Effects of Prolonged Whey Protein Supplementation and Resistance Training on Biomarkers of Vitamin B12 Status: A 1-Year Randomized Intervention in Healthy Older Adults (the CALM Study)
Abstract
:1. Introduction
2. Materials and Methods
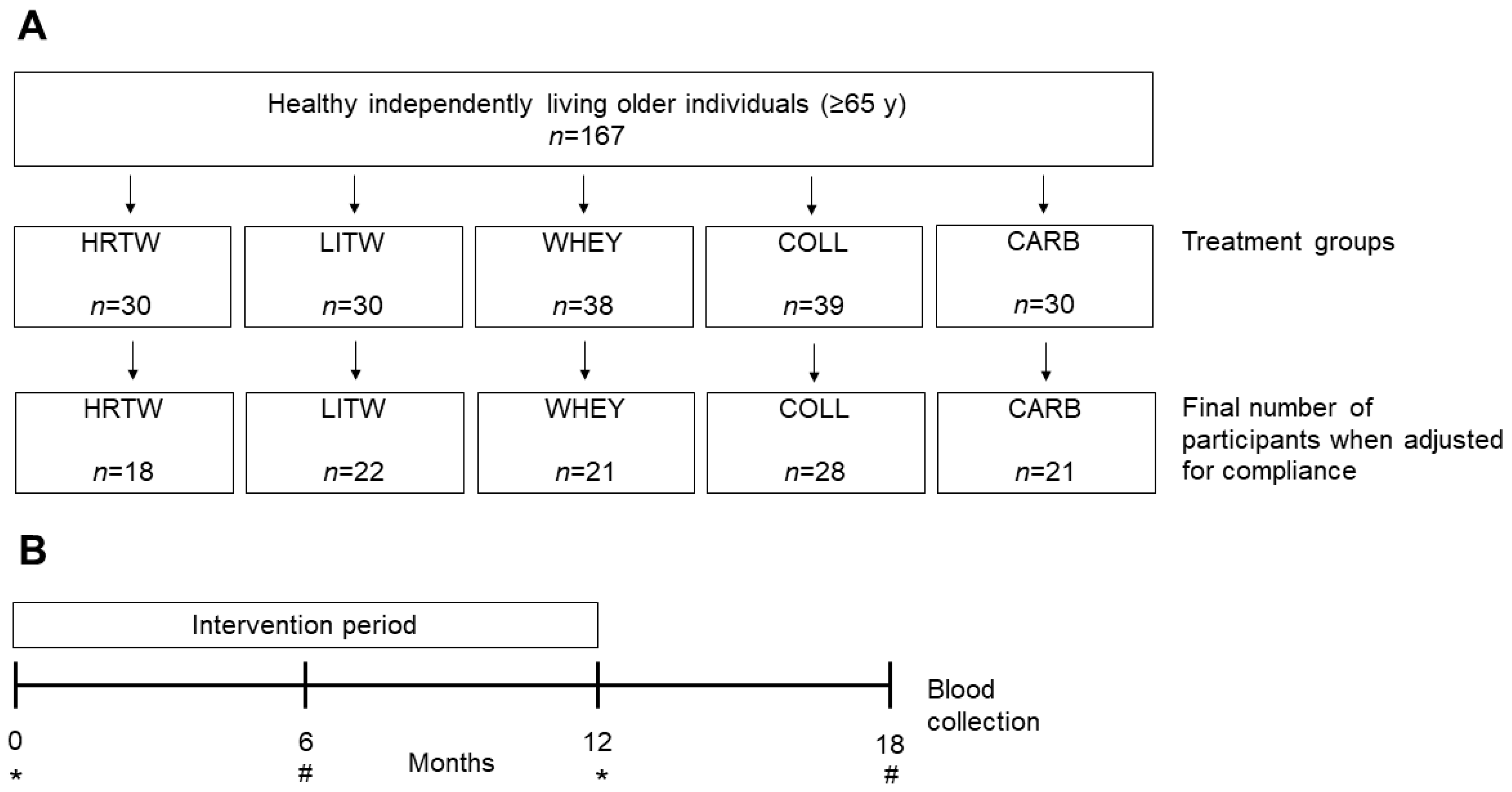
2.1. Participants and Study Design
2.2. Intervention
2.3. Biochemical Measurements
2.4. Intake of B12 from Diets and Supplements
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Dietary B12 Intake
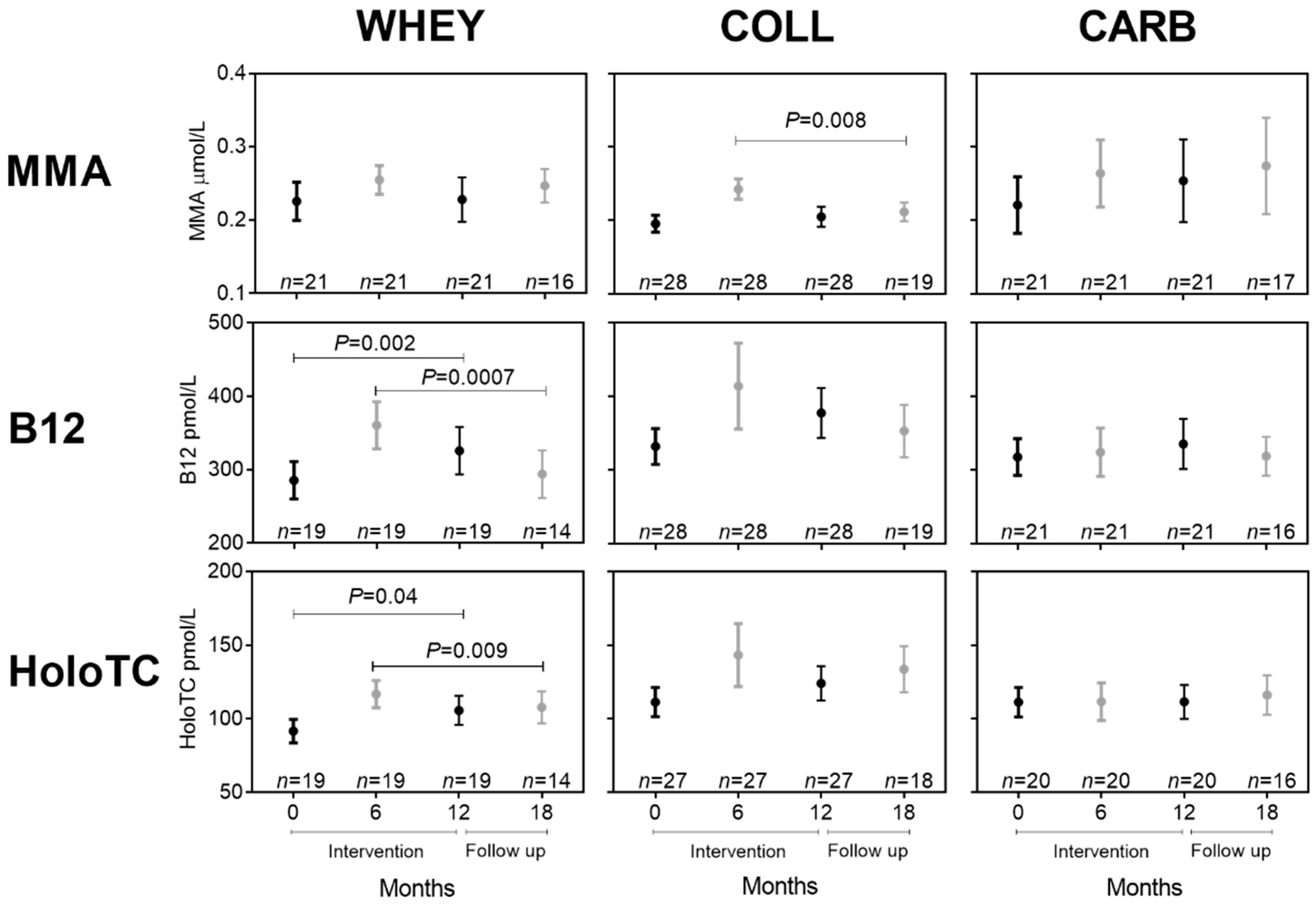
3.3. Effect of Whey Intervention on Biomarkers of B12 Status
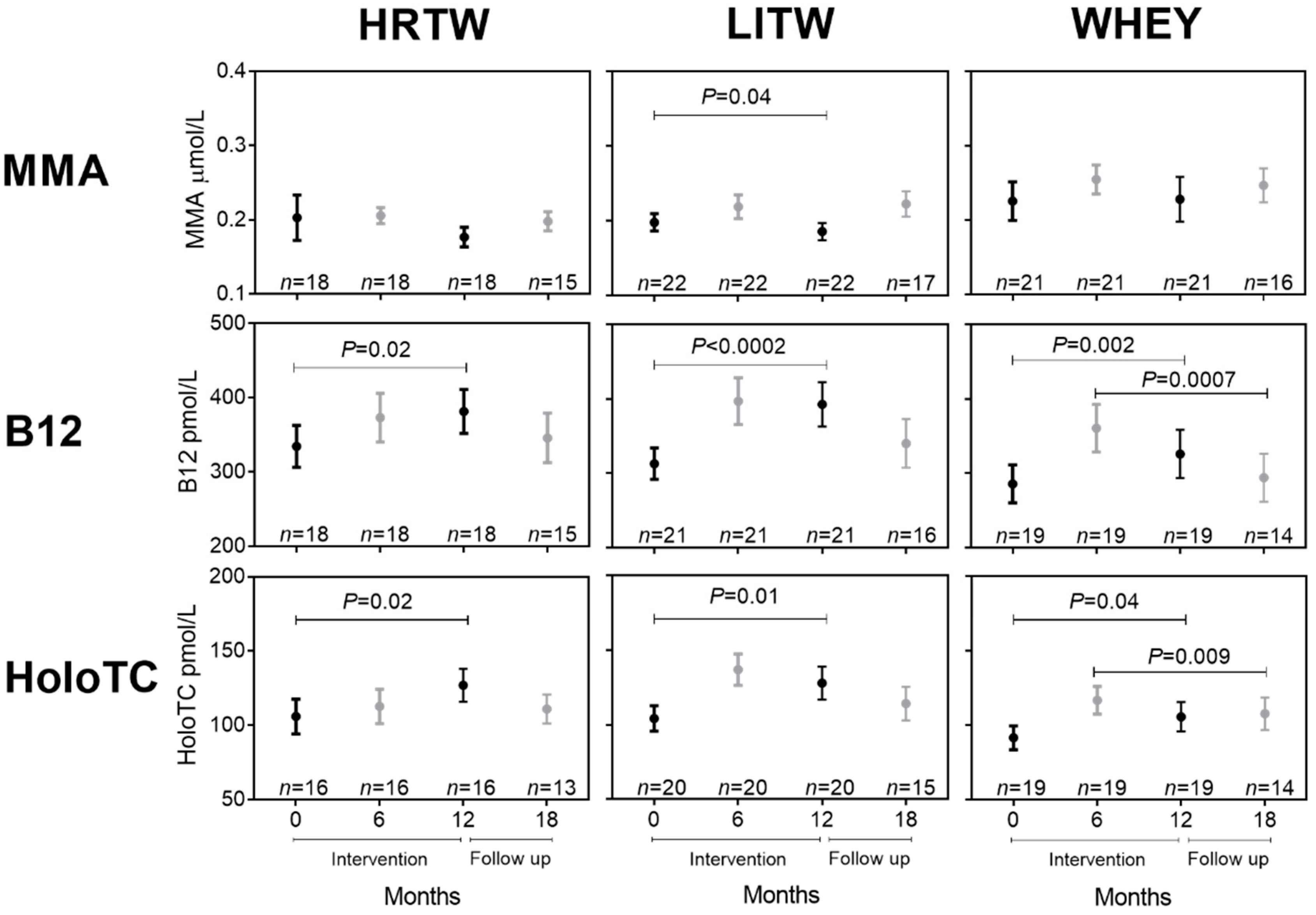
3.4. Effect of Training in Combination with Whey Intervention on Biomarkers of B12 Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.L.; Brito, A.; Guéant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B 12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P.; Lindenbaum, J.; Allen, R.H. Vitamin B-12 deficiency in the elderly: Current dilemmas. Am. J. Clin. Nutr. 1997, 66, 741–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stabler, S.P. Clinical practice. Vitamin B12 deficiency. N. Engl. J. Med. 2013, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Refsum, H.; De Jager, C.A.; Jacoby, R.; Nichols, T.E.; Smith, S.M.; Smith, A. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 9523–9528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, M.J. Vitamin B12 transport from food to the body’s cells—A sophisticated, multistep pathway. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 345–354. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Smith, A.D.; Nurk, E.; Berstad, P.; Drevon, C.A.; Ueland, P.M.; Vollset, S.E.; Tell, G.S.; Refsum, H.; Smith, A. Dietary sources of vitamin B-12 and their association with plasma vitamin B-12 concentrations in the general population: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2009, 89, 1078–1087. [Google Scholar] [CrossRef]
- Miller, D.R.; Specker, B.L.; Ho, M.L.; Norman, E.J. Vitamin B-12 status in a macrobiotic community. Am. J. Clin. Nutr. 1991, 53, 524–529. [Google Scholar] [CrossRef]
- Gao, X.; Yao, M.; McCrory, M.A.; Ma, G.; Li, Y.; Roberts, S.B.; Tucker, K.L. Dietary Pattern Is Associated with Homocysteine and B Vitamin Status in an Urban Chinese Population. J. Nutr. 2003, 133, 3636–3642. [Google Scholar] [CrossRef] [Green Version]
- Fedosov, S.; Nexø, E.; Heegaard, C.W. Binding of aquocobalamin to bovine casein and its peptides via coordination to histidine residues. Int. Dairy J. 2017, 76, 30–39. [Google Scholar] [CrossRef]
- Fedosov, S.; Nexø, E.; Heegaard, C.W. Vitamin B12 and its binding proteins in milk from cow and buffalo in relation to bioavailability of B12. J. Dairy Sci. 2019, 102, 4891–4905. [Google Scholar] [CrossRef]
- Matte, J.J.; Guay, F.; Girard, C.L. Bioavailability of vitamin B12 in cows’ milk. Br. J. Nutr. 2012, 107, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M. Bioavailability of vitamin B12. Eur. J. Clin. Nutr. 1997, 51, 49–53. [Google Scholar]
- Dhillon, V.S.; Zabaras, D.; Almond, T.; Cavuoto, P.; James-Martin, G.; Fenech, M. Whey protein isolate improves vitamin B12 and folate status in elderly Australians with subclinical deficiency of vitamin B12. Mol. Nutr. Food Res. 2017, 61, 1600915. [Google Scholar] [CrossRef] [PubMed]
- Mahalle, N.; Bhide, V.; Greibe, E.; Heegaard, C.W.; Nexø, E.; Fedosov, S.; Naik, S. Comparative Bioavailability of Synthetic B12 and Dietary Vitamin B12 Present in Cow and Buffalo Milk: A Prospective Study in Lactovegetarian Indians. Nutrients 2019, 11, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bechshøft, R.L.; Reitelseder, S.; Højfeldt, G.; Castro-Mejía, J.L.; Khakimov, B.; Ahmad, H.F.; Kjaer, M.; Engelsen, S.B.; Johansen, S.M.B.; Rasmussen, M.A.; et al. Counteracting Age-related Loss of Skeletal Muscle Mass: A clinical and ethnological trial on the role of protein supplementation and training load (CALM Intervention Study): Study protocol for a randomized controlled trial. Trials 2016, 17, 397. [Google Scholar] [CrossRef] [Green Version]
- Nexø, E.; Christensen, A.-L.; Hvas, A.-M.; Petersen, T.E.; Fedosov, S.N. Quantification of Holo-Transcobalamin, a Marker of Vitamin B12 Deficiency. Clin. Chem. 2002, 48, 561–562. [Google Scholar] [CrossRef] [Green Version]
- Schacht, S.R.; Lind, M.V.; Bechshøft, R.L.; Højfeldt, G.; Reitelseder, S.; Jensen, T.; Jespersen, A.P.; Nielsen, D.S.; Holm, L.; Tetens, I. Investigating Risk of Suboptimal Macro and Micronutrient Intake and Their Determinants in Older Danish Adults with Specific Focus on Protein Intake-A Cross-Sectional Study. Nutrients 2019, 11, 795. [Google Scholar] [CrossRef] [Green Version]
- Rustad, P.; Felding, P.; Franzson, L.; Kairisto, V.; Lahti, A.; Martensson, A.; Petersen, P.H.; Simonsson, P.; Steensland, H.; Uldall, A. The Nordic Reference Interval Project 2000: Recommended reference intervals for 25 common biochemical properties. Scand. J. Clin. Lab. Investig. 2004, 64, 271–284. [Google Scholar] [CrossRef]
- Nordin, G.; Martensson, A.; Swolin, B.; Sandberg, S.; Christensen, N.J.; Thorsteinsson, V.; Franzson, L.; Kairisto, V.; Savolainen, E. A multicentre study of reference intervals for haemoglobin, basic blood cell counts and erythrocyte indices in the adult population of the Nordic countries. Scand. J. Clin. Lab. Investig. 2004, 64, 385–398. [Google Scholar] [CrossRef]
- Rasmussen, K.; Møller, J.; Lyngbak, M.; Pedersen, A.M.; Dybkjaer, L. Age- and gender-specific reference intervals for total homocysteine and methylmalonic acid in plasma before and after vitamin supplementation. Clin. Chem. 1996, 42, 630–636. [Google Scholar] [CrossRef] [Green Version]
- Nexø, E. Variation with age of reference values for P-cobalamins. Scand. J. Haematol. 1983, 30, 430–432. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for cobalamin (vitamin B12). EFSA J. 2015, 13, 4150. [Google Scholar] [CrossRef] [Green Version]
- The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998.
- Hvas, A.-M.; Gravholt, C.H.; Nexø, E. Circadian variation of holo-transcobalamin (holo-TC) and related markers. Clin. Chem. Lab. Med. 2005, 43, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Orton, D.J.; Naugler, C.; Sadrzadeh, S.H. Fasting time and vitamin B12 levels in a community-based population. Clin. Chim. Acta 2016, 458, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Wallace, J.I.; Brodkin, K.I.; Beresford, S.A.; Allen, R.H.; Stabler, S.P. Response of Elevated Methylmalonic Acid to Three Dose Levels of Oral Cobalamin in Older Adults. J. Am. Geriatr. Soc. 2002, 50, 1789–1795. [Google Scholar] [CrossRef]
- Eussen, S.J.; De Groot, L.C.; Clarke, R.; Schneede, J.; Ueland, P.M.; Hoefnagels, W.H.L.; Van Staveren, W.A. Oral Cyanocobalamin Supplementation in Older People With Vitamin B12 Deficiency. Arch. Intern. Med. 2005, 165, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Obeid, R.; Scharhag, J.; Kindermann, W.; Herrmann, W. Altered vitamin B12 status in recreational endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 433–441. [Google Scholar] [CrossRef] [Green Version]
- De Jong, N.; Paw, M.J.C.A.; De Groot, L.C.; Rutten, R.A.; Swinkels, R.W.; Kok, F.J.; Van Staveren, W.A. Nutrient-dense foods and exercise in frail elderly: Effects on B vitamins, homocysteine, methylmalonic acid, and neuropsychological functioning. Am. J. Clin. Nutr. 2001, 73, 338–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Marker | Reference Intervals | Participants n = 167 |
|---|---|---|
| MMA µmol/L | 0.08–0.28 | 0.19 (0.11–1.60) n = 167 |
| B12 pmol/L | 200–600 | 301 (98–740) n = 161 |
| HoloTC pmol/L | 40–150 | 93 (22–264) n = 156 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greibe, E.; Reitelseder, S.; Bechshøft, R.L.; Bülow, J.; Højfeldt, G.W.; Schacht, S.R.; Knudsen, M.L.; Tetens, I.; Ostenfeld, M.S.; Mikkelsen, U.R.; et al. Effects of Prolonged Whey Protein Supplementation and Resistance Training on Biomarkers of Vitamin B12 Status: A 1-Year Randomized Intervention in Healthy Older Adults (the CALM Study). Nutrients 2020, 12, 2015. https://doi.org/10.3390/nu12072015
Greibe E, Reitelseder S, Bechshøft RL, Bülow J, Højfeldt GW, Schacht SR, Knudsen ML, Tetens I, Ostenfeld MS, Mikkelsen UR, et al. Effects of Prolonged Whey Protein Supplementation and Resistance Training on Biomarkers of Vitamin B12 Status: A 1-Year Randomized Intervention in Healthy Older Adults (the CALM Study). Nutrients. 2020; 12(7):2015. https://doi.org/10.3390/nu12072015
Chicago/Turabian StyleGreibe, Eva, Søren Reitelseder, Rasmus L. Bechshøft, Jacob Bülow, Grith W. Højfeldt, Simon R. Schacht, Mads L. Knudsen, Inge Tetens, Marie S. Ostenfeld, Ulla R. Mikkelsen, and et al. 2020. "Effects of Prolonged Whey Protein Supplementation and Resistance Training on Biomarkers of Vitamin B12 Status: A 1-Year Randomized Intervention in Healthy Older Adults (the CALM Study)" Nutrients 12, no. 7: 2015. https://doi.org/10.3390/nu12072015





