Wheat Sensitivity and Functional Dyspepsia: A Pilot, Double-Blind, Randomized, Placebo-Controlled Dietary Crossover Trial with Novel Challenge Protocol
Abstract
:1. Introduction
2. Methods
2.1. Participants
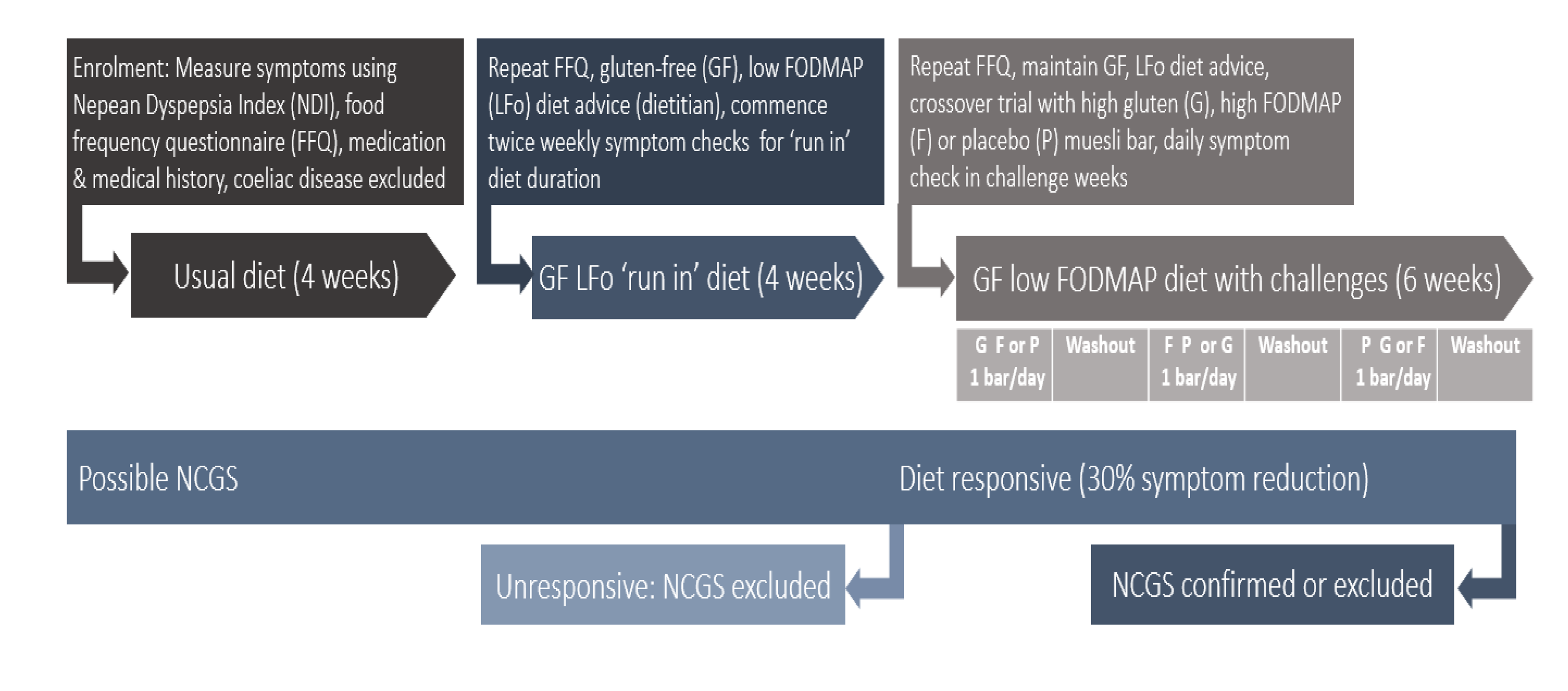
2.2. Protocol
2.3. Sample Size and Statistics
3. Results
4. Discussion
4.1. Limitations
4.2. Implication for Practice and Research
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Potter, M.D.; Walker, M.M.; Jones, M.P.; Koloski, N.A.; Keely, S.; Talley, N.J. Wheat Intolerance and Chronic Gastrointestinal Symptoms in an Australian Population-based Study: Association between Wheat Sensitivity, Celiac Disease and Functional Gastrointestinal Disorders. Am. J. Gastroenterol 2018, 113, 1036–1044. [Google Scholar] [CrossRef]
- Talley, N.J.; Ford, A.C. Functional Dyspepsia. N. Engl. J. Med. 2015, 373, 1853–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Wulffen, M.; Talley, N.J.; Hammer, J.; McMaster, J.; Rich, G.; Shah, A.; Koloski, N.; Kendall, B.J.; Jones, M.; Holtmann, G. Overlap of irritable bowel syndrome and functional dyspepsia in the clinical setting: Prevalence and risk factors. Dig. Dis. Sci. 2019, 64, 480–486. [Google Scholar] [CrossRef]
- Duncanson, K.R.; Talley, N.J.; Walker, M.M.; Burrows, T.L. Food and functional dyspepsia: A systematic review. J. Hum. Nutr. Diet. 2017, 31, 390–407. [Google Scholar] [CrossRef]
- Talley, N.J.; Walker, M.M.; Aro, P.; Ronkainen, J.; Storskrubb, T.; Hindley, L.A.; Harmsen, W.S.; Zinsmeister, A.R.; Agréus, L. Non-ulcer dyspepsia and duodenal eosinophilia: An adult endoscopic population-based case-control study. Clin. Gastroenterol. Hepatol. 2007, 5, 1175–1183. [Google Scholar] [CrossRef]
- Walker, M.M.; Aggarwal, K.R.; Shim, L.S.; Bassan, M.; Kalantar, J.S.; Weltman, M.D.; Jones, M.; Powell, N.; Talley, N.J. Duodenal eosinophilia and early satiety in functional dyspepsia: Confirmation of a positive association in an Australian cohort. J. Gastroenterol. Hepatol. 2014, 29, 474–479. [Google Scholar] [CrossRef]
- Carroccio, A.; Mansueto, P.; Iacono, G.; Soresi, M.; D’alcamo, A.; Cavataio, F.; Brusca, I.; Florena, A.M.; Ambrosiano, G.; Seidita, A.; et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: Exploring a new clinical entity. Am. J. Gastroenterol. 2012, 107, 1898–1906. [Google Scholar] [CrossRef] [Green Version]
- Vanheel, H.; Vicario, M.; Vanuytsel, T.; Van Oudenhove, L.; Martinez, C.; Keita, Å.V.; Pardon, N.; Santos, J.; Söderholm, J.D.; Tack, J.; et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut 2014, 63, 262–271. [Google Scholar] [CrossRef]
- Cirillo, C.; Bessissow, T.; Desmet, A.S.; Vanheel, H.; Tack, J.; Berghe, P.V. Evidence for neuronal and structural changes in submucous ganglia of patients with functional dyspepsia. Am. J. Gastroenterol. 2015, 110, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J. What causes functional gastrointestinal disorders? A proposed disease model. Am. J. Gastroenterol. 2020, 115, 41–48. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328. [Google Scholar] [CrossRef]
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef]
- Potter, M.D.E.; Walker, M.M.; Talley, N.J. Non-coeliac gluten or wheat sensitivity: Emerging disease or misdiagnosis? Med. J. Aust. 2017, 207, 211–215. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Rüssel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology 2017, 152, 1100–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volta, U.; Bardella, M.T.; Calabrò, A.; Troncone, R.; Corazza, G.R. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef] [Green Version]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; De Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E. Fructan, Rather than Gluten, Induces Symptoms in Patients with Self-reported Non-celiac Gluten Sensitivity. Gastroenterology 2017, 154, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Moshfegh, A.J.; Friday, J.E.; Goldman, J.P.; Ahuja, J.K. Presence of inulin and oligofructose in the diets of Americans. J. Nutr. 1999, 129, 1407S–1411S. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Gibson, P.R. Fructose malabsorption and symptoms of irritable bowel syndrome: Guidelines for effective dietary management. J. Am. Diet. Assoc. 2006, 106, 1631–1639. [Google Scholar] [CrossRef]
- Whitehead, W.E.; Palsson, O.; Thiwan, S.M.; Talley, N.J.; Chey, W.; Irvine, E.J.; Drossman, D.A.; Thompson, W.G.; Walker, L. Development and Validation of the Rome III Diagnostic Questionnaire. Rome III: The Functional Gastrointestinal Disorders, 3rd ed.; Degnon Associates, Inc.: McLean, VA, USA, 2006. [Google Scholar]
- Barrett, J.S.; Gibson, P.R. Development and validation of a comprehensive semi-quantitative food frequency questionnaire that includes FODMAP intake and glycemic index. J. Am. Diet. Assoc. 2010, 110, 1469–1476. [Google Scholar] [CrossRef]
- Talley, N.J.; Haque, M.; Wyeth, J.W.; Stace, N.H.; Tytgat, G.N.; Stanghellini, V.; Holtmann, G.; Verlinden, M.; Jones, M. Development of a new dyspepsia impact scale: The Nepean Dyspepsia Index. Aliment Pharm. Ther. 1999, 13, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Bianchi, P.I.; Marchese, A.; Trotta, L.; Vattiato, C.; Balduzzi, D.; Brusco, G.; Andrealli, A.; Cisaro, F.; Astegiano, M.; et al. A score that verifies adherence to a gluten-free diet: A cross-sectional, multicentre validation in real clinical life. Br. J. Nutr. 2012, 108, 1884–1888. [Google Scholar] [CrossRef] [PubMed]
- Assor, E.; Davies-Shaw, J.; Marcon, A.; Mahmud, H. Estimation of dietary gluten content using total protein in relation to gold standard testing in a variety of foods. J. Nutr. Food Sci. 2014, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- O’keeffe, M.; Jansen, C.; Martin, L.; Williams, M.; Seamark, L.; Staudacher, H.M.; Irving, P.M.; Whelan, K.; Lomer, M.C. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elli, L.; Tomba, C.; Branchi, F.; Roncoroni, L.; Lombardo, V.; Bardella, M.T.; Ferretti, F.; Conte, D.; Valiante, F.; Fini, L.; et al. Evidence for the Presence of Non-Celiac Gluten Sensitivity in Patients with Functional Gastrointestinal Symptoms: Results from a Multicenter Randomized Double-Blind Placebo-Controlled Gluten Challenge. Nutrients 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potter, M.D.E.; Duncanson, K.; Jones, M.P.; Walker, M.M.; Keely, S.; Talley, N.J. Wheat Sensitivity and Functional Dyspepsia: A Pilot, Double-Blind, Randomized, Placebo-Controlled Dietary Crossover Trial with Novel Challenge Protocol. Nutrients 2020, 12, 1947. https://doi.org/10.3390/nu12071947
Potter MDE, Duncanson K, Jones MP, Walker MM, Keely S, Talley NJ. Wheat Sensitivity and Functional Dyspepsia: A Pilot, Double-Blind, Randomized, Placebo-Controlled Dietary Crossover Trial with Novel Challenge Protocol. Nutrients. 2020; 12(7):1947. https://doi.org/10.3390/nu12071947
Chicago/Turabian StylePotter, Michael D. E., Kerith Duncanson, Michael P. Jones, Marjorie M. Walker, Simon Keely, and Nicholas J. Talley. 2020. "Wheat Sensitivity and Functional Dyspepsia: A Pilot, Double-Blind, Randomized, Placebo-Controlled Dietary Crossover Trial with Novel Challenge Protocol" Nutrients 12, no. 7: 1947. https://doi.org/10.3390/nu12071947
APA StylePotter, M. D. E., Duncanson, K., Jones, M. P., Walker, M. M., Keely, S., & Talley, N. J. (2020). Wheat Sensitivity and Functional Dyspepsia: A Pilot, Double-Blind, Randomized, Placebo-Controlled Dietary Crossover Trial with Novel Challenge Protocol. Nutrients, 12(7), 1947. https://doi.org/10.3390/nu12071947






