The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)—A Critical Review
Abstract
:1. Introduction
2. Clinical Picture of Inflammatory Bowel Disease
3. The Key-Players in Inflammatory Bowel Disease (IBD)
3.1. Genetic Aspect
3.2. Environmental Aspect
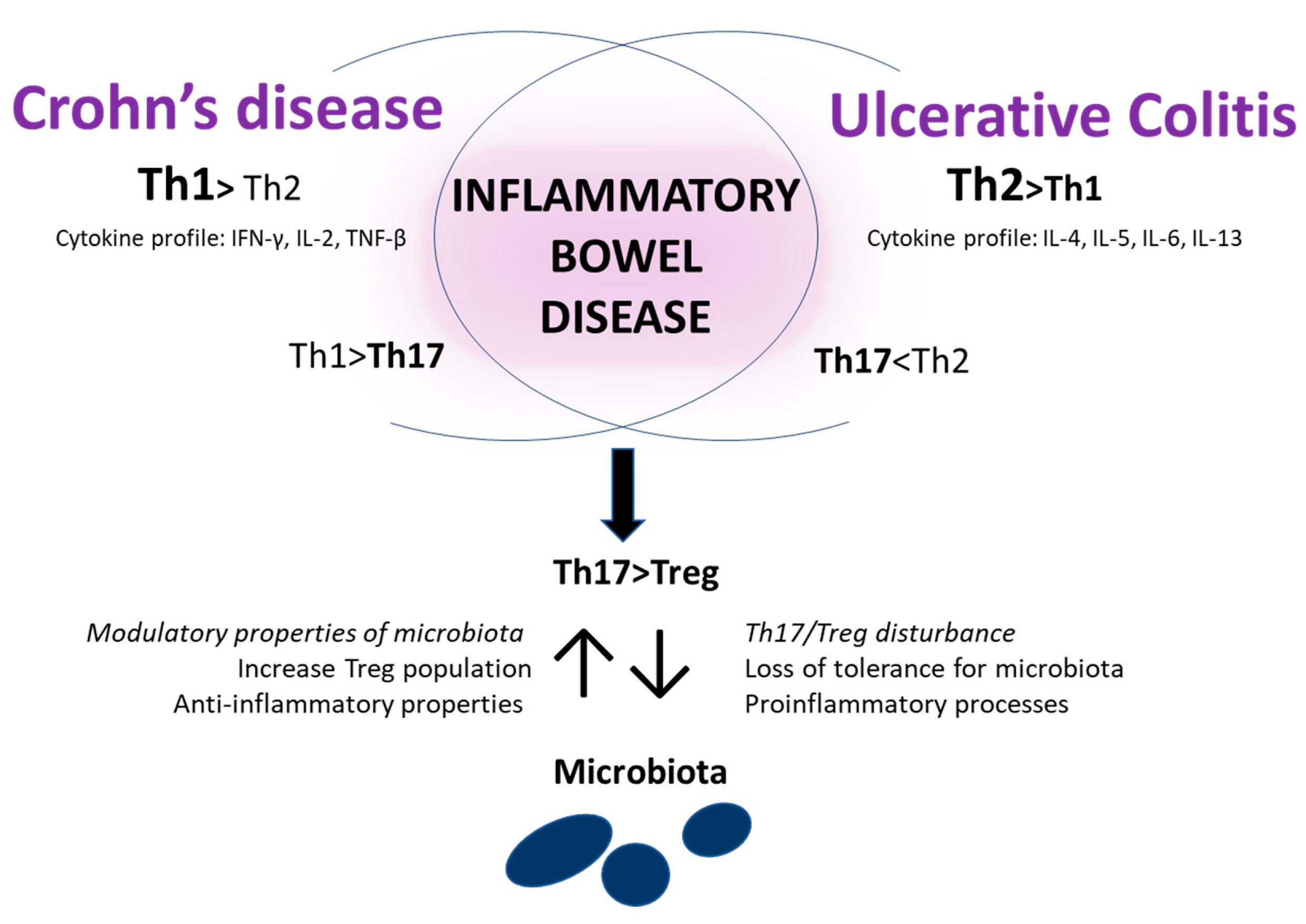
3.3. Immunological Aspects
3.4. The Gut Barrier and Microbiota
4. Probiotics. Treatment and Protection from IBD
4.1. Probiotic Effectiveness in Animal Model of Colitis
4.2. Clinical Study of Probiotic among IBD Patients
4.3. Probiotic Bacteria and IBD-Associated Cancer
4.4. The Effect of Components and Metabolites Produce by Probiotic Strains
4.5. Probiotic and Intestinal Epithelial Barrier Function
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lim, M.L.; Wallace, M.R. Infectious Diarrhea in History. Infect. Dis. Clin. North Am. 2004, 18, 261–274. [Google Scholar] [CrossRef]
- Mulder, D.J.; Noble, A.J.; Justinich, C.J.; Duffin, J.M. A Tale of Two Diseases: The History of Inflammatory Bowel Disease. J. Crohn’s Colitis. 2014, 8, 341–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirsner, J.B. Historical Origins of Current IBD Concepts. World J. Gastroenterol. 2001, 7, 175–184. [Google Scholar] [CrossRef] [PubMed]
- European Federation of Crohn’s & Ulcerative Colitis Associations. Available online: http://www.efcca.org (accessed on 1 April 2020).
- Centers of Disease Control and Prevention. Available online: https://www.cdc.gov (accessed on 1 April 2020).
- Crohn’s and Colitis Australia. Available online: https://www.crohnsandcolitis.com.au (accessed on 1 April 2020).
- Ng, S.C. Emerging Leadership Lecture: Inflammatory Bowel Disease in Asia: Emergence of a “Western” Disease. J. Gastroenterol. Hepatol. 2015, 30, 440–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, J.W.; Lai, H.C.; Chang, C.H.; Cheng, K.S.; Feng, C.L.; Chen, T.W. Epidemiology and Clinical Outcomes of Inflammatory Bowel Disease: A Hospital-Based Study in Central Taiwan. Gastroenterol. Res. Pract. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.R.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.F.; Gasche, C.; Geboes, K.; et al. Toward an Integrated Clinical, Molecular and Serological Classification of Inflammatory Bowel Disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005, 19, 5–36. [Google Scholar] [CrossRef]
- Levine, A.; Griffiths, A.; Markowitz, J.; Wilson, D.C.; Turner, D.; Russell, R.K.; Fell, J.; Ruemmele, F.M.; Walters, T.; Sherlock, M.; et al. Pediatric Modification of the Montreal Classification for Inflammatory Bowel Disease: The Paris Classification. Inflamm. Bowel Dis. 2011, 17, 1314–1321. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative Colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef] [Green Version]
- Bernett, C.N.; Krishnamurthy, K. Cutaneous Crohn Disease; StatPearls Publishing LLC, Tampa/St. Petersburg, Florida: 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470311/ (accessed on 1 April 2020).
- Ji, X.Q.; Wang, L.X.; Lu, D.G. Pulmonary Manifestations of Inflammatory Bowel Disease. World J. Gastroenterol. 2014, 20, 13501–13511. [Google Scholar] [CrossRef]
- Arvikar, S.L.; Fisher, M.C. Inflammatory Bowel Disease Associated Arthropathy. Curr. Rev. Musculoskelet. Med. 2011, 4, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Song, H.J.; Jeong, J.H.; Kim, H.U.; Boo, S.J.; Na, S.Y. Ophthalmologic Manifestations in Patients with Inflammatory Bowel Disease. Intest. Res. 2017, 15, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isene, R.; Bernklev, T.; Høie, O.L.E.; Munkholm, P.I.A.; Tsianos, E.; Stockbrügger, R.; Odes, S.; Palm, Ø.; Småstuen, M.; Moum, B. Extraintestinal Manifestations in Crohn’s Disease and Ulcerative Colitis: Results from a Prospective, Population-Based European Inception Cohort. Scand. J. Gastroenterol. 2014, 50, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Veloso, F.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Do They Influence Treatment and Outcome? World J. Gastroenterol. 2011, 17, 2702–2707. [Google Scholar] [CrossRef] [PubMed]
- Vide, J.; Osório, F.; Costa-Silva, M.; Lopes, S.; Azevedo, F.; Dias, C.C.; Magina, S.; Magro, F. Cutaneous Morbidity among Inflammatory Bowel Disease Patients: A Cohort Study. J. Crohn’s Colitis 2018, 12, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-Microbe Interactions Have Shaped the Genetic Architecture of Inflammatory Bowel Disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.Z.; Van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. Association Analyses Identify 38 Susceptibility Loci for Inflammatory Bowel Disease and Highlight Shared Genetic Risk across Populations. Nat. Genet. 2015, 47, 979–986. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; De Silva, P.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. Long-Term Intake of Dietary Fat and Risk of Ulcerative Colitis and Crohn’s Disease. Gut 2014, 63, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Niewiadomski, O.; Studd, C.; Wilson, J.; Williams, J.; Hair, C.; Knight, R.; Prewett, E.; Dabkowski, P.; Alexander, S.; Allen, B.; et al. Influence of Food and Lifestyle on the Risk of Developing Inflammatory Bowel Disease. Intern. Med. J. 2016, 46, 669–676. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Song, M.; Higuchi, L.M.; Richter, J.M.; Nimptsch, K.; Wu, K.; Chan, A.T. High School Diet and Risk of Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2015, 21, 2311–2319. [Google Scholar] [CrossRef] [Green Version]
- Racine, A.; Carbonnel, F.; Chan, S.S.M.; Hart, A.R.; Bas Bueno-De-Mesquita, H.; Oldenburg, B.; Van Schaik, F.D.M.; Tjønneland, A.; Olsen, A.; Dahm, C.C.; et al. Dietary Patterns and Risk of Inflammatory Bowel Disease in Europe: Results from the EPIC Study. Inflamm. Bowel Dis. 2016, 22, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Maconi, G.; Ardizzone, S.; Cucino, C.; Bezzio, C.; Russo, A.G.; Porro, G.B. Pre-Illness Changes in Dietary Habits and Diet as a Risk Factor for Inflammatory Bowel Disease: A Case-Control Study. World J. Gastroenterol. 2010, 16, 4297–4304. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.M.; Hernandez, V.; Bernigau, W.; Boeing, H.; Chan, S.S.M.; Luben, R.; Khaw, K.T.; Van Schaik, F.; Oldenburg, B.; Bueno-De-Mesquita, B.; et al. No Association of Alcohol Use and the Risk of Ulcerative Colitis or Crohn’s Disease: Data from a European Prospective Cohort Study (EPIC). Eur. J. Clin. Nutr. 2017, 71, 512–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuvlin, J.A.; Raza, S.S.; Bracamonte, S.; Julian, C.; Hanauer, S.B.; Nicolae, D.L.; King, A.C.; Cho, J.H. Smoking and Inflammatory Bowel Disease: Trends in Familial and Sporadic Cohorts. Inflamm. Bowel Dis. 2007, 13, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Bonner, G.F.; Fakhri, A.; Vennamanemi, S.R. A Long-Term Cohort Study of Nonsteroidal Anti-Inflammatory Drug Use and Disease Activity in Outpatients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2004, 10, 751–757. [Google Scholar] [CrossRef]
- Cornish, J.A.; Tan, E.; Simillis, C.; Clark, S.K.; Teare, J.; Tekkis, P.P. The Risk of Oral Contraceptives in the Etiology of Inflammatory Bowel Disease: A Meta-Analysis. Am. J. Gastroenterol. 2008, 103, 2394–2400. [Google Scholar] [CrossRef]
- Takeuchi, K.; Smale, S.; Premchand, P.; Maiden, L.; Sherwood, R.; Thjodleifsson, B.; Bjornsson, E.; Bjarnason, I. Prevalence and Mechanism of Nonsteroidal Anti-Inflammatory Drug-Induced Clinical Relapse in Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2006, 4, 196–202. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; McGinley, E.L.; Binion, D.G.; Saeian, K. Ambient Air Pollution Correlates with Hospitalizations for Inflammatory Bowel Disease: An Ecologic Analysis. Inflamm. Bowel Dis. 2011, 17, 1138–1145. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Hubbard, J.; Korzenik, J.; Sands, B.E.; Panaccione, R.; Ghosh, S.; Wheeler, A.J.; Villeneuve, P.J. The Inflammatory Bowel Diseases and Ambient Air Pollution: A Novel Association. Am. J. Gastroenterol. 2010, 105, 2412–2419. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, M.K.; Pratap, C.B.; Dixit, V.K.; Singh, T.B.; Shukla, S.K.; Jain, A.K.; Nath, G. Ulcerative Colitis and Its Association with Salmonella Species. Interdiscip. Perspect. Infect. Dis. 2016, 2016, 5854285. [Google Scholar] [CrossRef] [Green Version]
- Jodorkovsky, D.; Young, Y.; Abreu, M.T. Clinical Outcomes of Patients with Ulcerative Colitis and Co-Existing Clostridium Difficile Infection. Dig. Dis. Sci. 2010, 55, 415–420. [Google Scholar] [CrossRef]
- Rodemann, J.F.; Dubberke, E.R.; Reske, K.A.; Seo, D.H.; Stone, C.D. Incidence of Clostridium Difficile Infection in Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2007, 5, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; McGinley, E.L.; Binion, D.G. Excess Hospitalisation Burden Associated with Clostridium Difficile in Patients with Inflammatory Bowel Disease. Gut 2008, 57, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, J.V.; Elliott, D.E. Helminths and the IBD Hygiene Hypothesis. Inflamm. Bowel Dis. 2009, 15, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, D.; Bowcutt, R.; Lee, S.C.; Tang, M.S.; Kurtz, Z.D.; Ding, Y.; Honda, K.; Gause, W.C.; Blaser, M.J.; Bonneau, R.A.; et al. Helminth Infection Promotes Colonization Resistance via Type 2 Immunity. Science 2016, 352, 608–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walk, S.T.; Blum, A.M.; Ewing, S.A.S.; Weinstock, J.V.; Young, V.B. Alteration of the Murine Gut Microbiota during Infection with the Parasitic Helminth Heligmosomoides Polygyrus. Inflamm. Bowel Dis. 2010, 16, 1841–1849. [Google Scholar] [CrossRef]
- Weinstock, J.V.; Elliott, D.E. Translatability of Helminth Therapy in Inflammatory Bowel Diseases. Int. J. Parasitol. 2013, 43, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Summers, R.W.; Elliot, D.E.; Urban, J.F.; Thompson, R.; Weinstock, J.V. Trichuris Suis Therapy in Crohn’s Disease. Gut 2005, 54, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ueno, A.; Gasia, M.F.; Luider, J.; Wang, T.; Hirota, C.; Jijon, H.B.; Deane, M.; Tom, M.; Chan, R.; et al. Profiles of Lamina Propria T Helper Cell Subsets Discriminate between Ulcerative Colitis and Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 1779–1792. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Von Martels, J.Z.H.; De Vos, P.; Faber, K.N.; Dijkstra, G. Increased Fecal Calprotectin Levels in Crohn’s Disease Correlate with Elevated Serum Th1- and Th17-Associated Cytokines. PLoS ONE 2018, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, Y.; Bamba, T.; Fujiyama, Y. Increased Expression of Interleukin 17 in Inflammatory Bowel Disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef]
- Kobayashi, T.; Okamoto, S.; Hisamatsu, T.; Kamada, N.; Chinen, H.; Saito, R.; Kitazume, M.T.; Nakazawa, A.; Sugita, A.; Koganei, K.; et al. IL23 Differentially Regulates the Th1/Th17 Balance in Ulcerative Colitis and Crohn’s Disease. Gut 2008, 57, 1682–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 Cells in Human Disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Andoh, A.; Araki, Y.; Bamba, T.; Fujiyama, Y. Neutralization of Interleukin-17 Aggravates Dextran Sulfate Sodium-Induced Colitis in Mice. Clin. Immunol. 2004, 110, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.O.; Chang, S.H.; Park, H.; Nurieva, R.; Shah, B.; Acero, L.; Wang, Y.H.; Schluns, K.S.; Broaddus, R.R.; Zhu, Z.; et al. Regulation of Inflammatory Responses by IL-17F. J. Exp. Med. 2008, 205, 1063–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Kitani, A.; Fuss, I.; Strober, W. Cutting Edge: Regulatory T Cells Induce CD4 + CD25 − Foxp3 − T Cells or Are Self-Induced to Become Th17 Cells in the Absence of Exogenous TGF-β. J. Immunol. 2007, 178, 6725–6729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mucida, D.; Park, Y.; Kim, G.; Turovskaya, O.; Scott, I.; Kronenberg, M.; Cheroutre, H. Reciprocal TH17 and Regulatory T Cell Differentiation Mediated by Retinoic Acid. Science 2007, 317, 256–260. [Google Scholar] [CrossRef] [Green Version]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Eastaff-Leung, N.; Mabarrack, N.; Barbour, A.; Cummins, A.; Barry, S. Foxp3+ Regulatory T Cells, Th17 Effector Cells, and Cytokine Environment in Inflammatory Bowel Disease. J. Clin. Immunol. 2010, 30, 80–89. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A Microbial Symbiosis Factor Prevents Intestinal Inflammatory Disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef] [Green Version]
- Thibault, R.; Blachier, F.; Darcy-Vrillon, B.; De Coppet, P.; Bourreille, A.; Segain, J.P. Butyrate Utilization by the Colonic Mucosa in Inflammatory Bowel Diseases: A Transport Deficiency. Inflamm. Bowel Dis. 2010, 16, 684–695. [Google Scholar] [CrossRef]
- Leung, E.; Hong, J.; Fraser, A.G.; Merriman, T.R.; Vishnu, P.; Abbott, W.G.H.; Krissansen, G.W. Polymorphisms of CARD15/NOD2 and CD14 Genes in New Zealand Crohn’s Disease Patients. Immunol. Cell Biol. 2005, 83, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.; Karádi, O.; Rumi, G.; Rumi, G.J.; Pár, A.; Mózsik, G.; Czirják, L.; Süto, G. Crohn’s Disease Is Associated with Polymorphism of CARD15/NOD2 Gene in a Hungarian Population. Ann. N. Y. Acad. Sci. 2005, 1051, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Nelson, L.; Franke, A.; Poritz, L.; Li, T.Y.; Wu, R.; Wang, Y.; MacNeill, C.; Thomas, N.J.; Schreiber, S.; et al. OCTN1 Variant L503F Is Associated with Familial and Sporadic Inflammatory Bowel Disease. J. Crohn’s Colitis 2010, 4, 132–138. [Google Scholar] [CrossRef] [Green Version]
- McCole, D.F. IBD Candidate Genes and Intestinal Barrier Regulation. Inflamm. Bowel Dis. 2014, 20, 1829–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzanillo, P.; Mouchess, M.; Ota, N.; Dai, B.; Ichikawa, R.; Wuster, A.; Haley, B.; Alvarado, G.; Kwon, Y.; Caothien, R.; et al. Inflammatory Bowel Disease Susceptibility Gene C1ORF106 Regulates Intestinal Epithelial Permeability. ImmunoHorizons 2018, 2, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Kevans, D.; Turpin, W.; Madsen, K.; Meddings, J.; Shestopaloff, K.; Xu, W.; Moreno-Hagelsieb, G.; Griffiths, A.; Silverberg, M.S.; Paterson, A.; et al. Determinants of Intestinal Permeability in Healthy First-Degree Relatives of Individuals with Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 879–887. [Google Scholar] [CrossRef]
- Turpin, W.; Espin-Garcia, O.; Bedrani, L.; Madsen, K.; Meddings, J.B.; Raygoza Garay, J.A.; Silverberg, M.S.; Smith, M.I.; Griffiths, A.M.; Moayyedi, P.; et al. Analysis of Genetic Association of Intestinal Permeability in Healthy First-Degree Relatives of Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2019, 25, 1796–1804. [Google Scholar] [CrossRef]
- Schreiner, P.; Neurath, M.F.; Ng, S.C.; El-Omar, E.M.; Sharara, A.I.; Kobayashi, T.; Hisamatsu, T.; Hibi, T.; Rogler, G. Mechanism-Based Treatment Strategies for IBD: Cytokines, Cell Adhesion Molecules, JAK Inhibitors, Gut Flora, and More. Inflamm. Intest. Dis. 2019, 4, 79–96. [Google Scholar] [CrossRef]
- Velikova, T.; Kyurkchiew, D.; Ivanova-Todorova, E.; Spassova, Z.; Stanilova, S.; Altankova, I. Cytokines in Inflamed Mucosa of IBD Patients. IntechOpen 2012, 4, 71–92. [Google Scholar] [CrossRef]
- Edelblum, K.L.; Turner, J.R. The Tight Junction in Inflammatory Disease: Communication Breakdown. Curr. Opin. Pharmacol. 2009, 9, 715–720. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, A.H.; Yakymenko, O.; Olivier, I.; Håkansson, F.; Postma, E.; Keita, Å.V.; Söderholm, J.D. Faecalibacterium Prausnitzii Supernatant Improves Intestinal Barrier Function in Mice DSS Colitis. Scand. J. Gastroenterol. 2013, 48, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Laval, L.; Martin, R.; Natividad, J.N.; Chain, F.; Miquel, S.; Desclée de Maredsous, C.; Capronnier, S.; Sokol, H.; Verdu, E.F.; Van Hylckama Vlieg, J.E.T.; et al. Lactobacillus Rhamnosus CNCM I-3690 and the Commensal Bacterium Faecalibacterium Prausnitzii A2-165 Exhibit Similar Protective Effects to Induced Barrier Hyper-Permeability in Mice. Gut Microbes 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ekmekciu, I.; Von Klitzing, E.; Fiebiger, U.; Neumann, C.; Bacher, P.; Scheffold, A.; Bereswill, S.; Heimesaat, M.M. The Probiotic Compound VSL#3 Modulates Mucosal, Peripheral, and Systemic Immunity Following Murine Broad-Spectrum Antibiotic Treatment. Front. Cell. Infect. Microbiol. 2017, 7, 1–19. [Google Scholar] [CrossRef]
- Zoppi, G.; Cinquetti, M.; Benini, A.; Bonamini, E.; Minelli, E.B. Modulation of the Intestinal Ecosystem by Probiotics and Lactulose in Children during Treatment with Ceftriaxone. Curr. Ther. Res. Clin. Exp. 2001, 62, 418–435. [Google Scholar] [CrossRef]
- Wang, L.; Guo, M.J.; Gao, Q.; Yang, J.F.; Yang, L.; Pang, X.L.; Jiang, X.J. The Effects of Probiotics on Total Cholesterol. Medicine (United States) 2018, 97, e9679. [Google Scholar] [CrossRef]
- Reid, G.; Bruce, A.W.; Fraser, N.; Heinemann, C.; Owen, J.; Henning, B. Oral Probiotics Can Resolve Urogenital Infections. FEMS Immunol. Med. Microbiol. 2001, 30, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. Biomed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104, 1–14. [Google Scholar] [CrossRef]
- Loeuillard, E.; Bertrand, J.; Herranen, A.; Melchior, C.; Guérin, C.; Coëffier, M.; Aziz, M.; Déchelotte, P.; Savoye, G.; Marion-Letellier, R. 2,4,6-Trinitrobenzene Sulfonic Acid-Induced Chronic Colitis with Fibrosis and Modulation of TGF-Β1 Signaling. World J. Gastroenterol. 2014, 20, 18207–18215. [Google Scholar] [CrossRef]
- Alex, P.; Zachos, N.C.; Nguyen, T.; Gonzales, L.; Chen, T.E.; Conklin, L.S.; Centola, M.; Li, X. Distinct Cytokine Patterns Identified from Multiplex Profiles of Murine DSS and TNBS-Induced Colitis. Inflamm. Bowel Dis. 2009, 15, 341–352. [Google Scholar] [CrossRef]
- Javed, N.H.; Alsahly, M.B.; Khubchandani, J. Oral Feeding of Probiotic Bifidobacterium Infantis: Colonic Morphological Changes in Rat Model of TNBS-Induced Colitis. Scientifica (Cairo) 2016, 2016, 9572596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duranti, S.; Gaiani, F.; Mancabelli, L.; Milani, C.; Grandi, A.; Bolchi, A.; Santoni, A.; Lugli, G.A.; Ferrario, C.; Mangifesta, M.; et al. Elucidating the Gut Microbiome of Ulcerative Colitis: Bifidobacteria as Novel Microbial Biomarkers. FEMS Microbiol. Ecol. 2016, 92, fiw191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satish Kumar, C.S.V.; Kondal Reddy, K.; Boobalan, G.; Gopala Reddy, A.; Sudha Rani Chowdhary, C.H.; Vinoth, A.; Jayakanth, K.; Srinivasa Rao, G. Immunomodulatory Effects of Bifidobacterium Bifidum 231 on Trinitrobenzenesulfonic Acid-Induced Ulcerative Colitis in Rats. Res. Vet. Sci. 2017, 110, 40–46. [Google Scholar] [CrossRef]
- Kennedy, R.J.; Hoper, M.; Deodhar, K.; Kirk, S.J.; Gardiner, K.R. Probiotic Therapy Fails to Improve Gut Permeability in a Hapten Model of Colitis. Scand. J. Gastroenterol. 2000, 35, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.M.; Heo, W.; Cho, H.T.; Lee, D.H.; Kim, J.H.; Rhee, M.S.; Park, T.S.; Kim, Y.K.; Lee, J.H.; Kim, Y.J. Effects of Orally-Administered Bifidobacterium Animalis Subsp. Lactis Strain BB12 on Dextran Sodium Sulfate-Induced Colitis in Mice. J. Microbiol. Biotechnol. 2018, 28, 1800–1805. [Google Scholar] [CrossRef]
- Elian, S.D.A.; Souza, E.L.S.; Vieira, A.T.; Teixeira, M.M.; Arantes, R.M.E.; Nicoli, J.R.; Martins, F.S. Bifidobacterium Longum Subsp. Infantis BB-02 Attenuates Acute Murine Experimental Model of Inflammatory Bowel Disease. Benef. Microbes 2015, 6, 277–286. [Google Scholar] [CrossRef]
- Veiga, P.; Gallini, C.A.; Beal, C.; Michaud, M.; Delaney, M.L.; DuBois, A.; Khlebnikov, A.; Van Hylckama Vlieg, J.E.T.; Punit, S.; Glickman, J.N.; et al. Bifidobacterium Animalis Subsp. Lactis Fermented Milk Product Reduces Inflammation by Altering a Niche for Colitogenic Microbes. Proc. Natl. Acad. Sci. USA 2010, 107, 18132–18137. [Google Scholar] [CrossRef] [Green Version]
- Santos Rocha, C.; Lakhdari, O.; Blottière, H.M.; Blugeon, S.; Sokol, H.; Bermúdez-Humarán, L.G.; Azevedo, V.; Miyoshi, A.; Doré, J.; Langella, P.; et al. Anti-Inflammatory Properties of Dairy Lactobacilli. Inflamm. Bowel Dis. 2012, 18, 657–666. [Google Scholar] [CrossRef]
- Traina, G.; Proietti, P.C.; Menchetti, L.; Leonardi, L.; Tomasello, G.; Barbato, O.; Piro, F.; Brecchia, G. Colon microbial composition is correlated with the severity of colitis induced by 2,4,6-trinitrobenzenesulfonic acid in mice. EuroMediterr. Biomed. J. 2016, 11, 165–175. [Google Scholar] [CrossRef]
- Biagioli, M.; Laghi, L.; Carino, A.; Cipriani, S.; Distrutti, E.; Marchianò, S.; Parolin, C.; Scarpelli, P.; Vitali, B.; Fiorucci, S. Metabolic Variability of a Multispecies Probiotic Preparation Impacts on the Anti-Inflammatory Activity. Front. Pharmacol. 2017, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hrdý, J.; Alard, J.; Couturier-Maillard, A.; Boulard, O.; Boutillier, D.; Delacre, M.; Lapadatescu, C.; Cesaro, A.; Blanc, P.; Pot, B.; et al. Lactobacillus Reuteri 5454 and Bifidobacterium Lactis 5764 Improve Colitis While Differentially Impacting Dendritic Cells Maturation and Antimicrobial Responses. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia Vilela, E.; De Lourdes De Abreu Ferrari, M.; Oswaldo Da Gama Torres, H.; Guerra Pinto, A.; Carolina Carneiro Aguirre, A.; Paiva Martins, F.; Marcos Andrade Goulart, E.; Sales Da Cunha, A. Influence of Saccharomyces Boulardii on the Intestinal Permeability of Patients with Crohn’s Disease in Remission. Scand. J. Gastroenterol. 2008, 43, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Mizuno, S.; Umesaki, Y.; Ishii, Y.; Sugitani, M.; Imaoka, A.; Otsuka, M.; Hasunuma, O.; Kurihara, R.; Iwasaki, A.; et al. Randomized Placebo-Controlled Trial Assessing the Effect of Bifidobacteria-Fermented Milk on Active Ulcerative Colitis. Aliment. Pharmacol. Ther. 2004, 20, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Frič, P.; Pokrotnieks, J.; Lukáš, M.; Fixa, B.; Kaščák, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining Remission of Ulcerative Colitis with the Probiotic Escherichia Coli Nissle 1917 Is as Effective as with Standard Mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, S.K.; El-bedewy, M.M. Effect of Probiotics on Pro-Inflammatory Cytokines and NF- κ B Activation in Ulcerative Colitis. World J. Gastroenterol. 2010, 16, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M.M. Bifidobacterium Infantis 35624 Modulates Host Inflammatory Processes beyond the Gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, H.; Matsumoto, S.; Ohashi, Y.; Imaoka, A.; Setoyana, H.; Umesaki, Y.; Tanaka, R.; Otani, T. Beneficial Effects of Probiotic Bifidobacterium and Galacto-Oligosaccharide in Patients with Ulcerative Colitis: A Randomized Controlled Study. Digestion 2011, 84, 128–133. [Google Scholar] [CrossRef]
- Matsuoka, K.; Uemura, Y.; Kanai, T.; Kunisaki, R.; Suzuki, Y.; Yokoyama, K.; Yoshimura, N.; Hibi, T. Efficacy of Bifidobacterium Breve Fermented Milk in Maintaining Remission of Ulcerative Colitis. Dig. Dis. Sci. 2018, 63, 1910–1919. [Google Scholar] [CrossRef] [Green Version]
- Wildt, S.; Nordgaard, I.; Hansen, U.; Brockmann, E.; Rumessen, J.J. A Randomised Double-Blind Placebo-Controlled Trial with Lactobacillus Acidophilus La-5 and Bifidobacterium Animalis Subsp. Lactis BB-12 for Maintenance of Remission in Ulcerative Colitis. J. Crohn’s Colitis 2011, 5, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Tamaki, H.; Nakase, H.; Inoue, S.; Kawanami, C.; Itani, T.; Ohana, M.; Kusaka, T.; Uose, S.; Hisatsune, H.; Tojo, M.; et al. Efficacy of Probiotic Treatment with Bifidobacterium Longum 536 for Induction of Remission in Active Ulcerative Colitis: A Randomized, Double-Blinded, Placebo-Controlled Multicenter Trial. Dig. Endosc. 2016, 28, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, V.D.; Romeo, M.; Gammazza, A.M.; Carini, F.; Damiani, P.; Damiano, G.; Buscemi, S.; Lo Monte, A.I.; Gerges-Geagea, A.; Jurjus, A.; et al. The Long-Term Effects of Probiotics in the Therapy of Ulcerative Colitis: A Clinical Study. Biomed. Pap. 2016, 160, 372–377. [Google Scholar] [CrossRef]
- Wang, C.S.E.; Li, W.B.; Wang, H.Y.; Ma, Y.M.; Zhao, X.H.; Yang, H.; Qian, J.M.; Li, J.N. VSL#3 Can Prevent Ulcerative Colitis-Associated Carcinogenesis in Mice. World J. Gastroenterol. 2018, 24, 4254–4262. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Midha, V.; Makharia, G.K.; Ahuja, V.; Singal, D.; Goswami, P.; Tandon, R.K. The Probiotic Preparation, VSL#3 Induces Remission in Patients With Mild-to-Moderately Active Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2009, 7, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Brandimarte, G.; Papa, A.; Giglio, A.; Elisei, W.; Giorgetti, G.M.; Forti, G.; Morini, S.; Hassan, C.; Pistoia, M.A.; et al. Treatment of Relapsing Mild-to-Moderate Ulcerative Colitis with the Probiotic VSL#3 as Adjunctive to a Standard Pharmaceutical Treatment: A Double-Blind, Randomized, Placebo-Controlled Study. Am. J. Gastroenterol. 2010, 105, 2218–2227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballero-Franco, C.; Keller, K.; De Simone, C.; Chadee, K. The VSL#3 Probiotic Formula Induces Mucin Gene Expression and Secretion in Colonic Epithelial Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, 315–322. [Google Scholar] [CrossRef]
- Huynh, H.Q.; DeBruyn, J.; Guan, L.; Diaz, H.; Li, M.; Girgis, S.; Turner, J.; Fedorak, R.; Madsen, K. Probiotic Preparation VSL#3 Induces Remission in Children with Mild to Moderate Acute Ulcerative Colitis: A Pilot Study. Inflamm. Bowel Dis. 2009, 15, 760–768. [Google Scholar] [CrossRef]
- Fedorak, R.N.; Feagan, B.G.; Hotte, N.; Leddin, D.; Dieleman, L.A.; Petrunia, D.M.; Enns, R.; Bitton, A.; Chiba, N.; Paré, P.; et al. The Probiotic VSL#3 Has Anti-Inflammatory Effects and Could Reduce Endoscopic Recurrence after Surgery for Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2015, 13, 928–935. [Google Scholar] [CrossRef]
- Kowalska-Duplaga, K.; Gosiewski, T.; Kapusta, P.; Sroka-Oleksiak, A.; Wędrychowicz, A.; Pieczarkowski, S.; Ludwig-Słomczyńska, A.H.; Wołkow, P.P.; Fyderek, K. Differences in the Intestinal Microbiome of Healthy Children and Patients with Newly Diagnosed Crohn’s Disease. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium Prausnitzii Is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [Green Version]
- Eaden, J.A.; Abrams, K.R.; Maynerry, J.F. The Risk of Colorectal Cancer in Ulcerative Colitis: A Meta-Analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [Green Version]
- Jess, T.; Gamborg, M.; Matzen, P.; Munkholm, P.; Sørensen, T.I.A. Increased Risk of Intestinal Cancer in Crohn’s Disease: A Meta-Analysis of Population-Based Cohort Studies. Am. J. Gastroenterol. 2005, 100, 2724–2729. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Beaugerie, L.; Maynadié, M.; Laharie, D.; Dupas, J.L.; Flourié, B.; Lerebours, E.; Peyrin-Biroulet, L.; Allez, M.; Simon, T.; et al. Excess Primary Intestinal Lymphoproliferative Disorders in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2012, 18, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Gulamhusein, A.F.; Eaton, J.E.; Tabibian, J.H.; Atkinson, E.J.; Juran, B.D.; Lazaridis, K.N. Duration of Inflammatory Bowel Disease Is Associated with Increased Risk of Cholangiocarcinoma in Patients with Primary Sclerosing Cholangitis and IBD. Am. J. Gastroenterol. 2016, 111, 705–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.W.; Kim, H.M.; Yang, K.M.; Kim, S.A.; Kim, S.K.; An, M.J.; Park, J.J.; Lee, S.K.; Kim, T.I.; Kim, W.H.; et al. Bifidobacterium Lactis Inhibits NF-ΚB in Intestinal Epithelial Cells and Prevents Acute Colitis and Colitis-Associated Colon Cancer in Mice. Inflamm. Bowel Dis. 2010, 16, 1514–1525. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Viladomiu, M.; Pedragosa, M.; De Simone, C.; Hontecillas, R. Immunoregulatory Mechanisms Underlying Prevention of Colitis-Associated Colorectal Cancer by Probiotic Bacteria. PLoS ONE 2012, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kahouli, I.; Malhotra, M.; Westfall, S.; Alaoui-Jamali, M.A.; Prakash, S. Design and Validation of an Orally Administrated Active L. Fermentum-L. Acidophilus Probiotic Formulation Using Colorectal Cancer Apc Min/+ Mouse Model. Appl. Microbiol. Biotechnol. 2017, 101, 1999–2019. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, C.B.; Cruz, M.L.; Isidro, A.A.; Arthur, J.C.; Jobin, C.; De Simone, C. Pretreatment with the Probiotic VSL#3 Delays Transition from Inflammation to Dysplasia in a Rat Model of Colitis-Associated Cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, 1004–1013. [Google Scholar] [CrossRef] [Green Version]
- Arthur, J.C.; Gharaibeh, R.Z.; Uronis, J.M.; Perez-Chanona, E.; Sha, W.; Tomkovich, S.; Mühlbauer, M.; Fodor, A.A.; Jobin, C. VSL#3 Probiotic Modifies Mucosal Microbial Composition but Does Not Reduce Colitis-Associated Colorectal Cancer. Sci. Rep. 2013, 3, 12–14. [Google Scholar] [CrossRef] [Green Version]
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A Four-Probiotics Regimen Reduces Postoperative Complications after Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World J. Surg. 2015, 39, 2776–2783. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgård, L.; Wettergren, Y. Intestinal Microbiota Is Altered in Patients with Colon Cancer and Modified by Probiotic Intervention. BMJ Open Gastroenterol. 2017, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Martignoni, M.; Groothuis, G.M.M.; De Kanter, R. Species Differences between Mouse, Rat, Dog, Monkey and Human CYP-Mediated Drug Metabolism, Inhibition and Induction. Expert Opin. Drug Metab. Toxicol. 2006, 2, 875–894. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, S.M.; Mariadassou, M.; Nicolas, P.; Parayre, S.; Le Guellec, R.; Chuat, V.; Peton, V.; Le Maréchal, C.; Burati, J.; Loux, V.; et al. Identification of Proteins Involved in the Anti-Inflammatory Properties of Propionibacterium Freudenreichii by Means of a Multi-Strain Study. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.R.; Cui, Y.J.; Liu, J.X.; Luo, X.; Wang, H.F. Lactobacillus Rhamnosus GG Components SLP, GDNA and CpG Exert Protective Effects on Mouse Macrophages upon Lipopolysaccharide Challenge. Lett. Appl. Microbiol. 2020, 70, 118–127. [Google Scholar] [CrossRef] [PubMed]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evidence Based Complement. Altern. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, X.; Zhu, Y.; Ma, J.; Ma, H.; Zhang, H. Probiotic Mixture Protects Dextran Sulfate Sodium-Induced Colitis by Altering Tight Junction Protein Expressions and Increasing Tregs. Mediators Inflamm. 2018, 2018, 9416391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meini, S.; Laureano, R.; Fani, L.; Tascini, C.; Galano, A.; Antonelli, A.; Rossolini, G.M. Breakthrough Lactobacillus Rhamnosus GG Bacteremia Associated with Probiotic Use in an Adult Patient with Severe Active Ulcerative Colitis: Case Report and Review of the Literature. Infection 2015, 43, 777–781. [Google Scholar] [CrossRef]
- Kumar, M.; Kissoon-Singh, V.; Coria, A.L.; Moreau, F.; Chadee, K. Probiotic Mixture VSL#3 Reduces Colonic Inflammation and Improves Intestinal Barrier Function in Muc2 Mucin-Deficient Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, 34–45. [Google Scholar] [CrossRef]
- Palumbo, P.; Lombardi, F.; Cifone, M.G.; Cinque, B. The Epithelial Barrier Model Shows That the Properties of VSL#3 Depend from Where It Is Manufactured. Endocrine Metab. Immune Disord. Drug Targets 2018, 19, 199–206. [Google Scholar] [CrossRef]
- Zhai, Q.; Tian, F.; Zhao, J.; Zhang, H.; Narbad, A.; Chen, W. Oral Administration of Probiotics Inhibits Absorption of the Heavy Metal Cadmium by Protecting the Intestinal Barrier. Appl. Environ. Microbiol. 2016, 82, 4429–4440. [Google Scholar] [CrossRef] [Green Version]
- Zakostelska, Z.; Kverka, M.; Klimesova, K.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Hornova, M.; Srutkova, D.; Hudcovic, T.; Ridl, J.; et al. Lysate of Probiotic Lactobacillus Casei DN-114 001 Ameliorates Colitis by Strengthening the Gut Barrier Function and Changing the Gut Microenvironment. PLoS ONE 2011, 6, e27961. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W.; et al. A Novel Postbiotic From Lactobacillus Rhamnosus GG With a Beneficial Effect on Intestinal Barrier Function. Frotneriers Microbiol. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, M.; Yan, X.; Weng, W.; Yang, Y.; Gao, R.; Liu, M.; Pan, C.; Zhu, Q.; Li, H.; Wei, Q.; et al. Micro Integral Membrane Protein (MIMP), a Newly Discovered Anti-Inflammatory Protein of Lactobacillus Plantarum, Enhances the Gut Barrier and Modulates Microbiota and Inflammatory Cytokines. Cell. Physiol. Biochem. 2018, 45, 474–490. [Google Scholar] [CrossRef] [PubMed]

| CD Classification | UC Classification | ||
|---|---|---|---|
| Age at diagnosis | A1:< 17 years | Severity | S0: remission, no symptoms |
| A2: 17–40 years | S1: mild symptoms | ||
| A3: > 40 years | S2: moderate symptoms | ||
| S3: severe symptoms | |||
| Location, endoscopic or macroscopic estimation | L1: terminal ileal | Extensity | E1: ulcerative proctitis |
| L2: colon | E2: left-sided UC; distal colitis | ||
| L3: ileocolon | E3: extensive UC, pancolitis | ||
| L4: upper GI modifier: proximal disease with distal disease, such as L1 + L4, L2 + L4, L3 + L4) | |||
| Behavior over time | B1: non-stricturing, non-penetrating | ||
| B2: stricturing | |||
| B3: penetrating | |||
| P: perianal disease modifiers, such as B1p, B2p, B3p | |||
| Strain | IBD | Research Model and Study Scheme | Results | Ref. |
|---|---|---|---|---|
| Bifidobacterium infantis (BabyLife, Solaray) | CD-like | Rat model (TNBS) 10 days supplementation with 0.205 g of B. infantis dissolved in 1.0 mL distilled water prior colitis. Colitis was induced by enema of 1.0 mL 5% (w/v) TNBS which lasted for 7 days. | Reduction in the symptoms, weaker damage to the mucosal architecture, protective function for mucus goblet cells and the epithelial cell layer; | [75] |
| Summary: a beneficial effect was observed | ||||
| Bifidobacterium bifidum PRL2010 | CD-like | Murine model (TNBS) 7 days oral supplementation of 109 of B. bifidum PRL 2010. Colitis (TNBS 2.5 mg/mice) was induced in the 5th day of probiotic strain feeding. | Reduction in the edema, reduction in the macroscopic damage and histological scores, reduction in weight-loss, anti-inflammatory effect; | [76] |
| Summary: a beneficial effect was observed | ||||
| Bifidobacterium bifidum 231 | CD-like | Rat model (TNBS) Colitis was induced by 31 mg/kg of TNBS. 14 days supplementation of 1.4 × 1011 CFU/rat/day B. bifidum (in saline) after colitis induction | Reduction in the edema, reduction in the macroscopic damage and histological scores, reduction in weight-loss, anti-inflammatory effect; | [77] |
| Summary: a beneficial effect was observed | ||||
| Lactobacillus plantarum species 299 | CD-like | Rat model (TBSN) Colitis was induced by 30 mg (0.6 mL of 5% aqueous solution) of TNBS. 7 days supplementation of 109 colony forming units (CFU) of Lactobacillus plantarum (in oat fiber) after colitis induction. | No beneficial effects on the rat’s gut permeability, weight changes, colon microscopic scores, and the level of blood albumins; | [78] |
| Summary: Lack of positive effect | ||||
| Bifidobacterium animalis subsp. lactis BB12 | UC-like | Murine model (DSS) 7 days supplementation (twice a day) with 1.2 × 1010 CFU Bifidobacterium animalis subsp. lactis BB12 by oral gavage prior to colitis. Colitis was induced by 3% DSS added to drinking water for 6 days. | Protection against a reduction in colon length, better picture of the colon histology, reduction in apoptosis in the epithelial layer, decrease in the level of TNF-α; | [79] |
| Summary: a beneficial effect was observed | ||||
| Bifidobacterium longum subsp. infantis BB-02 | UC-like | Murine model (DSS) 10 days supplementation (by oral gavage) once a day with 0.1 mL of a suspension containing 9.0 log10 CFU/mL in phosphate-buffered saline prior to colitis induction and continued during its development for next 7 days. Colitis was induced by DSS 3.5% (w/v) in drinking water ad libitum for 7 days. | Reduction in the clinical symptoms of the disease, protection of the colonic structure, reduction in edema; | [80] |
| Summary: a beneficial effect was observed | ||||
| Bifidobacterium lactis from fermented milk (Activia; Danone) | UC-like | Murine model (T-bet−/−Rag2−/−) 100 mg of diary product was orally instilled daily, and additional 100 mg per mouse in its cage was provided for consumption. | Reduction in severity of colitis and inflammation in an early stage, decrease in the level of Enterobacteriaceae (colitogenic) | [81] |
| Summary: a beneficial effect was observed | ||||
| Lactobacillus delbrueckii | UC-like | Murine model (DSS) Colitis was induced by the addition of 3% (w/v) DSS in the drinking water for 7 days. Administration of probiotic (5 × 109 bacteria/mouse/day started 1 day) before colitis induction and lasted until sacrifice. | Modulation of the NF-kB pathway, reduction in the inflammatory state; | [82] |
| Summary: a beneficial effect was observed | ||||
| VSL#3 (L. paracasei, L. plantarum, L. acidophilus, L. delbrueckii subspecies bulgaricus, B. longum, B. breve, and B. infantis, Streptococcus thermophilus) | UC-like | Murine model (DSS) 8 days supplementation (by oral gavage) with 0.1 mL of a suspension containing 5 × 1010 probiotic CFU/kg of body weight dissolved in saline solution after colitis induction. Colitis was induced by 5% DSS (w/v) in drinking water for 8 days. | Discrepancy in results (two different batches of VSL#3, contrary data); VSL#3 batch A: reduction in macroscopic scores, intestinal permeability, -reduction in expression of TNFα, IL-1β, IL-6 mRNAs, increase in the expression of TGFβ, IL-10, occludin, zonula occludens-1 (ZO-1) mRNAs, shift of colonic macrophages from a M1 to M2 phenotype- lack of effect in VSL#3 batch B | [84] |
| CD-like | Murine model (TBNS) Eight days supplementation (by oral gavage) with 0.1 mL of a suspension containing 5 × 1010 probiotic CFU/kg of body weight dissolved in saline solution after colitis induction. Colitis was induced by 1 mg of TNBS fasted for 12 h. | [84] | ||
| Summary: positive effect only one batch of the same product | ||||
| Bifidobacterium animalis spp. lactis Bl 5764 | CD-like | Murine model (TNBS) 5 days supplementation (by oral gavage) of a 5 × 108 CFU/day/mice prior to colitis induction and continued during its development for next 2 days. Colitis was induced by TNBS (110 mg/kg, dissolved in 0.9% NaCl/ethanol (50/50 v/v)). | Lower body weight-loss, better macroscopic indicators of inflammation (Wallace scores), histopathological analysis (Ameho score), and level of lipocalin-2, promotion in the bone marrow-derived dendritic cell maturation and IL-17A secretion | [85] |
| Lactobacillus reuteri Lr 5454 | CD-like | Murine model (TNBS) 5 days supplementation (by oral gavage) of a 5 × 108 CFU/day/mice prior to colitis induction and continued during its development for next 2 days. Colitis was induced by TNBS (110 mg/kg, dissolved in 0.9% NaCl/ethanol (50/50 v/v)). | Lower body weight-loss, better macroscopic indicators of inflammation (Wallace scores), histopathological analysis (Ameho score), and level of lipocalin-2; greater involvement in the development of tolerogenic DC, induce Tregs population and expression of Reg3b in a NOD2-independent manner; | [85] |
| Summary: a beneficial effect was observed | ||||
| Saccharomyces boulardii (Floratil®) | CD | Human model Patients with CD in remission (based on Crohn’s disease activity index) were supplemented with S. boulardii about 4 × 108 cells every 8 h as an oral capsule formulation during 3 months. | Help in maintaining remission and bowel sealing; | [86] |
| Summary: a beneficial effect was observed | ||||
| Bifidobacterium breve strain Yakult, Bifidobacterium bifidum strain Yakult, Lactobacillus acidophilus strain Yakult (Yakult Co. Ltd. Japan) | UC | Human model Patients with mild to moderate active UC were supplemented with 100 mL/day (10 billion cells) of bifidobacterial-fermented milk for 3 months. | Promising usefulness in sustaining the remission phase, improvement in clinical activity index score and histological scores; | [87] |
| Summary: a beneficial effect was observed | ||||
| Escherichia coli Nissle1917 (Mutaflor 100 mg; Ardeypharm GmbH, Herdecke, Germany) | UC | Human model Randomized, double blind, double dummy trial, patients with UC remission were supplemented with 2.5–25 × 109 viable bacteria for 12 months. | Promising behavior in sustaining the remission phase, prevention from inflammatory state; | [88] |
| Summary: the beneficial effect was demonstrated | ||||
| Lactobacillus fermentum, Lactobacillus delbruekii. (Lacteol Fort; Rameda, Egypt) | UC | Human model Patients with mild to moderate UC assessed by Mayo score were supplemented with 10 billion CFU of probiotic cells (powder to dissolve in 50 mL fresh water) for 8 weeks. | Decrease in the IL-6 and TNF-alpha levels and lowering regulation of NF-kB; | [89] |
| Summary: a beneficial effect was observed | ||||
| Bifidobacterium infantis 35624 | UC | Human model Randomized, double-blind placebo-controlled studies, patients with mild to moderate active UC (based on a clinical activity index) were supplemented with 1 × 1010 CFU viable probiotic cells for 8 weeks. | Reduction in the levels CRP and TNF-α in both gastrointestinal and non-gastrointestinal inflammatory disorders, but did not particularly affect UC disease; | [90] |
| Summary: Lack of effect for UC group, positive effect for gastrointestinal, and non-gastrointestinal inflammatory disorders group | ||||
| Bifidobacterium breve strain Yakult | UC | Human model Patients with UC (active and inactive) were supplemented with 1 g of the freeze-dried powder containing probiotic (109 CFU/g) for 1 year. | Better endoscopic scores, decrease of MPO level, modulation of luminal environmental factors such as intestinal microflora and pH | [91] |
| Summary: a beneficial effect was observed | ||||
| Bifidobacterium breve strain Yakult, Lactobacillus acidophilus strain Yakult (Yakult fermented milk (Mil–Mil)) | UC | Human model Multileft, randomized, placebo-controlled, double-blind parallel-group study; patients with UC in remission were supplemented with 100 mL/day (10 billion cells of Bifidobacterium and 1 billion of cells of Lactobacillus) of fermented milk for 12 months. | No beneficial effect | [92] |
| Summary: no beneficial effect was observed, the study was discontinued | ||||
| Lactobacillus acidophilus strain LA-5 and Bifidobacterium animalis subsp. lactis BB12 (Probio-Tec AB25) | UC | Human model Randomized, double blind, placebo-controlled study; patients with UC in remission were supplemented with 1.5 × 1011 CFU daily (2 capsules 3 times daily) for 52 weeks. | Maintaining remission in colitis | [93] |
| Summary: a beneficial effect was observed | ||||
| Bifidobacterium longum 536 (Morinaga Milk Industry Co. Ltd, Tokyo, Japan) | UC | Human model A randomized, double-blinded, placebo-controlled multileft trial study; patients with mild to moderate UC (based on disease activity index) were supplemented with 2–3 × 1011 freeze-dried viable probiotic capsule 3 times daily for 8 weeks. | Decrease in the disease activity index and downscale the rectal bleeding, clinical remission; | [94] |
| Summary: a beneficial effect was observed | ||||
| Lactobacillus salivarius, Lactobacillus acidophilus, Bifidobacterium bifidum strain BGN4; Acronelle®, Bromatech SRL, Milan, Italy) | UC | Human model Patients with moderate to severe UC (based on disease activity index) were supplemented with probiotic blend for 24 months. | Reduction in recovery time, weaker activity of the disease, better endoscopic picture; | [95] |
| Summary: a beneficial effect was observed | ||||
| VSL#3 | UC | Murine model UC associated carcinogenesis model was based on a single injection of 12.5 mg/kg body weight azoxymethane intraperitoneally, 1 week later 2.5% DSS was added to drinking water for 5 days, followed by 10 weeks and 2 days of regular drinking water. Probiotic mixture (1.5 × 109 CFU/mice) was supplemented alone or together with mesalazine. | Decrease in the level of TNF-α and IL-6, reduction of number of pathogenic microbiota, increase in the population of Bifidobacterium and other non-pathogenic species in the intestinal mucosa; | [96] |
| Summary: preventive effect for UC associated carcinogenesis | ||||
| VSL#3 | UC | Human model Patients with mild to moderate, active UC (based on Activity Index) were supplemented with the probiotic mixture twice daily for 12 weeks. | Improvement in rectal bleeding and stool frequency, mucosal appearance and overall physician’s evaluation; | [97] |
| Summary: a beneficial effect was observed | ||||
| VSL#3 | UC | Human model A multileft, double-blind, randomized, placebo-controlled, parallel study, patients with mild to moderately active UC (based on Activity Index) were supplemented with the probiotic mixture for 8 weeks in addition to standard therapy. | Reduction in UCDAI scores and frequency of rectal bleeding; - no differences in parameters such as the physician’s rate of disease activity, or endoscopic scores; | [98] |
| Summary: a beneficial effect was observed | ||||
| VSL#3 | UC | Human model, children population Patients with mild to moderately active UC (based on activity index) were supplemented with the probiotic twice daily for 8 weeks with a dose of probiotic based on their age (from one-half sachet to two and one-half). | Remission in colitis, improvement in microbiota composition, decrease in level of IFN-γ, TNF-α, CRP, ESR; | [100] |
| Summary: a beneficial effect was observed | ||||
| Faecalibacterium prausnitzii | CD n.a | Murine model (TNBS) A double-blind controlled trial study, 5 days before colitis induction mice were supplemented with 109–1010 CFU bacterial suspension or bacterial medium. Colitis was induced by TNBS (100 mg/kg body weight), which was administrated intrarectally. The observation took 20 days.Cell line CaCo-2 | Anti-inflammatory effect, blocking of NF-kB pathway and IL-8 production, anti-inflammatory effect; | [103] |
| Summary: a beneficial effect was observed | ||||
| Bifidobacterium lactis | Cancer model | Murine model 6 days of supplementation with probiotic (in different dose) prior to colitis. An acute colitis was induced by 3.5% DSS in drinking water for 7 days. Colitis associated cancer was induced by azoxymethane (10 mg/kg) prior to 5 days DSS challenge. Cell line model (HT-29) Cell line HT-29 was incubated with the different concentration of Bifidobacterium lactis. | Inhibition of NF-kB and NF-kB-regulated genes in epithelial cells and prevention meaning for the acute colitis and cancer model, reduction in number and size of the tumors; | [108] |
| Summary: a beneficial effect was observed | ||||
| Lactobacillus acidophilus Lactobacillus fermentum | Cancer model | Murine cancer model 12 weeks supplementation with probiotic (0.5 × 1010 CFU of each strain)among ApcMin/+ mice. Cell line model (CaCo-2) Bacteria (alone and as a mixture) prepared in simulated artificial intestinal juice were incubated with Caco-2 (up to 72 h). | Reduction in cancer cells proliferation, increase in apoptosis level, protection of normal colon cell growth from toxic treatment; | [110] |
| Summary: a beneficial effect was observed | ||||
| VSL#3 | Cancer model | Murine cancer model (IL10−/−) Supplementation with 1.2 billion bacteria per mouse/day) or conjugated linoleic acid. Cancer was induced by azoxymethane, DSS (the first step) and a single dose of 5 × 107 CFU Helicobacter typhlonius by oral gavage (the second step cancer induction). Observation took 68 days. | Shorter recovery time, lower disease severity in an active phase of cancer; VSL#3 treatment- higher mRNA expression of TNF-α, increase in the angiostatin level and Tcells subpopulations; CLA treatment- decreased the level of COX-2; | [109] |
| Summary: a beneficial effect was observed | ||||
| VSL#3 | Cancer model | Murine cancer model/IL-10−/− Mice with IL-10 deficient were supplemented by VSL# from the day of cancer induction for 17 weeks (once a day without the weekends). Cell line model (CaCo-2) Bacteria (alone and as a mixture) prepared in simulated artificial intestinal juice were incubated with Caco-2 (up to 72 h). | No protection against inflammatory processes and tumor development, increase in the tumor penetrance, worsening histologic dysplasia scores, extension of Clostridium population; | [112] |
| Summary: lack of beneficial effect, worsening of the neoplastic processes | ||||
| VSL#3 | Cancer model | Rat cancer model (TNBS) Supplementation with VSL#3 1 week before the colorectal cancer induction by TNBS. Then rats were supplemented with probiotic in drinking water until end of experiment (17 weeks). | Delay in the carcinoma growing processes, improvement in the histological picture of the colon, increase in the level of angiostatin vitamin D receptor (VDR); | [111] |
| Summary: a beneficial effect was observed | ||||
| Propionibacterium freudenreichii, Isolated: SlpB, SlpE, two proteins with SLH domains, HsdM3 | n.a | Proteomic and transcriptomic study | Potential connection with IL-10 increase and anti-inflammatory value | [116] |
| Summary: anti-inflammatory effect as a results of combination cytoplasmatic and surface protein | ||||
| Lactobacillus rhamnosus GG, isolated antigens: surface layer protein, genomic DNA and unmethylated cytosine-phosphate-guanine-containing oligodeoxynucleotides, alone or in combination. | n.a | Cell line RAW 264.7 Cell line was incubated with bacterial cells, components, or their combination (2 h), then was challenged with lipopolysaccharide (0.5 h). | Suppression in the inflammatory paths, inhibition of TLR, MAPK and NF-κB signaling pathways | [117] |
| Summary: anti-inflammatory properties were observed | ||||
| bacteria’s cells-free growth medium, Lactobacillus acidophilus, Lactobacillus casei, Lactococcus lactis, Lactobacillus reuteri, and Saccharomyces boulardii | n.a | Cell line HT-29 After LPS challenge (4 h), cell line was incubated with cell free bacterial medium (18 h.) | Anti-inflammatory properties, modulation of the level of IL-10, IL-1β, TNF-α, PGE-2, IL-8; | [118] |
| Summary: anti-inflammatory properties were observed | ||||
| Bifidobacterium, Lactobacillus acidophilus, Enterococcus (Bifico, Shanghai Sine Pharmaceutical) | CD-like | Murine model (IL-10-deficient, DSS) Colitis was induced by 4% DSS in drinking water by 14 days. Mice were supplemented with 3 × 107 CFU probiotic in all study groups (with colitis or not) for 14 days. | Increase in the number of Treg, decrease in the total number of T cells in the colon and the peripheral blood, positive influence on the tight junctions; | [119] |
| Summary: a beneficial effect was observed | ||||
| Lactobacillus rhamnosus GG. | UC | Case report A female patient affected by UC with severe active pancolonic involvement. | Symptoms of bacteremia; | [120] |
| Summary: probiotic strain as a cause of bacteremia | ||||
| VSL#3 (Seaford Pharmaceuticals) | n.a | Rat model 7 days intragastrical supplementation with 3 × 109 bacteria. | Influence on the mucus structure, stimulation of expression of the Muc2 gene, increase in secretion of the non-mucin glycoprotein, improvement in the permeability of the intestinal barrier; | [99] |
| Summary: a beneficial effect on secretion and mucus within epithelial barrier | ||||
| VSL#3 | UC-like | Murine model (Muc2−/− and Muc2+/+; DSS) 15 days of supplementation with VSL#3. Colitis among Muc2−/− group was induced by 1% DSS added to drinking water for 3 days. | Induction of mucus secretion in the crypts’ goblet cells in the colon, reduction in the wall thickness and MPO level - lack of protection from colitis severity | [121] |
| Summary: a beneficial effect on mucus secretion and oblet cells, lack of usage in colitis severity protection | ||||
| VSL#3 | n.a | Cell line Caco-2 Cell line monolayer incubated with 108 CFU/mL (4 h). | Discrepancy in results (two different manufacturers of VSL#3, contrary data) - Italy-made product: increased the permeability of the barrier, and decreased the ZO-1/occludin expression - US-made product: increased the occludin level, pretreatment with VSL#3 prevented the heat-induced epithelial barrier integrity loss | [122] |
| Summary: different usefulness of the same product but produced by two different manufacturers | ||||
| Lactobacillus casei DN-114 001, lysate | CD-like | Murine model (DSS) Mice were supplemented with 6 x 108 CFU of heat-killed bacteria, by gavage. The administration of bacteria was repeated every 7 days (4 doses together) prior to colitis. Colitis was induced 7 days later by 3% DSS dissolved in tap water for 7 days. | Protective behavior of probiotic only on the BALB/c mice, increase in the barrier function by the upregulation of ZO-1, increase in the amount of Treg, decrease in the level of proinflammatory factors, TNF-α, INF-γ, IL-10, influence on microbiota composition; | [124] |
| Summary: a beneficial effect was observed | ||||
| Lactobacillus rhamnosus GG, surface layer protein HM0539 | n.a | Cell line CaCo-2 Caco-2 cell line was incubated with HM0539 (50 ng/mL) for 12 h, then cell line was stimulated using TNF-a, (10 ng/mL) or lipopolysaccharide (1 mg/mL) for 6 h. | Increase in the level of the tight junction, increase in mucin secretion; | [125] |
| Summary: a beneficial effect was observed | ||||
| Lactobacillus plantarum, isolated MIMP | n.a | Murine model (DSS) Mice were supplemented with MIMP (0.1 μg/20 g) for 7 days prior colitis. Colitis was induced by DSS administered in drinking water for 7 days. Cell line Caco-2 Cell line was incubated with MIMP in different concentrations for 48 h. | Reduction in the permeability, increase in expression of JAM-1, occludin, ZO-1; | [126] |
| Summary: a beneficial effect was observed | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubczyk, D.; Leszczyńska, K.; Górska, S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)—A Critical Review. Nutrients 2020, 12, 1973. https://doi.org/10.3390/nu12071973
Jakubczyk D, Leszczyńska K, Górska S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)—A Critical Review. Nutrients. 2020; 12(7):1973. https://doi.org/10.3390/nu12071973
Chicago/Turabian StyleJakubczyk, Dominika, Katarzyna Leszczyńska, and Sabina Górska. 2020. "The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)—A Critical Review" Nutrients 12, no. 7: 1973. https://doi.org/10.3390/nu12071973
APA StyleJakubczyk, D., Leszczyńska, K., & Górska, S. (2020). The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)—A Critical Review. Nutrients, 12(7), 1973. https://doi.org/10.3390/nu12071973






