.15 | 1.37 ± 1.18 | 0.72 | 1.34 ± 0.92 | 1.24 ± 0.72 | 0.83 |
| Fatigue | 1.51 ± 1.31 | 1.32 ± 0.73 | 0.42 | 3.98 ± 2.06 | 4.15 ± 1.74 | 0.44 | 3.67 ± 1.39 | 3.76 ± 1.80 | 0.93 |
| Baseline | Day 1 | Day 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein | Placebo | p Value | Protein | Placebo | p Value | Protein | Placebo | p Value | |
| Calves | |||||||||
| No pain | 44 (88) | 49 (92) | 0.52 | 31 (69) | 34 (67) | 1.00 | 33 (70) | 34 (38) | 0.87 |
| Mild pain | 6 (12) | 4 (8) | 14 (28) | 16 (31) | 13 (28) | 15 (30) | |||
| Moderate pain | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 1 (2) | 1 (2) | |||
| Severe pain | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Thighs | |||||||||
| No pain | 46 (92) | 50 (94) | 0.71 | 31 (67) | 38 (74) | 29 (63) | 31 (62) | 0.67 | |
| Mild pain | 4 (8) | 3 (6) | 15 (33) | 11 (22) | 0.26 | 16 (35) | 19 (38) | ||
| Moderate pain | 0 (0) | 0 (0) | 0 (0) | 2 (4) | 1 (2) | 0 (0) | |||
| Severe pain | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Glutes | |||||||||
| No pain | 43 (86) | 51 (96) | 0.09 | 36 (82) | 43 (84) | 0.88 | 40 (85) | 43 (86) | 1.00 |
| Mild pain | 7 (14) | 2 (4) | 8 (19) | 7 (14) | 7 (15) | 7 (14) | |||
| Moderate pain | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | |||
| Severe pain | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Baseline | Day 1 | Day 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein n = 50 | Placebo n = 54 | p Value | Protein n = 50 | Placebo n = 54 | p Value | Protein n = 50 | Placebo n = 54 | p Value | |
| Blood analysis | |||||||||
| CRP, mg/L | 2.9 (2.9–2.9) | 2.9 (2.9–2.9) | 0.97 | 2.9 (2.9–2.9) | 2.9 (2.9–2.9) | 0.92 | 9.0 (4.0–16.8) | 10.0 (4.8–14.6) | 0.73 |
| IL-6, pg/mL | 0.69 (0.46–0.90) | 0.69 (0.43–0.97) | 0.93 | 5.58 (3.39–11.05) | 6.49 (4.65–10.93) | 0.33 | 1.86 (1.20–2.83) | 2.40 (1.41–5.46) | 0.06 |
| IL-10, pg/mL | 0.20 (0.13–0.32) | 0.21 (0.12–0.30) | 0.87 | 0.33 (0.19–0.67) | 0.38 (0.25–0.64) | 0.35 | 0.19 (0.10–0.26) | 0.24 (0.16–0.38) | 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
ten Haaf, D.S.M.; Bongers, C.C.W.G.; Hulshof, H.G.; Eijsvogels, T.M.H.; Hopman, M.T.E. The Impact of Protein Supplementation on Exercise-Induced Muscle Damage, Soreness and Fatigue Following Prolonged Walking Exercise in Vital Older Adults: A Randomized Double-Blind Placebo-Controlled Trial. Nutrients 2020, 12, 1806. https://doi.org/10.3390/nu12061806
ten Haaf DSM, Bongers CCWG, Hulshof HG, Eijsvogels TMH, Hopman MTE. The Impact of Protein Supplementation on Exercise-Induced Muscle Damage, Soreness and Fatigue Following Prolonged Walking Exercise in Vital Older Adults: A Randomized Double-Blind Placebo-Controlled Trial. Nutrients. 2020; 12(6):1806. https://doi.org/10.3390/nu12061806
Chicago/Turabian Styleten Haaf, Dominique S. M., Coen C. W. G. Bongers, Hugo G. Hulshof, Thijs M. H. Eijsvogels, and Maria T. E. Hopman. 2020. "The Impact of Protein Supplementation on Exercise-Induced Muscle Damage, Soreness and Fatigue Following Prolonged Walking Exercise in Vital Older Adults: A Randomized Double-Blind Placebo-Controlled Trial" Nutrients 12, no. 6: 1806. https://doi.org/10.3390/nu12061806





